Abstract
To investigate the impact of mipomersen, an apolipoprotein B-100 (apoB) synthesis inhibitor, on intra-hepatic triglyceride content (IHTG content), we conducted a randomized, double-blind, placebo-controlled study in 21 patients with familial hypercholesterolemia (FH). Subjects received a weekly subcutaneous dose of 200 mg mipomersen or placebo for 13 weeks while continuing conventional lipid lowering therapy. The primary endpoint was change in IHTG content from week 0 to week 15 as measured by localized proton magnetic resonance spectroscopy (1H-MRS). Thirteen weeks of mipomersen administration reduced LDL-cholesterol by 22.0 (17.8) % and apoB by 19.9 (17.4) % (both P < 0.01). One of 10 patients (10%) in the mipomersen-treated group developed mild hepatic steatosis at week 15, which was reversible following mipomersen discontinuation. For the group, there was a trend toward an increase in IHTG content [placebo; baseline: 1.2% and week 15: 1.1%; change −0.1 (0.9). Mipomersen; baseline: 1.2% and week 15: 2.1%; change 0.8 (1.7) (P = 0.0513)]. Mipomersen administration for 13 weeks to subjects with FH is associated with a trend toward an increase in IHTG content. Future studies evaluating the effects of long-term use of mipomersen reaching more profound reductions in apoB are required prior to broader use of this compound.
Keywords: antisense oligonucleotides, drug therapy, familial hypobetalipoproteinemia, hepatic steatosis, LDL-cholesterol, localized proton magnetic resonance spectroscopy, mipomersen
ApoB is the main structural component of all atherogenic lipid particles and is obligatory for the secretion of VLDL from the liver (1, 2). As a consequence, apoB synthesis inhibition has been put forward as a potentially valuable approach to achieve target levels for LDL-cholesterol in subjects with heterozygous familial hypercholesterolemia (FH), who are characterized by profoundly elevated baseline cholesterol levels and are unable to achieve target levels with conventional lipid lowering therapy (3, 4). In previous clinical trials, mipomersen, a second-generation antisense oligonucleotide targeted to human apoB, has been shown to induce dose-dependent reductions in all apoB-containing lipoproteins. A drug elimination half-life of ∼30 days resulted in a prolonged pharmacological effect and the drug was overall well tolerated (5, 6).
Intrinsic to its mechanism of action, apoB synthesis inhibition may result in an impaired secretion of triglyceride-rich VLDL particles from the liver, potentially leading to accumulation of triglycerides in the liver. In fact, inhibition of microsomal triglyceride transfer protein (MTP), which is involved in VLDL synthesis downstream of apoB, has been shown to result in hepatic steatosis in both experimental animals and humans (7, 8). Indeed, hepatic enzyme increases have been observed during treatment with mipomersen, particularly following treatment with higher doses. In line with this observation, during follow up of some patients with elevated liver enzymes, hepatic steatosis was detected (9). Since baseline values in these patients were absent and hepatic steatosis is prevalent in the general population (10), a definite causal relationship between administration of mipomersen and hepatic steatosis could not be established.
Insight into the long-term sequelae of apoB synthesis inhibition can be obtained indirectly from the lessons learned from a “natural variant” of low apoB concentrations known as familial hypobetalipoproteinemia (FHBL). FHBL is primarily caused by mutations in the apoB gene, resulting in truncated forms of apoB (11). FHBL subjects are characterized by extremely low levels of apoB and plasma LDL-cholesterol and appear to be protected against atherosclerotic disease (12). The majority of patients with FHBL are characterized by severe hepatic steatosis (13, 14). In contrast, hepatic steatosis is absent in a particular subset of patients with FHBL who are not characterized by truncated forms of apoB (13). Therefore, the relation between decreased availability of apoB and IHTG content is not completely clear.
To evaluate the effects of mipomersen on IHTG content, a randomized, double-blind, placebo-controlled study was designed in patients with heterozygous familial hypercholesterolemia (FH) on conventional lipid-lowering therapy. In addition we evaluated IHTG content over time in a “positive control group” of subjects with FHBL.
METHODS
Study participants
Twenty-one subjects with heterozygous FH were selected for the treatment group. Men and women between the age of 18 and 75 with a diagnosis of heterozygous FH were eligible. FH had to be diagnosed either by genotyping or by fulfilling the criteria for the diagnosis of FH as outlined by the World Health Organization (15). LDL-cholesterol was > 2.6 mmol/l; plasma triglyceride levels < 2.26 mmol/l; HbA1c < 6%; plasma glucose ≤ 5.8 mmol/l; alanine amino transferase (ALT) ≤1.5 × ULN; and total bilirubin ≤1.0 × ULN at screening. All patients had been using stable lipid-lowering therapy for at least 3 months prior to screening. Alcohol consumption had to be less than three units (30 g) per day and 12 units (120 g) per week for male subjects; and less than two units (20 g) per day and eight units (80 g) per week for female subjects. Patients with IHTG content exceeding 5% at baseline were excluded from the trial.
In addition, six subjects with FHBL were included as a “positive control” group that did not receive treatment. The participants in the FHBL group had to have documented disease due to an apoB-gene mutation; triglyceride levels < 2.26 mmol/l; HbA1c < 6.0%; ALT ≤ 3.0 × ULN; and total bilirubin ≤ 1.0 × ULN at screening.
