Abstract
Cysteinyl-leukotrienes (cysteinyl-LT) are rapidly generated at sites of inflammation and, in addition to their role in asthma, rhinitis, and other immune disorders, are increasingly regarded as significant inflammatory factors in cancer, gastrointestinal, cardiovascular diseases. We recently demonstrated that in monocyte/macrophage–like U937 cells, extracellular nucleotides heterologously desensitize CysLT1 receptor (CysLT1R)-induced Ca2+ transients. Given that monocytes express a number of other inflammatory and chemoattractant receptors, this study was aimed at characterizing transregulation between these different stimuli. We demonstrate that in U937 cells and in primary human monocytes, a series of inflammatory mediators activating Gi-coupled receptor (FPR1, BLT1) desensitize CysLT1R-induced Ca2+ response unidirectionally through activation of PKC. Conversely, PAF-R, exclusively coupled to Gq, cross-desensitizes CysLT1R without the apparent involvement of any kinase. Interestingly, Gs-coupled receptors (β2AR, H1/2R, EP2/4R) are also able to desensitize CysLT1R response through activation of PKA. Heterologous desensitization seems to affect mostly the Gi-mediated signaling of the CysLT1R. The hierarchy of desensitization among agonists may be important for leukocyte signal processing at the site of inflammation. Considering that monocytes/macrophages are likely to be the major source of cysteinyl-LT in many immunological and inflammatory processes, shedding light on how their receptors are regulated will certainly help to better understand the role of these cells in orchestrating this complex network of integrated signals.
Keywords: Cysteinyl-leukotriene, CysLT1 receptor, heterologous desensitization, inflammation, macrophage, PKA, PKC
Cysteinyl-leukotrienes (cysteinyl-LT) (i.e., LTC4, LTD4, and LTE4) are members of a large family of biologically active lipid mediators rapidly generated at sites of inflammation from arachidonic acid via the 5-lipoxygenase (5-LO) pathway (1). They are synthesized by inflammatory cells, such as eosinophils, basophils, mast cells, and alveolar macrophages, in response to different immune and inflammatory stimuli (2). Beyond their well-recognized role in asthma and immune disorders, cysteinyl-LTs are increasingly regarded as significant inflammatory factors in cancer, gastrointestinal, and cardiovascular diseases (3). In the past few years, the use of LT receptor antagonists as cardiovascular drugs has become a matter of considerable interest (4–6), as the presence of 5-LO, FLAP, and LT receptors transcripts has been shown in atherosclerotic lesions at various stages of development in human aorta, coronary arteries, and carotid arteries (3).
The effects of cysteinyl-LTs are mediated through two officially recognized receptor subtypes: CysLT1 and CysLT2 (2, 3), which belong to the G-protein-coupled receptor (GPCR) gene superfamily and particularly to the purine receptor cluster (within the δ group) of the rhodopsin family (7). Over the years, several data in the literature suggested the existence of additional CysLT receptor (CysLT-R) subtypes in humans (3), and very recently new pharmacological targets have been identified for cysteinyl-LTs, namely, two possible new receptor entities (8, 9) and a CysLT1/CysLT2 heterodimer (10).
The activation of a number of receptors does not always lead to a direct effect on a particular signaling pathway but, rather, to amplification (11, 12) or, more often, to inhibition of the response produced by distinct but convergent signals (13–16). In this respect, heterologous desensitization (i.e., the reduced response to activation of an unrelated or apparently unrelated receptor that has not been exposed to an agonist) plays an important role (17, 18). This latter process is generally ascribed to the action of different kinases, such as PKA and PKC, that participate as a feedback mechanisms in which the second messenger activates a kinase that, in turn, modulates the activity of other receptors.
Monocytes/macrophages, besides providing innate immune surveillance for every tissue in the body, are generally believed to sustain and precipitate pathological states. Cytokines, 5-LO metabolites, and several other agents selectively home monocytes to the parenchymal tissue where they participate in the innate immune response and lipid homeostasis (19). Of particular interest is the ability of macrophages to produce large amounts of proinflammatory mediators including cytokines (20), prostaglandins, and LTs (2), as well as to express inflammatory surface receptors such as CysLT1 (3). This correlates with the recent finding that cysteinyl-LTs might contribute to inflammatory reactions by induction of MCP-1 (21) and increasing CCR2B expression in human monocytes/macrophages (22).
The past decade has revealed that macrophage-derived foam cells are integral to the development and progression of atherosclerosis (23), while a very recent paper demonstrated that montelukast (Singulair), a specific CysLT1R antagonist widely used for asthma treatment, prevented ROS production and reduced atherosclerotic plaque formation in apolipoprotein E–deficient knockout mice in vivo (24). Monocyte/macrophage lineage may play a potential role in the pathophysiology of COPD (25) as well in tumor initiation and various key steps in growth and metastasis (26). Therefore, elucidation of molecular mechanisms of macrophage function and their pharmacological modulation represents an important strategy for the modulation of immune response, prevention, and treatment of diseases.
We have recently demonstrated that in monocytes/macrophages like U937 cells, a CysLT1R-induced increase in cytosolic free Ca2+ concentration ([Ca2+]i) can be modulated by extracellular nucleotides, such as ATP and UDP that, following activation of different P2Y receptors, heterologously desensitize the CysLT1R through PKC (16). Given that monocytes/macrophages besides CysLT1R and P2Y-R express a number of other inflammatory and chemoattractant receptors, such as those for LTB4 (BLT1R), fMLF (previously known as fMLP, [27]), FPR1, platelet activating factor (PAF-R), histamine H (H1/2R), and prostaglandin E2 (EP2/4R) or inflammatory-related receptors, such as β2 adrenoreceptor (β2AR) (20), this study was aimed at characterizing transregulation between these different stimuli (13, 28). Although much has been learned about cellular activation and regulation by single receptors, mechanisms of receptor cross-regulation leading to priming or desensitization are only beginning to be unraveled. The fine tuning of CysLT1R function by a series of mediators that largely accumulate at sites of inflammation might shed light on a new network of integrated signaling in inflammatory responses.