All study participants were enrolled at the Academic Medical Centre in Amsterdam. The study protocol was approved by the local institutional review board. All subjects gave written, informed consent. The study was performed in compliance with the standards of Good Clinical Practice (CPMP/ICH/135/95) and the declaration of Helsinki (Washington, 2002).
Study design
Subjects with FH were selected to investigate the effects of mipomersen, 200 mg/wk for 13 weeks, on IHTG content assessed using 1H-MRS. Patients were randomized at a 1:1 ratio (active: placebo). Patients, investigators, and study staff were blinded to the treatment assignment with the exception of the pharmacist who prepared the study drug. Study drug was administered subcutaneously on days 1,8,15, 22, 29, 36, 43, 50, 57, 64, 71, 78 and 85 (13-week treatment period). The 200 mg/wk dose was selected based on safety and efficacy data from previous clinical trials.
IHTG content was assessed at baseline, week 4, and week 15. Since ALT elevations observed in previous clinical trials clustered around week 4, this time was chosen for the first follow-up 1H-MRS. The main safety endpoint included change in IHTG content from baseline to week 15. Prespecified efficacy endpoints included percentage reduction in LDL-cholesterol and apoB from baseline. Due to the long half-life of mipomersen, efficacy and safety endpoints were analyzed 2 weeks after the last dose of study drug at week 15. The treatment period was followed by a 5-month evaluation period with monthly visits.
As a comparator for the 1H-MRS values observed in patients using apoB antisense inhibition, we simultaneously assessed IHTG content in six patients with FHBL.
Lipid and lipoprotein analysis
Fasting blood samples were analyzed for lipids and lipoproteins by MedPace (Cincinnati, OH). ApoB, apoA1, and lipoprotein (a) (Lpa) concentrations were determined by rate nephelometry. Total cholesterol (TC) and triglycerides (TG) were measured by standard enzyme-based colorimetric assays. HDL-cholesterol was determined by an enzyme-based colorimetric assay after dextran-sulfate precipitation. LDL- cholesterol and non–HDL-cholesterol were calculated.
1H-MRS
1H-MRS was used to quantify IHTG concentration. 1H-MRS is a noninvasive technique by which liver triglyceride concentrations can be quantified. This technique has been shown to be well correlated with liver biopsy data in healthy individuals and patients with hepatic steatosis (16). 1H-MRS was performed at screening, at the end of week 4 and at week 15, 2 weeks after the last dose of mipomersen. Two voxels of 20 × 20 × 20 mm (8000 mm3) were positioned in the right lobe of the liver. Spectra were acquired on a 3.0 Tesla MRI scanner (Philips Medical Systems, Best, The Netherlands) using first order iterative shimming, a PRESS sequence with TE/TR = 35/2000 ms and 64 signal acquisitions. The water and fat resonance peaks, located at 4.65 and 1.3 ppm, were integrated using jMRUI software (17). Relative fat content was expressed as a ratio of the fat peak area over the cumulative water and fat peak areas. Calculated peak areas of water and fat were corrected for T2 relaxation (T2 water = 34 ms, T2 fat = 68 ms) (18), and the percentage hepatic fat content was calculated according to Szczepaniak et al. (10). Reproducibility of 1H-MRS to measure IHTG content using this method has been shown to be high for “between weeks” measurements in fatty livers (19). An IHTG concentration of > 5.6% was accepted as the ULN (10).
Safety monitoring
Safety and tolerability of mipomersen was assessed by determining the incidence, severity, and possible relationship to the study drug of adverse events and laboratory parameters, including blood chemistry, routine hematology, coagulation, and urinalysis. Vital functions were recorded on visits for days 36, 60, 64, and 78. Full physical examination was performed at screening, week 15, and week 25. A 12-lead electrocardiogram was recorded at screening, week 4, and week 15. Subjects returned to the study center 2 weeks after the last dose of study drug for evaluation of liver triglyceride content by 1H-MRS. If IHTG content appeared to be > 10% or showed an increase of 100% relative to screening values or if ALT levels were > 2.0 × ULN, results were submitted to the Data and Safety Monitoring Board (DSMB). Subsequently the DSMB recommended whether another 1H-MRS evaluation should be performed at week 25.
Statistical analysis
Study endpoints were analyzed on the intention-to-treat population, consisting of all 21 subjects randomized as well as on the 6 FHBL positive controls. Missing lipid parameter and liver triglyceride content values at day 99 were replaced by the last observation carried forward method (LOCF). Demographic and baseline characteristics were summarized using descriptive statistics. Baseline was defined as the last value prior to the first dose both for lipid parameters and IHTG content. Efficacy endpoints were analyzed 2 weeks after the last dose, at day 99 with a window of +6/−7 days. Percentage change from baseline for lipid parameters and absolute change from baseline for IHTG content were compared between the two treatment groups using the analysis Exact Wilcoxon Rank Sum test with a 0.050 two-sided significance level. Sample size was based upon a standard deviation of 0.65 in the absolute percentage change for IHTG -content and analysis of the data between the two treatment groups (19). Under these assumptions, a sample size of 7 per group would provide 80% power to detect a 2% difference in change for IHTG content with a 0.050 two-sided significance level. Software utilized for the analyses was SAS version 8.2 (SAS Institute, Cary, NC).