MATERIALS AND METHODS
Materials
RPMI 1640, FBS, BSA, EGTA, EDTA, penicillin, streptomycin, L-glutamine, dimethylsulphoxyde, probenecid, penicillamine, forskolin, phorbol 12-myristate 13-acetate (PMA), ionomycin, UTP, PAF, fMLF, histamine, isoproterenol, propranolol, and U75302 were from Sigma Chem. Co. All salts for saline and digitonin were from Merck. Ficoll-Paque was from Amersham.
LTD4, LTB4, and PGE2, and LY255283 were purchased from Cayman Chemical Co.; GF109203X (GFX), H89, pertussis toxin (PTX), KN92, KN93, and KT 5823 were from Calbiochem. YM-254890 was a kind gift from Dr. J. Takasaki. Fluo3/AM, and pluronic F-127 purchased from Molecular Probes. WEB 2086 was from BIOMOL International, and BOC-PLPLP was from GenWay Biotech. Disposable culture flasks and filters were from Corning Glassworks. HTRF cAMP Dynamic 2 Kit was from Cisbio International.
Cell culture
U937 cells (ATCC) were routinely cultured into flasks in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin at 37C° (5% CO2) and differentiated for 96 h with 1.3% dimethylsulphoxyde, as previously described (29).
Isolation of primary human monocytes
Blood from healthy donors was collected into sodium citrate 3.8% or in citrate phosphate dextrose (CPD) and centrifuged at 200 g for 15 min. The obtained plasma reach platelet (PRP) was discharged and the blood was diluted (1:2) with RPMI-1640 culture medium or PBS, layered on a cushion of Ficoll Paque (3:1), and then centrifuged at 500 g for 20 min. The mononuclear lymphocyte cells layer was transferred and washed twice in PBS +0.5% BSA + 0.1% glucose + 5 mM EDTA, once in PBS + 0.1% glucose + 5 mM EDTA and then resuspended in RPMI-1640 culture medium. The cells were seeded (2–3 × 106/mL) on coverslips coated with poly-D-lysine and incubated for 2 h at 37°C. The lymphocytes, which do not adhere to coverslip, were washed with PBS and the adherent monocytes were incubated overnight in RPMI-1640 culture medium at 37°C.
Determination of cytosolic free Ca2+ levels
Determination of free [Ca2+]i in U937 cells was performed as previously described (16, 29, 30). Briefly, cells were incubated for 30 min at 30°C in the dark with 2 µM Fluo3/AM. After loading, Fluo3/AM was removed and cells were further incubated for 30 min at 30°C to complete the hydrolysis of Fluo3/AM. Then cells were centrifuged, resuspended, diluted to the concentration of 106 cells/mL, transferred to the spectrofluorimeter (Perkin Elmer LS50), and fluorescence monitored at 37°C (506 nm excitation, 530 nm emission). Primary monocytes were incubated for 1 h at 25°C in the dark with 2µM Fura2/AM. After loading, cells were washed twice with a saline solution, transferred to the spectrofluorimeter, and fluorescence monitored at 37°C (505 nm emission, 340 and 380 nm excitation). Calibration was performed by adding 2 µM ionomycin and 100 µM digitonin (Fmax, maximal fluorescence of the system) and by adding 5 mM EGTA and 60 mM Tris-base (Fmin minimal fluorescence of the system). [Ca2+]i elevation has been expressed as the ratio of stimulated over basal (s/b).
The heterologous desensitization protocol was performed as previously described (16). Briefly, the second challenge was dispensed 3 min after the first challenge or when [Ca2+]i levels returned to baseline without washing the sample.
Statistical analysis
Statistical analysis of concentration-response curves was performed using GraphPad Prism version 4, which use the four parameters logistic model as described in the ALLFIT program (31). All curves are computer generated. Statistical comparison of multiple groups was performed using one-way ANOVA followed by Bonferroni's multiple comparison test. Data are expressed as mean ± SEM.
RESULTS
Inflammatory-related stimuli elicited second messenger generation in DMSO-differentiated U937 cells
It is widely known that LTD4 elicits a cytosolic Ca2+ transient ([Ca2+]i) in DMSO-differentiated U937 (dU937) cells through the activation of the CysLT1R promiscuously coupled to both Gq and Gi (16, 29, 30). In the same cells, the ability to increase [Ca2+]i is shared also by other inflammatory stimuli through different molecular signaling pathways. Indeed, LTB4, fMLF, UTP, and PAF were found to increase [Ca2+]i through the activation of specific receptors exclusively coupled either to Gq/11 (PAF-R) or Gi (FPR1) or promiscuously coupled to both G proteins (BLT1R and P2Y2/6R) (Table 1). In addition, a number of other inflammatory or inflammatory-related stimuli (e.g., PGE2, histamine and isoproterenol) were found to increase cAMP levels in dU937 cells through activation of their Gs protein-coupled receptors (EP2/4R, H1/2R, and β2AR, respectively) (Table 1). Therefore, these heterogeneous stimuli were studied to expand the previous notion of extracellular nucleotide regulation of CysLT1R activity (16), examining the possibility that they might participate in CysLT1R regulation through specific molecular mechanisms and possibly specific hierarchical organization.
TABLE 1.
Inflammatory stimuli elicited second messenger generation in dU937 cells
| [Ca2+]i fold increase ± SEM |
cAMP pmol/106 cellsb | |||
|---|---|---|---|---|
| Stimulus | −PTX | +PTXa | Predominant Coupling | |
| LTD4, 10nM | 5.56 ± 0.44 | 3.54 ± 0.34 (P < 0.01) | Gi/Gq | |
| LTB4, 1 μM | 2.22 ± 0.26 | 1.23 ± 0.09 (P < 0.01) | Gi | |
| fMLF, 1 μM | 2.4 ± 0.24 | 1 (P < 0.01) | Gi | |
| UTP, 10 μM | 3.87 ± 0.62 | 2.81 ± 0.5 (P < 0.05) | Gi/Gq | |
| PAF, 36 nM | 6.78 ± 0.98 | 6.41 ± 0.75 | Gq | |
| isoproterenol,1 μM | — | — | 43.7 ± 6.7 | Gs |
| PGE2, 1 μM | — | — | 71.5 ± 15.5 | Gs |
| histamine, 1 μM | 39.3 ± 1.7 | Gs | ||
PTX 300 ng/mL −18 h pretreatment.
Basal cAMP, 2.38 ± 0.47 pmol/106 cells.
Effect of Gi-coupled receptors on CysLT1R-induced [Ca2+]i transient
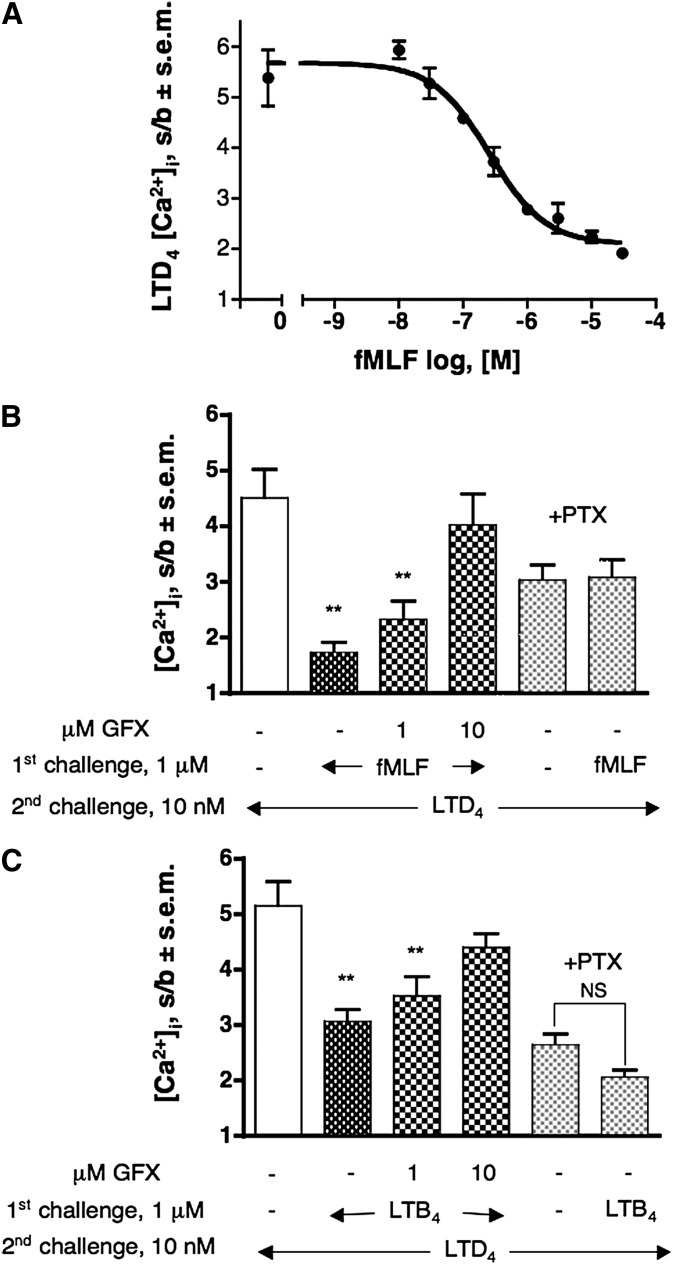
We examined modulation of CysLT1R activation by a “pure” Gi-coupled receptor measuring LTD4-induced [Ca2+]i transient following a first challenge with fMLF. Fig. 1A shows the concentration-dependent desensitization curve of fMLF on the response elicited by 10 nM LTD4 in dU937 cells. Maximum degree of desensitization (77%, P < 0.01) was obtained starting from 3 μM fMLF (Fig. 1A). This effect can be largely prevented by a 5 min preincubation with increasing concentration of the PKC inhibitor GFX (Fig. 1B), suggesting the involvement of the second messenger-activated PKC in the heterologous desensitization of CysLT1R. Pretreatment of cells with the PKC inhibitor only marginally increase LTD4-induced response, suggesting a minimal tonic desensitization of the CysLT1R by PKC in resting conditions (data not shown). Overnight pretreatment of cells with 300 ng/mL PTX completely abolished fMLF-induced desensitization, demonstrating specificity of the fMLF effect (Fig. 1B).
Fig. 1.
Desensitization of CysLT1R by fMLF- and LTB4-activated Gi-coupled receptors. (A) Effect of increasing concentration of fMLF (1st challenge) on 10 nM LTD4-induced [Ca2+]i transient. (B) Reversal of fMLF-induced heterologous CysLT1R desensitization by pretreatment with GFX (5 min) and complete prevention PTX (300 ng/ml, overnight).( C) Effect of pretreatment with GFX (5 min) and PTX (300 ng/ml, overnight) on the LTB4-induced densensitization of the [Ca2+]i transient evoked by 10 nM LTD4 (2nd challenge). Values shown represent means of [Ca2+]i stimulation over basal (s/b) ± SEM of at least three independent experiments. Statistical comparison of multiple groups was performed using one-way ANOVA followed by Bonferroni's multiple comparison test (**P < 0.01, versus control, unless otherwise indicated). GFX, GF109203X.