RESULTS
Study participants
Twenty-one subjects with heterozygous FH and fulfilling all other entry criteria were randomly assigned to either mipomersen or placebo treatment. Demographics and baseline lipid-lowering therapy by treatment group are summarized in Table 1. All subjects were on stable lipid-lowering therapy. Baseline lipid parameters were comparable between the treatment groups (Table 2). Twenty subjects completed the study protocol. One mipomersen-treated subject discontinued treatment after the first dose due to flu-like symptoms.
TABLE 1.
Demographics and baseline lipid-lowering therapy
| Placebo N = 11 | Mipomersen N = 10 | FHBL N = 6 | |
|---|---|---|---|
| Gender (M:F)a | 3:8 | 6:4 | 5:1 |
| Age | 46 (1)a | 49 (12)a | 49 (16)a |
| BMI | 26a | 27a | 30a |
| Statin (mg/day) | |||
| Atorvastatin | |||
| 20 | 1 (10)b | ||
| 40 | 1 (9)b | 2 (20)b | |
| 80 | 4 (36)b | 1 (10)b | |
| Rosuvastatin | |||
| 5 | 2 (18)b | ||
| 20 | 1 (9)b | 1 (10)b | |
| 40 | 2 (18)b | 4 (40)b | |
| Simvastatin | |||
| 80 | 1 (10)b | ||
| Pravastatin | |||
| 40 | 1 (9)b | ||
| Ezetemibe | |||
| 10 | 7 (64)b | 9 (90)b | |
| Other | 1 (9)b | 1 (10)b |
BMI, body mass index; F, female; FHBL, familial hypobetalipoproteinemia; M, male.
Values are the mean and (standard deviation).
Values are the number of subjects (and percentage) by drug type and dose.
TABLE 2.
Baseline lipid parameters
| Lipids (mg/dl) | Placebo (N = 11) | Mipomersen (N = 10) | FHBL (N = 6) |
|---|---|---|---|
| LDL-C | 155 ± 31 | 155 ± 37 | 46 ± 18 |
| VLDL-C | 18 ± 6 | 20 ± 7 | 8 ± 3 |
| Non-HDL-C | 173 ± 35 | 175 ± 38 | 54 ± 19 |
| HDL-C | 46 ± 10 | 47 ± 13 | 56 ± 6 |
| TC | 219 ± 29 | 222 ± 37 | 110 ± 20 |
| TG | 91 (52–133)a | 102 (51–166)a | 32 (23–63)a |
| Apo A1 | 144 ± 19 | 149 ± 26 | 156 ± 20 |
| Apo B | 124 ± 24 | 131 ± 29 | 35 ± 10 |
| Lp(a) | 50 ± 62 | 78 ± 74 | 22 ± 22 |
Data are presented as the mean ± standard deviation. Apo, apolipoprotein; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; LP(a), lipoprotein (a); TC, total cholesterol TG, triglycerides; VLDL-C, VLDL-cholesterol.
Data are presented as the median (min–max).
Efficacy
Thirteen weeks of treatment with mipomersen 200 mg/wk resulted in significant reductions in apoB of 19.9% (P < 0.001) with a mean apoB at week 15 of 104.7 mg/dl and concomitant reductions in LDL-cholesterol of 22% (P < 0.01) with a mean LDL-cholesterol at week 15 of 118.1 mg/dl (Table 3).
TABLE 3.
Percentage change in lipid parameters after 13 weeks of treatment
| Change from baseline lipid parameter (%) | Placebo (N = 11) | Mipomersen (N = 10) |
|---|---|---|
| LDL-C | 1.0 ± 16.5 | −22.0 ± 17.8a |
| VLDL-C | −3.1 ± 24.8 | −13.2 ± 13.8 |
| Non-HDL-C | 0.4 ± 16.3 | −21.3 ± 16.6b |
| HDL-C | 7.5 ± 12.7 | 4.0 ± 9.2 |
| TC | 1.9 ± 14.9 | −16.4 ± 13.4a |
| TG | −7 (−45.9–42.3)c | −16.3 (−35.1–9.8)c |
| Apo A1 | 2.2 ± 12.0 | −1.3 ± 8.6 |
| Apo B | 5.7 ± 13.1 | −19.9 ± 17.4d |
| Lp(a) | 8.0 ± 16.1 | −19.6 ± 14.9d |
Data was analyzed at week 15. Data are presented as the mean ± standard deviation. Apo, apolipoprotein; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; LP(a), lipoprotein (a); TC, total cholesterol TG, triglycerides; VLDL-C, VLDL-cholesterol.
P < 0.01 compared with placebo using the Exact Wilcoxon Rank sum test.
P < 0.05 compared with placebo using the Exact Wilcoxon Rank sum test.
Data are presented as the median (min–max).
P < 0.001 compared with placebo using the Exact Wilcoxon Rank sum test.