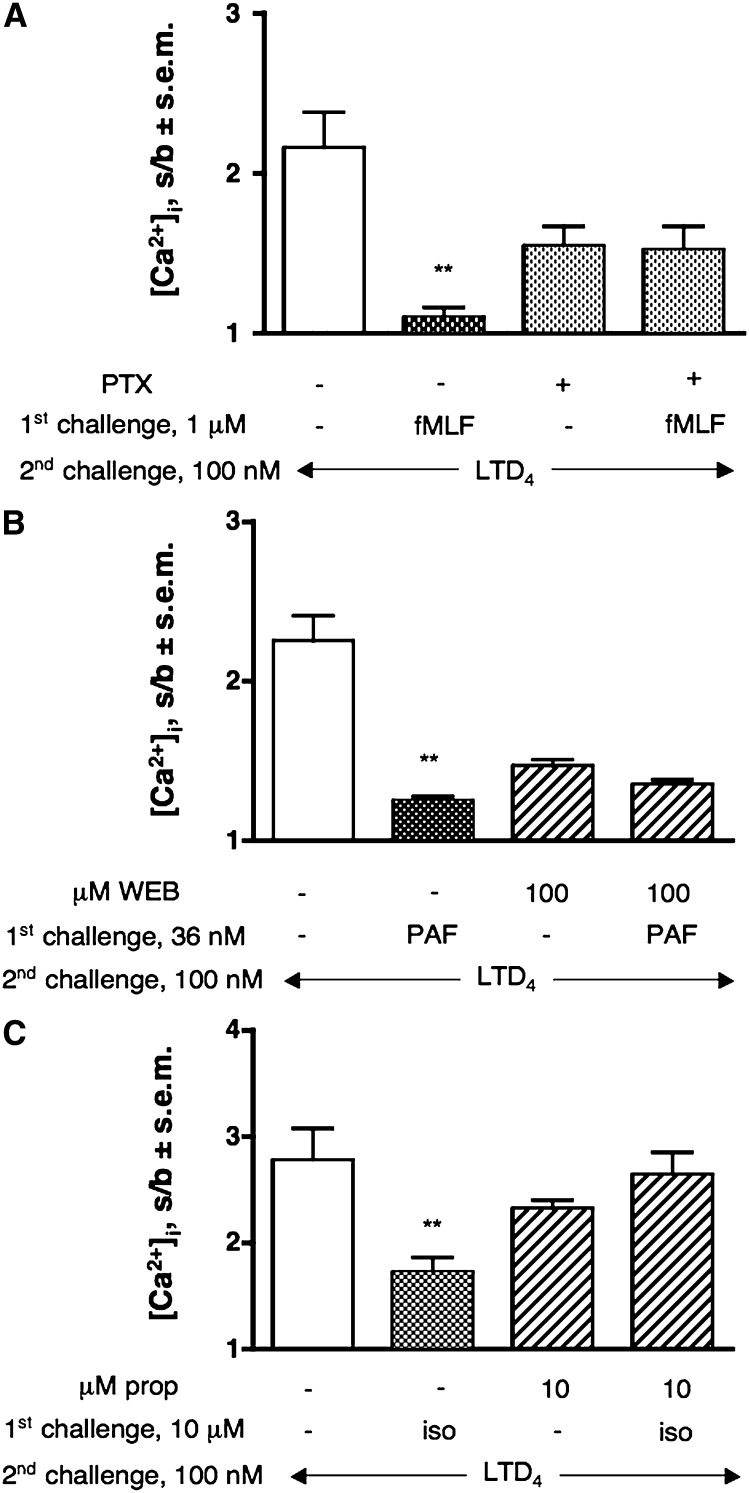
Similar results have been obtained with the chemotactic lipid mediator LTB4. Fig. 1C shows that a first challenge with 1 μM LTB4 provoked a reduction in CysLT1R-induced Ca2+ response, reaching a maximal CysLT1R desensitization of 50% (P < 0.01). It is also clear that this effect can be prevented by preincubation with increasing concentrations of GFX. Pretreatment of cells with PTX strongly impaired, but did not abolish, LTB4-induced desensitization, in agreement with the effect of PTX on LTB4 signaling (Table 1). LTB4 effects can be fully ascribed to the activation of the BLT1R, as the specific BLT2 antagonist LY255283 up to 1 μM was completely ineffective in inhibiting LTB4-induced response in dU937 cells (data not shown). The same is also true for primary human monocytes (HM) (see below).