1H-MRS
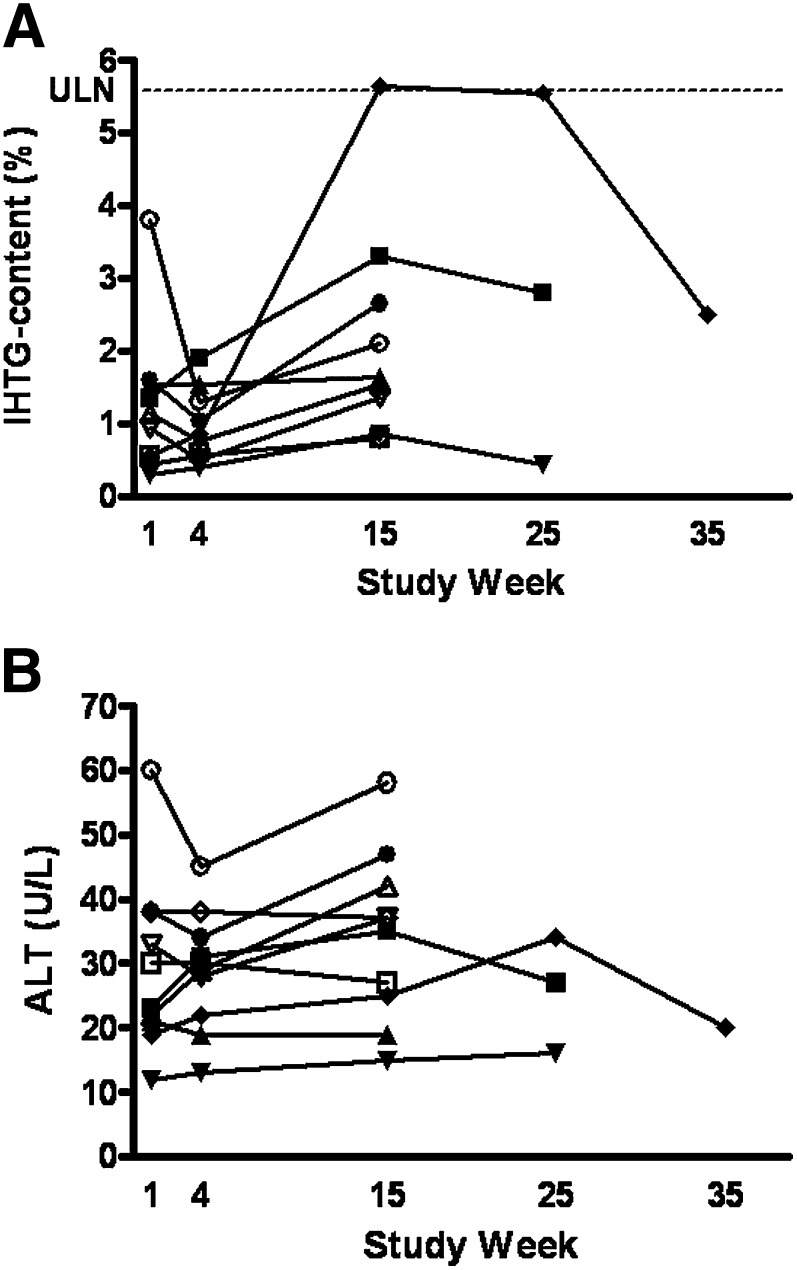
Baseline IHTG content was similar between the two treatment groups with a mean of 1.2% and a range of 0.2–3.2% for the placebo and 0.3–3.8% for the treatment group (Table 4). Thirteen weeks of treatment with mipomersen resulted in a mean change from baseline for IHTG content of 0.8 percentage points compared with −0.1 percentage points in the placebo group at week 15 (P = 0.0513). One subject treated with mipomersen exceeded the upper limit of normal for IHTG content of 5.6%. In this particular subject, IHTG content increased from 0.6% at baseline to 5.7% at week 15. During follow-up IHTG content dropped to 5.6% at week 25 and to 2.5% at week 35 (Fig. 1A). In two other subjects in the mipomersen group and in three subjects in the placebo group, additional IHTG content measurements at week 25 were performed on recommendation of the DSMB because of increases in IHTG content > 100% relative to screening values. Both subjects in the mipomersen treatment group showed reduction in IHTG content at week 25 compared with week 15 (Fig. 1A and Fig. 2A).
TABLE 4.
Changes in intra-hepatic triglyceride content
| IHTG content (%) | FHBL no-dosing (n = 6) | HeFH placebo (n = 11) | Mipomersen (n = 10) |
|---|---|---|---|
| Baseline | |||
| Mean ± SD | 21.9 ± 6.5 | 1.2 ± 1.0 | 1.2 ± 1.0 |
| Range | 13.2 – 30.1 | 0.2 – 3.2 | 0.3 – 3.8 |
| Week 4 | |||
| Mean ± SD | 21.7 ± 5.9 | 1.2 ± 1.1 | 1.0 ± 0.5 |
| Range | 11.9 – 27.6 | 0.2 – 3.3 | 0.4 – 1.9 |
| Week 15 | |||
| Mean ± SD | 22.6 ± 6.8 | 1.1 ± 0.8 | 2.1 ± 1.5 |
| Range | 12.7 – 31.0 | 0.2 – 2.5 | 0.8 – 5.7 |
| Change from baseline to week 15 | |||
| Mean ± SD | 0.7 ± 2.1 | −0.1 ± 0.9 | 0.8 ± 1.7a |
| Range | −2.0 – 4.3 | −1.5 – 1.3 | −1.7 – 5.1 |
FHBL, familial hypobetalipoproteinemia; HeFH, heterozygous familial hypercholesterolemia; IHTG, intra-hepatic triglyceride content.
P = 0.0513 compared with placebo using the Exact Wilcoxon Rank sum test.
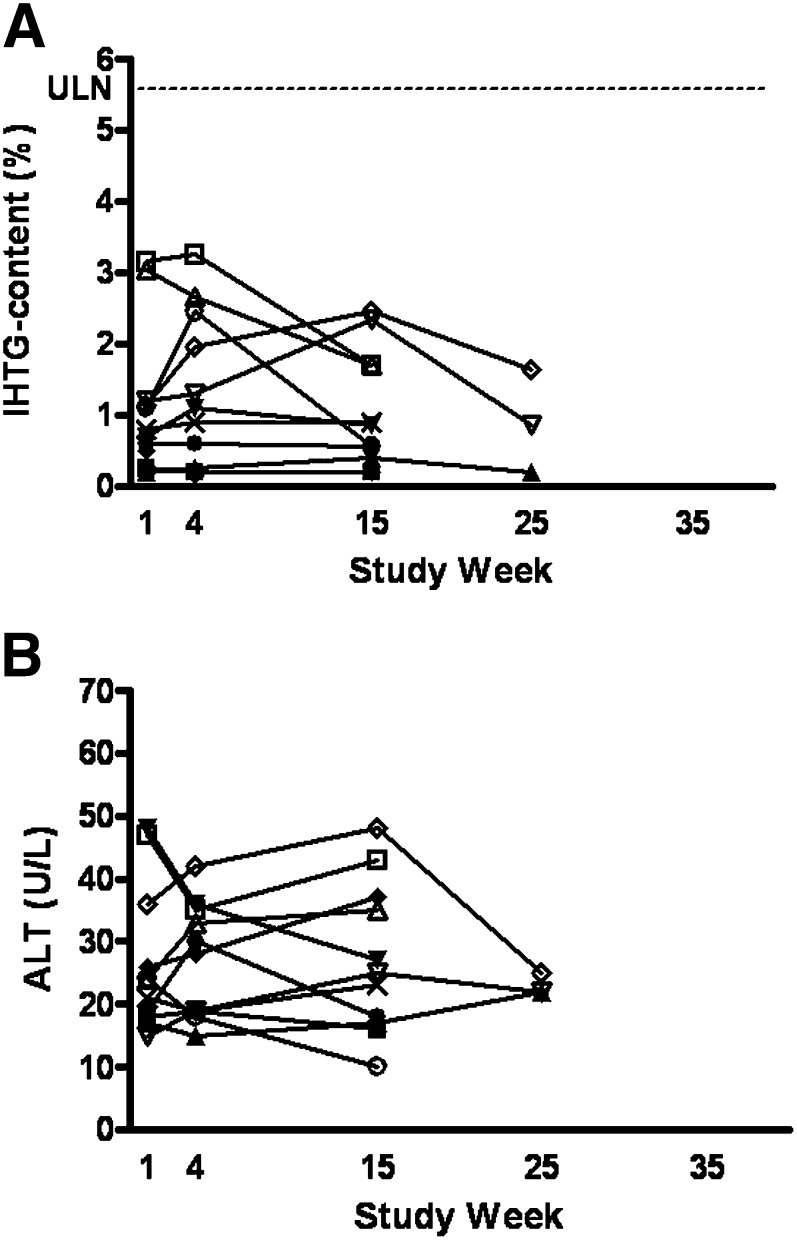
Fig. 1.
IHTG content (A) en ALT (B) in time for the placebo treatment group. Dotted line represents ULN = 5.6% for IHTG content. ALT, alanine aminotransferase; IHTG, intra-hepatic triglyceride content; ULN, upper limit of normal.
Fig. 2.
IHTG content (A) and ALT (B) in time for mipomersen treatment group. Dotted line represents ULN = 5.6% for IHTG content. ALT, alanine aminotransferase; IHTG, intra-hepatic triglyceride content; ULN, upper limit of normal.
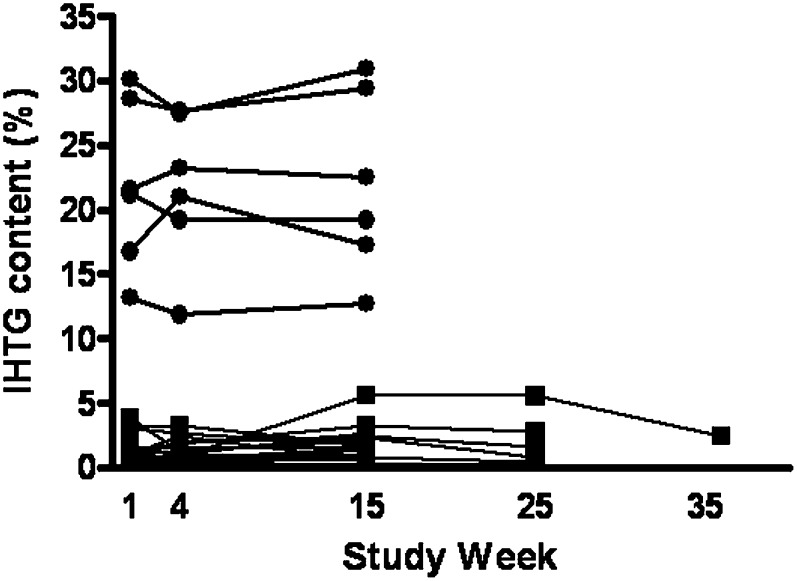
1H-MRS measurements in FHBL subjects at baseline showed a mean IHTG content of 21.9% with a range of 13.2–30.1%. Mean change from baseline to week 15 for this group was 0.7 percentage points (Table 4 and Fig. 3).
Fig. 3.
IHTG content in time for FHBL subjects and both placebo and mipomersen treatment groups. ULN = 5.6% for IHTG content. Closed circles represent FHBL subjects. Closed squares represent treatment group. FHBL, familial hypobetalipoproteinemia; IHTG, intra-hepatic triglyceride content; ULN, upper limit of normal.