Effect of a Gq-coupled receptor on CysLT1-induced [Ca2+]i transient
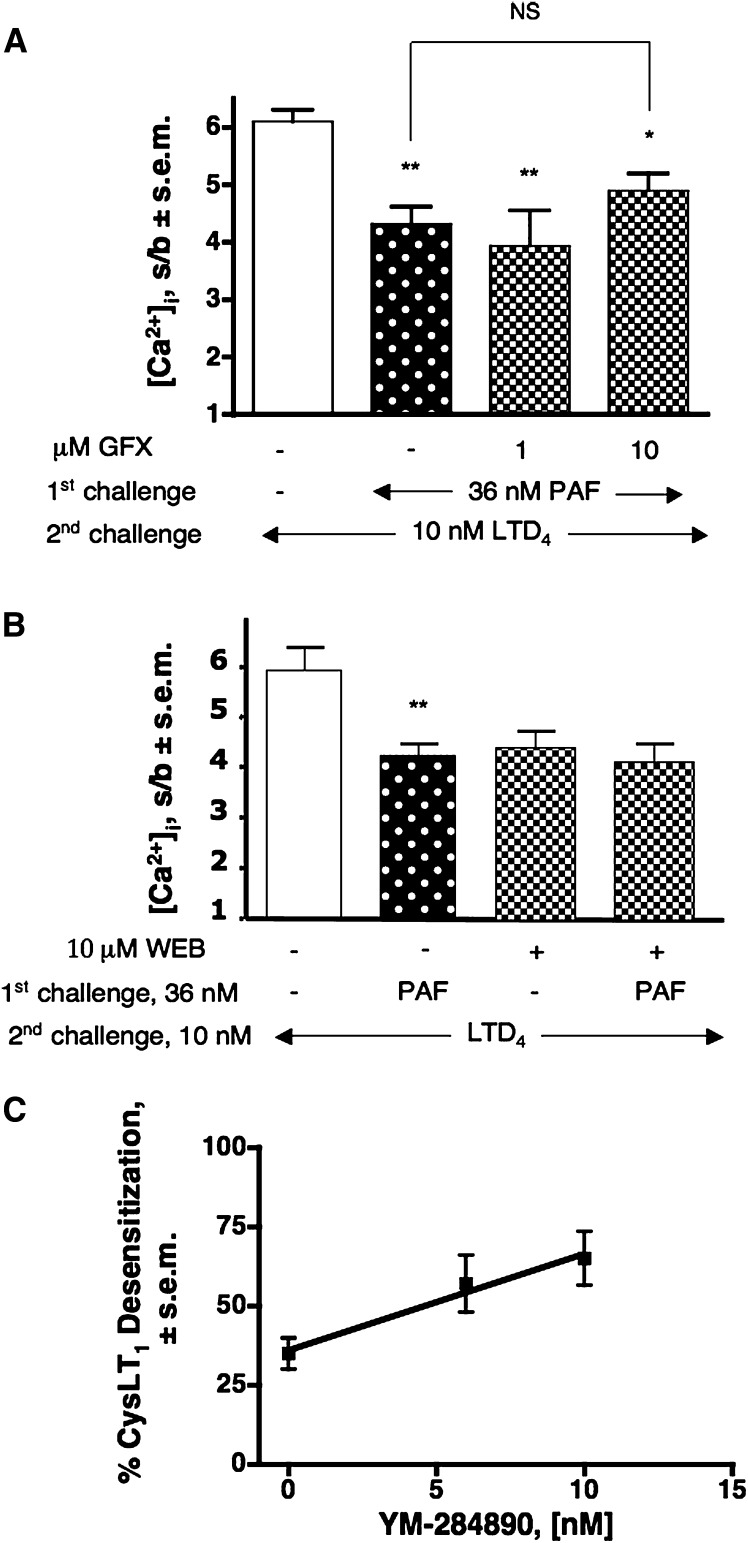
We also examined the possible effect of a “pure” Gq/11-coupled receptor, such as PAF-R, on the regulation of CysLT1R activity. Fig. 2A shows that a first challenge with 36 nM PAF affected LTD4-induced response up to 35% (P < 0.01) and that this effect cannot be substantially prevented by increasing concentration of GFX. In addition, none of the following were able to completely revert PAF-induced CysLT1 desensitization: pretreatment with 10 μM H89, a PKA inhibitor; pretreatment with 10 μM KN93, a Ca2+/calmodulin–dependent kinase II (CaMKII) inhibitor; and pretreatment with 10 μM KT-5823, a PKG inhibitor, alone or in addition to GFX (data not shown).
Fig. 2.
Desensitization of CysLT1R by PAF-activated Gq-coupled receptor. (A) Desensitization of the LTD4-induced [Ca2+]i response (2nd challenge) by 36 nM PAF and lack of effect of pretreatment with increasing concentrations of GFX (5 min). (B) Effect of pretreatment with 10 μM WEB 2086 (WEB, 5 min) on the PAF-induced desensitization of the [Ca2+]i transient evoked by 10 nM LTD4 (2nd challenge). Values shown represent means of [Ca2+]i stimulation over basal (s/b) ± SEM of at least three independent experiments. Statistical comparison of multiple groups was performed using one-way ANOVA followed by Bonferroni's multiple comparison test (*P < 0.05; **P < 0.01, versus control, unless otherwise indicated). (C) Effect of increasing concentrations of YM-254890 on PAF-induced CysLT1R heterologous desensitization. Values shown represent means ± SEM of three independent experiments. GFX, GF109203X.
Specificity of PAF response was assessed by demonstrating that 10 μM of the PAF-R antagonist WEB 2086 completely prevented PAF signal (data not shown) and PAF-induced CysLT1R desensitization (Fig. 2B).
Effect of YM-254890 on PAF-induced CysLT1R heterologous desensitization
We previously reported that use of YM-254890 to selectively inhibit the Gq signaling pathway produced important augmentation of P2Y-induced CysLT1 desensitization compared with untreated cells, suggesting that the majority of P2Y-derived desensitization might preferentially affect the Gi signaling pathway of the CysLT1R (16). Here we found that the increase in the extent of PAF-induced desensitization of the CysLT1R directly correlated with the concentration of YM-254890 (administered in between the first challenge with PAF and the second challenge with LTD4), again indicating a preferential desensitization of the Gi-coupled pathway of the CysLT1R (Fig. 2C). As a control, 10 nM of YM-254890 did not statistically affect fMLF receptor signaling (a pure Gi coupled receptor), while it completely inhibited PAF signaling (data not shown).