Safety
No serious adverse events occurred in this study. The most common adverse events were injection site reactions (ISR) following subcutaneous administration of mipomersen (Table 5). ISRs were generally characterized by erythema that occurred within 24 h after the injection. ISRs did not worsen on repeated dosing. All subjects treated with mipomersen experienced at least one ISR. Of all injections with mipomersen 19% (22/118) resulted in ISR compared with 9% (13/142) in the placebo group. Other adverse events with > 10% incidence in the mipomersen treatment group were influenza-like illness, influenza, nasopharyngitis, headache, fatigue, mylagia, back pain, abdominal pain, nausea, and cough.
TABLE 5.
Treatment-emergent adverse events (>10% Mipomersen)
| Event | Placebo (n = 11) | Mipomersen (n = 10) |
|---|---|---|
| Injection site reaction | 8 (73) | 10 (100) |
| Influenza-like illness | 2 (18) | 7 (70) |
| Influenza | 1 (9) | 3 (30) |
| Nasopharyngitis | 4 (36) | 3 (30) |
| Headache | 6 (55) | 3 (30) |
| Fatigue | 5 (46) | 2 (20) |
| Myalgia | 1 (9) | 2 (20) |
| Back pain | 0 (0) | 2 (20) |
| Upper abdominal pain | 0 (0) | 2 (20) |
| Nausea | 0 (0) | 2 (20) |
| Cough | 0 (0) | 2 (20) |
The numbers in parentheses are percentages.
There were no clinically significant increases in ALT (>3 × ULN) (Figs. 1B and 2B) or other measures of liver function, such as prothrombin time, albumin, or bilirubin. Vital signs, electrocardiogramm and urinalysis did not show any clinically significant changes.
DISCUSSION
Mipomersen at a dose of 200 mg/wk for 13 weeks in patients with FH achieved incremental reductions in LDL-cholesterol with a concomitant trend toward increased IHTG content, which did not reach statistical significance. New-onset hepatic steatosis was observed in one patient, which was reversible after discontinuation of the compound. There were no increases in hepatic transaminase levels exceeding 3 × ULN.
The findings in this study may have divergent implications. On the one hand, there was no profound change in IHTG content following 13 weeks of apoB synthesis inhibition. This observation is in apparent contradiction with the severe hepatic steatosis observed in patients with FHBL as well as patients receiving MTP inhibition (7, 8). With respect to the FHBL population, this disorder is genotypically and phenotypically heterogeneous (20). The vast majority of FHBL variants are caused by mutations in the apoB gene, resulting in the formation of truncated forms of apoB. The truncated apoB particles are secreted at lower rate and more rapidly cleared from the plasma, resulting in circulating apoB levels approximately 25% of normal (21–23) with a concomitant 3–5-fold increase in hepatic triglyceride content (13, 14). In contrast, mutations linked to the 3p21 locus result in qualitatively normal and only quantitatively abnormal apoB. Due to decreased secretion of apoB, this FHBL variant is associated with circulating apoB plasma levels of approximately 44% of normal (23). Interestingly, in these FHBL patients hepatic steatosis is not present (24). The genes involved in this particular FHBL variant have not been identified yet and unfortunately the mechanism for the absence of fatty liver in FHBL linked to 3p21 remains unknown (25). Therefore, the relation between decreased availability of apoB and IHTG content is not fully elucidated.
The second comparison concerns the use of MTP inhibitors. MTP mediates the transfer of triglycerides on to the apoB particles prior to export from the liver (26). In both experimental models and clinical trials, the inhibition of MTP was associated with profound increases in IHTG content (7, 8). These findings fueled the concept that interference with triglyceride-rich VLDL secretion from the liver may invariably contribute to hepatic steatosis. Yet, although apoB acts in the same pathway as MTP, the present observation does not support severe steatotic responses following mipomersen administration. In line with these findings, apoB synthesis inhibition did not result in hepatic triglyceride accumulation in specific mouse and monkey models, in contrast to the use of MTP inhibitors in these same models (27, 28). The latter has mechanistically been ascribed to a compensatory decrease in fatty acid synthesis combined with an increase in fatty acid oxidation during apoB synthesis inibition, as was suggested by results from microarray analysis of liver mRNA from these experimental models (28). Such effects may in part explain the heterogeneous findings between these two treatment strategies (27).
On the other hand, in the current trial a trend toward an increased IHTG content was observed, accompanied by one subject progressing into new-onset hepatic steatosis in spite of a relatively modest group size. In this respect, it should be noted that the present study was designed to detect large differences in IHTG content between the two treatment groups, similar to the severe increases reported following MTP inhibition (7). In addition, lipid reductions were less than those reported in previous clinical trials with mipomersen. In an earlier study, treatment with mipomersen 200 mg/wk resulted in reductions of apoB of 36% compared with 19.9% in the present study. In addition increases in ALT (>3 × ULN) were observed in 50% of the study subjects in this cohort (9). In the latter trial, however, a loading dose consisting of two additional doses of mipomersen was administered during week 1. A dose of 200 mg of mipomersen weekly without a loading dose regimen has been suggested to produce steady-state tissue concentrations no earlier than after 26 weeks of treatment (29). Since tissue concentrations after 13 weeks of treatment are thought to be in the range of 70% of steady state, an additional reduction in apoB can be expected following prolonged exposure. Taking into account that the degree of apoB synthesis inhibition may determine the extent of hepatic triglyceride accumulation, the present findings do not rule out the possibility of a steatotic response following prolonged treatment with mipomersen. Steatotic changes following inhibition of VLDL excretion by the liver may, however, differ from hepatic steatosis observed in patients with nonalcoholic fatty liver disease (NAFLD) (30). In fact, although long-term follow up data on liver safety in FHBL is scarce, hepatic fat accumulation in patients with FHBL has not been associated with liver disease (31). Conversely, in NAFLD, progression to fibrosis, cirrhosis, and even hepatocellular carcinoma has been reported (32, 33). Although the present observation of minor changes in IHTG content does not raise safety concerns, these findings do require further validation following prolonged treatment with mipomersen, particularly in subjects at increased risk of hepatic steatosis.
SUMMARY
ApoB synthesis inhibition is an attractive target to establish incremental LDL-cholesterol lowering on top of statin therapy in patients with FH. However, the tendency toward an increase in IHTG content combined in one patient with new onset hepatic steatosis as observed in the mipomersen group in the present study, underscores the need for additional safety studies. Specifically, the effect of mipomersen on IHTG content needs to be evaluated following prolonged treatment duration and in a larger number of subjects, including those at increased risk of hepatic steatosis.
Footnotes
Abbreviations:
- 1H-MRS
- magnetic resonance spectroscopy
- ALT
- alanine aminotransferase
- Apo
- apolipoprotein
- FH
- familial hypercholesterolemia
- FHBL
- familial hypobetalipoproteinemia
- IHTG
- intra-hepatic triglyceride content
- ISR
- injection site reaction
- MTP
- microsomal triglyceride transfer protein
- NAFLD
- nonalcoholic fatty liver disease
- TC
- total cholesterol
- TG
- triglycerides
- ULN
- upper limit of normal
This study was funded by ISIS Pharmaceuticals, Inc., Carlsbad, CA This study is registered at clinicaltrials.gov as NCT00362180.
REFERENCES
- 1.Elovson J., Chatterton J. E., Bell G. T., Schumaker V. N., Reuben M. A., Puppione D. L., Reeve J. R., Jr., Young N. L. 1988. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J. Lipid Res. 29: 1461–1473. [PubMed] [Google Scholar]
- 2.Davis R. A. 1999. Cell and molecular biology of the assembly and secretion of apolipoprotein B-containing lipoproteins by the liver. Biochim. Biophys. Acta. 1440: 1–31. [DOI] [PubMed] [Google Scholar]
- 3.Stein E., Stender S., Mata P., Sager P., Ponsonnet D., Melani L., Lipka L., Suresh R., Maccubbin D., Veltri E. 2004. Achieving lipoprotein goals in patients at high risk with severe hypercholesterolemia: efficacy and safety of ezetimibe co-administered with atorvastatin. Am. Heart J. 148: 447–455. [DOI] [PubMed] [Google Scholar]
- 4.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults Adult Treatment Panel III final report. 2002. Circulation.. 106: 3143–3421. [PubMed] [Google Scholar]
- 5.Akdim F., Stroes E. S. G., Sijbrands E. J. G., Tribble D. L., Trip M. D., Jukema J. W., Flaim J. D., Su J., Yu R. Z., Baker B. F., et al. 2009. Efficacy and safety of mipomersen, an apolipoprotein b synthesis inhibitor, in hypercholesterolemic subjects on stable statin therapy. J. Am. Coll. Cardiol. In press. [DOI] [PubMed] [Google Scholar]
- 6.Kastelein J. J., Wedel M. K., Baker B. F., Su J., Bradley J. D., Yu R. Z., Chuang E., Graham M. J., Crooke R. M. 2006. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 114: 1729–1735. [DOI] [PubMed] [Google Scholar]
- 7.Cuchel M., Bloedon L. T., Szapary P. O., Kolansky D. M., Wolfe M. L., Sarkis A., Millar J. S., Ikewaki K., Siegelman E. S., Gregg R. E., et al. 2007. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356: 148–156. [DOI] [PubMed] [Google Scholar]
- 8.Chandler C. E., Wilder D. E., Pettini J. L., Savoy Y. E., Petras S. F., Chang G., Vincent J., Harwood H. J., Jr. 2003. CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J. Lipid Res. 44: 1887–1901. [DOI] [PubMed] [Google Scholar]
- 9.Akdim F., Visser M. E., Tribble D. L., Baker B. F., Stroes E. S. G., Yu R., Flaim J. D., Su J., Stein E. A., Kastelein J. J. P. 2010. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density- lipoprotein cholesterol in patients with familial hypercholesterolemia. Am. J. Cardiol. In press. [DOI] [PubMed] [Google Scholar]
- 10.Szczepaniak L. S., Nurenberg P., Leonard D., Browning J. D., Reingold J. S., Grundy S., Hobbs H. H., Dobbins R. L. 2005. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 288: E462–E468. [DOI] [PubMed] [Google Scholar]
- 11.Schonfeld G. 1995. The hypobetalipoproteinemias. Annu. Rev. Nutr. 15: 23–34. [DOI] [PubMed] [Google Scholar]
- 12.Sankatsing R.R., Fouchier S. W., de Haan S., Hutten B. A., de Groot E., Kastelein J. J., Stroes E. S. 2005. Hepatic and cardiovascular consequences of familial hypobetalipoproteinemia. Arterioscler. Thromb. Vasc. Biol. 25: 1979–1984. [DOI] [PubMed] [Google Scholar]
- 13.Schonfeld G., Patterson B. W., Yablonskiy D. A., Tanoli T. S., Averna M., Elias N., Yue P., Ackerman J. 2003. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J. Lipid Res. 44: 470–478. [DOI] [PubMed] [Google Scholar]
- 14.Tanoli T., Yue P., Yablonskiy D., Schonfeld G. 2004. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J. Lipid Res. 45: 941–947. [DOI] [PubMed] [Google Scholar]
- 15.Familial Hypercholesterolemia (FH). report of the World Health Organization, 1997. http://www.medped.org/who/.