Effect of LTD4 pretreatment on fMLF-, LTB4-, and PAF-induced [Ca2+]i transient
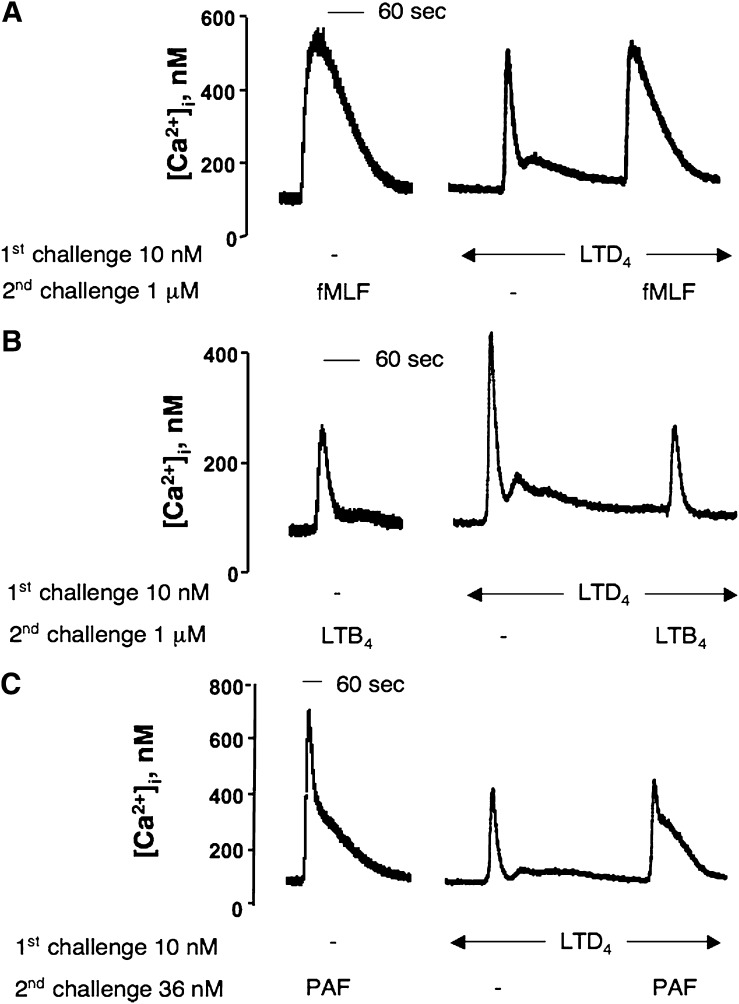
To verify a possible influence of CysLT1 activation on FPR1, BLT1R, and PAF-R responses, we measured the fMLF-, LTB4-, and PAF-induced [Ca2+]i transient subsequent to a first challenge with LTD4. Fig. 3A and 3B show that fMLF- and LTB4-induced responses were only marginally (P > 0.05) affected by a prior challenge with 10 nM LTD4, suggesting that transregulation between CysLT1R and FPR1 or BLT1R occurs only in a unidirectional way. At variance, LTD4 was able to partially desensitize PAF response (Fig. 3C) (44% reduction, P < 0.01), indicating the existence of cross-desensitization between CysLT1R and PAF-R in dU937 cells. Moreover, Fig. 3A and 3B clearly demonstrate that after a first challenge with LTD4, cells can still be responsive to the second challenge with a different agonist, ruling out the possibility that the heterologous desensitization observed might depend upon an altered ability of the cells to mobilize calcium.
Fig. 3.
Impact of CysLT1R activation on fMLF-, LTB4- and PAF-induced [Ca2+]i transient. Representative traces of the [Ca2+]i transient induced by 1 μM fMLF (A), 1 μM LTB4 (B) and 36 nM PAF (C) (2nd challenge) before or after an initial challenge with 10 nM LTD4.
Effect of isoproterenol challenge on CysLT1-induced [Ca2+]i transient
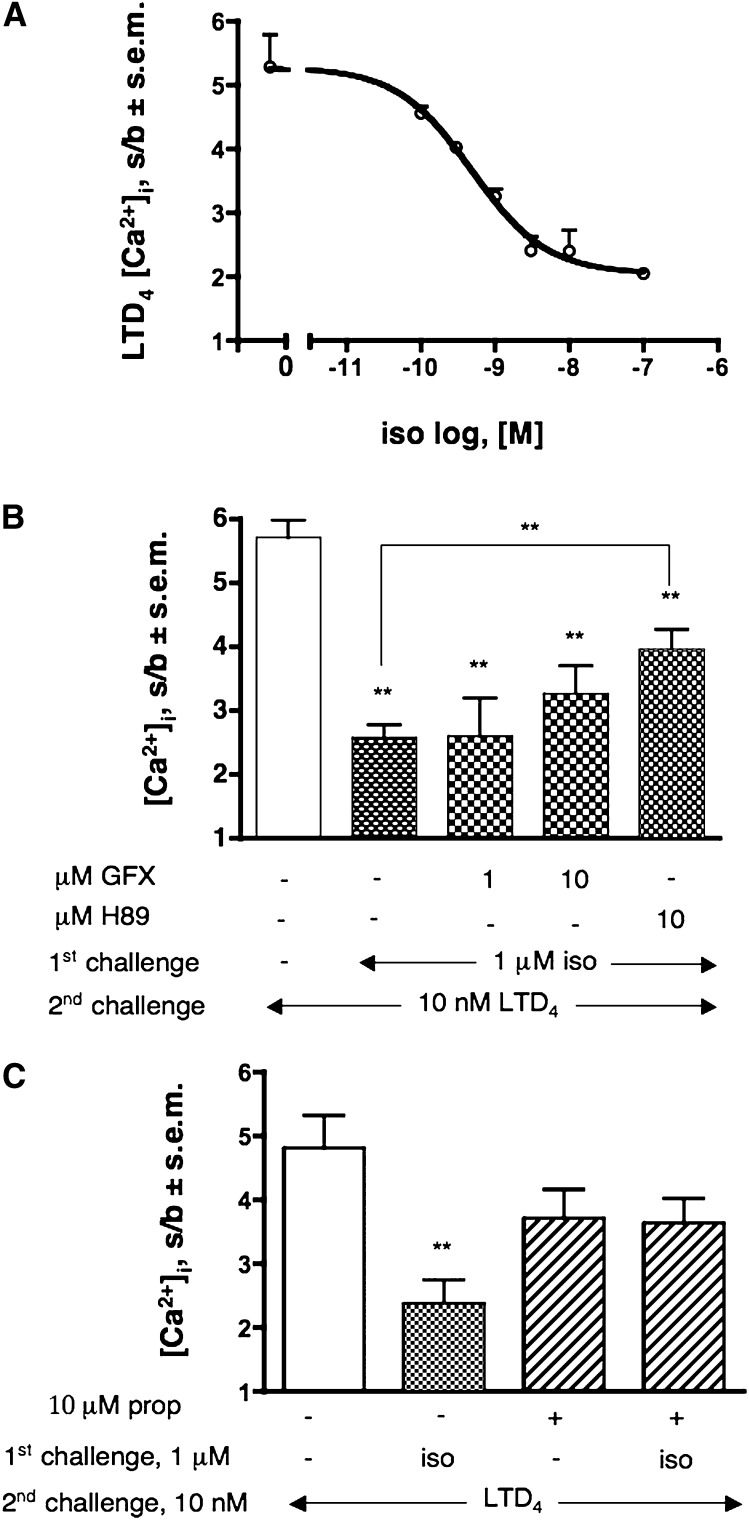
As previously mentioned, dU937 cells express a number of receptors for inflammatory-related stimuli that, at variance with the stimuli previously examined, are not coupled to Gq/11 or Gi/o, but rather to Gs (Table 1). To verify whether cAMP increase could affect LTD4-induced response through activation of PKA, we challenged dU937 cells with increasing concentrations of the β2AR agonist isoproterenol. Indeed, Fig. 4A shows that isoproterenol dose-dependently impaired the CysLT1R response up to 70% and that this effect can be only partially reverted by pretreatment of cells with 10 μM of the widely used PKA inhibitor H89 (53% recovery, P < 0.01) (Fig. 4B). It is also clear from Fig. 4B that while GFX at a concentration of 1 μM (a concentration at which the compound is specific for PKC) had no effect on the isoproterenol-induced CysLT1R desensitization, at 10 μM (a concentration at which GFX start to inhibit also PKA [32]), it had an effect similar to that of H89. Pretreatment of cells with the PKA inhibitor did not alter the LTD4-induced response (data not shown). Finally, to assess the specificity of the observed responses, we demonstrated that pretreatment with propranolol, a competitive antagonist at the β2AR, prevented isoproterenol-induced CysLT1R desensitization (Fig. 4C). As expected, PTX pretreatment was totally ineffective on isoproterenol-induced CysLT1R desensitization (data not shown).
Fig. 4.
Desensitization of CysLT1R by isoproterenol-activated Gs-coupled receptors. (A) Effect of increasing concentration of isoproterenol (iso) (1st challenge) on 10 nM LTD4-induced [Ca2+]i transient. (B) Reversal of 1 μM iso-induced heterologous CysLT1R desensitization by pretreatment with GFX or H89 (5 min). C) Effect of pretreatment with 10 μM propanolol (prop) 5 min on 1 μM iso-induced desensitization of the [Ca2+]i transient evoked by 10 nM LTD4 (2nd challenge). Values shown represent means of [Ca2+]i stimulation over basal (s/b) ± SEM of at least three independent experiments. Statistical comparison of multiple groups was performed using one-way ANOVA followed by Bonferroni's multiple comparison test (**P < 0.01, vs. control, unless otherwise indicated). GFX, GF109203X.
Effect of PGE2 and histamine challenge on CysLT1-induced [Ca2+]i transient
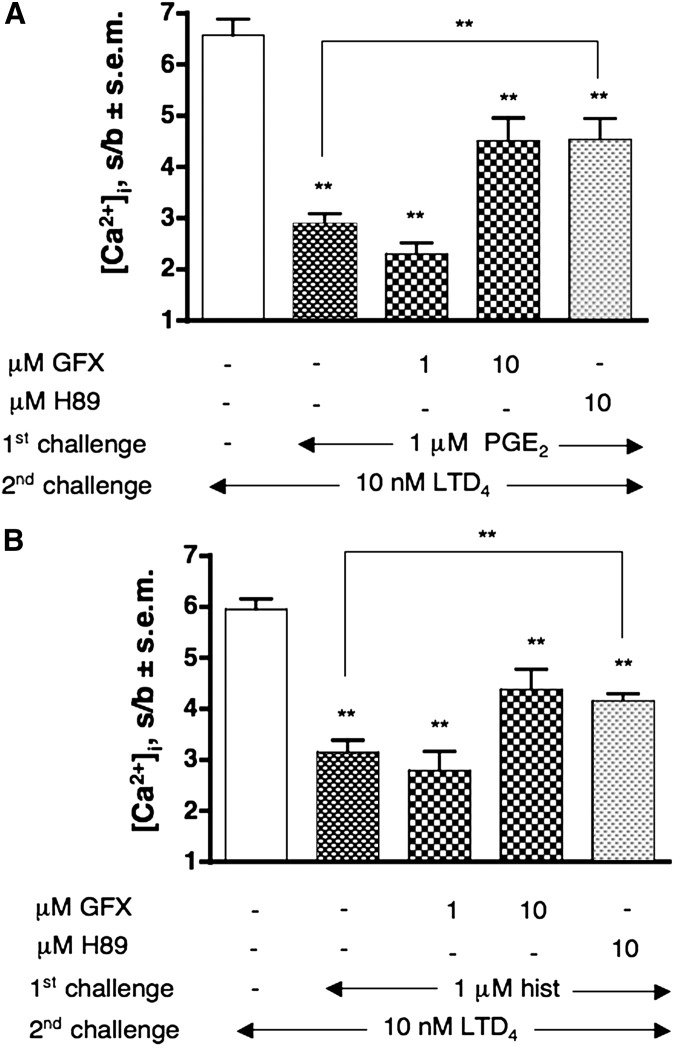
Similar to isoproterenol, PGE2 and histamine also, through their specific receptors coupled to cAMP production (EP2/4 and H1/2 receptors, respectively) (Table 1), induced desensitization of the CysLT1R up to 61% and 56%, respectively (P < 0.01) (Fig. 5A, B). Again, both effects could only be partially reverted by 10 μM GFX or 10 μH89. Finally, similar to isoproterenol data, there was no effect of PTX pretreatment on PGE2 and histamine-induced desensitization (data not shown).
Fig. 5.