- 16.Longo R., Pollesello P., Ricci C., Masutti F., Kvam B. J., Bercich L., Croce L. S., Grigolato P., Paoletti S., de Bernard B., et al. 1995. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J. Magn. Reson. Imaging. 5: 281–285. [DOI] [PubMed] [Google Scholar]
- 17.Naressi A., Couturier C., Devos J. M., Janssen M., Mangeat C., de Beer R., Graveron-Demilly D. 2001. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 12: 141–152. [DOI] [PubMed] [Google Scholar]
- 18.de Bazelaire C. M., Duhamel G. D., Rofsky N. M., Alsop D. C. 2004. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 230: 652–659. [DOI] [PubMed] [Google Scholar]
- 19.van Werven J. R., Hoogduin J. M., Nederveen A. J., van Vliet A. A., Wajs E., Vandenberk P., Stroes E. S., Stoker J. 2009. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J. Magn. Reson. Imaging. 30: 444–448. [DOI] [PubMed] [Google Scholar]
- 20.Schonfeld G., Lin X., Yue P. 2005. Familial hypobetalipoproteinemia: genetics and metabolism. Cell. Mol. Life Sci. 62: 1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguilar-Salinas C. A., Barrett P. H., Parhofer K. G., Young S. G., Tessereau D., Bateman J., Quinn C., Schonfeld G. 1995.. Apoprotein B-100 production is decreased in subjects heterozygous for truncations of apoprotein B. Arterioscler. Thromb. Vasc. Biol. 15: 71–80. [DOI] [PubMed] [Google Scholar]
- 22.Parhofer K. G., Barrett P. H., Aguilar-Salinas C. A., Schonfeld G. 1996. Positive linear correlation between the length of truncated apolipoprotein B and its secretion rate: in vivo studies in human apoB-89, apoB-75, apoB-54.8, and apoB-31 heterozygotes. J. Lipid Res. 37: 844–852. [PubMed] [Google Scholar]
- 23.Elias N., Patterson B. W., Schonfeld G. 2000. In vivo metabolism of ApoB, ApoA-I, and VLDL triglycerides in a form of hypobetalipoproteinemia not linked to the ApoB gene. Arterioscler. Thromb. Vasc. Biol. 20: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 24.Yue P., Tanoli T., Wilhelm O., Patterson B., Yablonskiy D., Schonfeld G. 2005. Absence of fatty liver in familial hypobetalipoproteinemia linked to chromosome 3p21. Metabolism. 54: 682–688. [DOI] [PubMed] [Google Scholar]
- 25.Tarugi P., Averna M., Di L. E., Cefalu A. B., Noto D., Magnolo L., Cattin L., Bertolini S., Calandra S. 2007. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 195: e19–e27. [DOI] [PubMed] [Google Scholar]
- 26.Wetterau J. R., Lin M. C., Jamil H. 1997. Microsomal triglyceride transfer protein. Biochim. Biophys. Acta. 1345: 136–150. [DOI] [PubMed] [Google Scholar]
- 27.Crooke R. M., Baker B. F., Wedel M. 2007.. Cardiovascular therapeutic applications. In Antisense Drug Technology: Principles, Strategies and Applications 2nd ed.Crooke S. T., ISIS Pharmaceuticals: Carlsbad, CA; 601–639. [Google Scholar]
- 28.Crooke R. M., Graham M. J., Lemonidis K. M., Whipple C. P., Koo S., Perera R. J. 2005. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J. Lipid Res. 46: 872–884. [DOI] [PubMed] [Google Scholar]
- 29.Kastelein J. 2007. Antisense ApoB mRNA inhibition: From Promise To Practice. J.Clin. Lipidol 1:378 Presented at the DALM 2007 New York.
- 30.Lonardo A., Lombardini S., Scaglioni F., Carulli L., Ricchi M., Ganazzi D., Adinolfi L. E., Ruggiero G., Carulli N., Loria P. 2006. Hepatic steatosis and insulin resistance: does etiology make a difference? J. Hepatol. 44: 190–196. [DOI] [PubMed] [Google Scholar]
- 31.Ballestri S., Lonardo A., Losi L., Pellegrini E., Bertolotti M., Loria P. 2009. Do diabetes and obesity promote hepatic fibrosis in familial heterozygous hypobetalipoproteinemia? Intern. Emerg. Med. 4: 71–73. [DOI] [PubMed] [Google Scholar]
- 32.Adams L. A., Lymp J. F., St. Sauver J., Sanderson S. O., Lindor K. D., Feldstein A., Angulo P. 2005. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 129: 113–121. [DOI] [PubMed] [Google Scholar]
- 33.Ong J. P., Pitts A., Younossi Z. M. 2008. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 49: 608–612. [DOI] [PubMed] [Google Scholar]