Desensitization of CysLT1R by PGE2- and histamine- activated Gs-coupled receptors. Effect of pretreatment with GFX and H89 (5 min) on 1 μM PGE2 (A) or histamine (hist) (B) induced desensitization of the [Ca2+]i transient evoked by 10 nM LTD4 (2nd challenge). Values shown represent means of [Ca2+]i stimulation over basal (s/b) ± SEM of at least three independent experiments. Statistical comparison of multiple groups was performed using one-way ANOVA followed by Bonferroni's multiple comparison test (**P < 0.01, vs. control, unless otherwise indicated). GFX, GF109203X.
Effect of forskolin pretreatment on CysLT1-induced [Ca2+]i transient
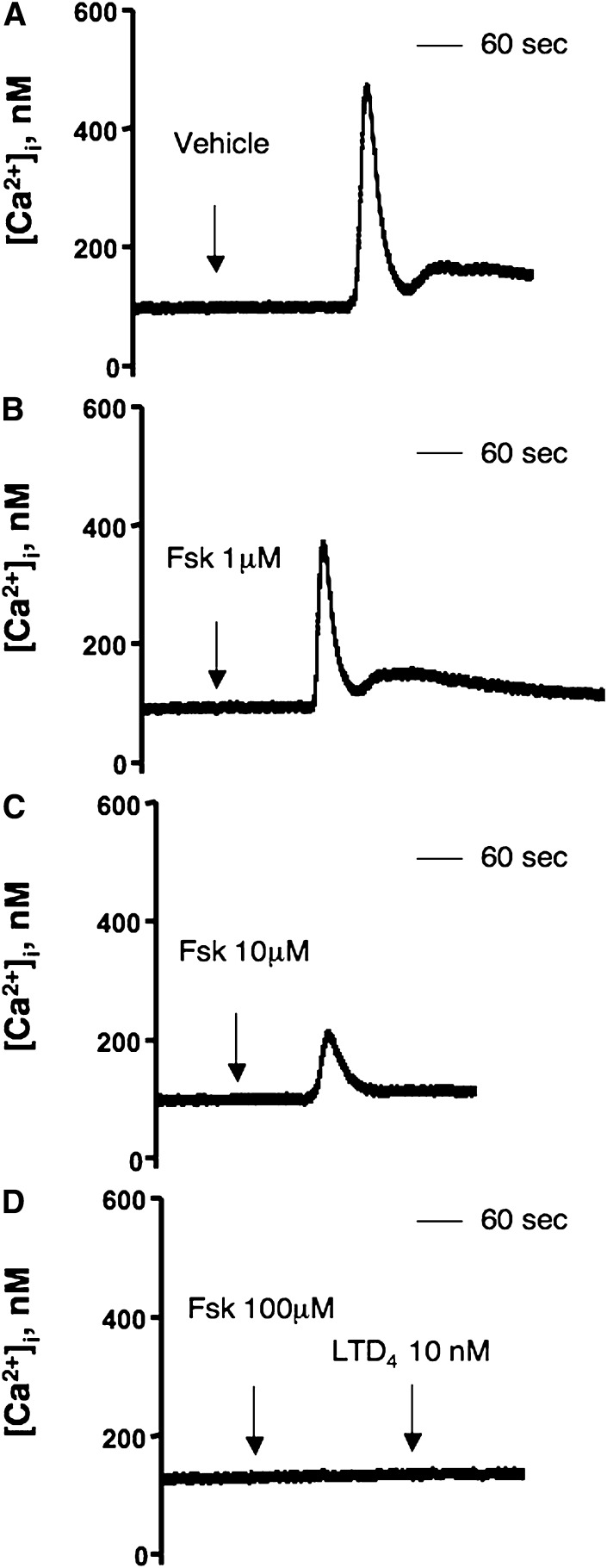
To confirm cAMP-induced CysLT1 transregulation, we investigated the effect of a non-specific activator of adenylate cyclase. 100 μM forskolin completely desensitized the CysLT1R (Fig. 6), confirming the involvement of cAMP in regulation of the activity of CysLT1R and strongly indicating that also the second messenger–activated PKA is able to heterologously desensitize the CysLT1R.
Fig. 6.
Effect of forskolin (fsk) pretreatment on LTD4-induced [Ca2+]i transient. Representative traces of the effect of a prior challenge with vehicle (A), 1 μM (B), 10 μM (C) and 100 μM (D) forskolin on [Ca2+]i transient induced by 10 nM LTD4.
Heterologous desensitization of CysLT1 receptor in primary human monocytes
To be sure that dU937 cells accurately represented the spectrum of receptors expressed by monocytes/macrophages, we repeated some key experiments in monocytes. Fig. 7 shows that the same results as obtained in dU937 were achieved in freshly isolated primary HM. Agonist and antagonist concentrations were adjusted to the different sensitivity of adherent cells. fMLF, PAF, and isoproterenol specifically desensitize 100 nM LTD4-induced [Ca2+]i transient (91%, 79%, and 59%, respectively; P < 0.01). fMLF and PAF used the same molecular mechanism observed in dU937 cells; i.e., unidirectional for fMLF and complete cross-desensitization for PAF (34% inhibition, data not shown). Finally, as previously observed, the PAF effect was not prevented by pretreatment with 10 μM GFX (data not shown).
Fig. 7.
Impact of CysLT1R activation on fMLF-, PAF- and isoproterenol-induced [Ca2+]i transient in human primary monocytes. (A) Effect of 1 μM fMLF (1st challenge) on 100 nM LTD4-induced [Ca2+]i transient and effect of pretreatment with PTX (300 ng/ml, overnight). (B) Effect of 36 nM PAF (1st challenge) on 100 nM LTD4-induced [Ca2+]i transient and effect of pretreatment with 100 μM WEB 2086 (WEB, 5 min). (C) Effect of 10 μM isoproterenol (iso) (1st challenge) on 100 nM LTD4-induced [Ca2+]i transient and effect of pretreatment with 10 μM propanolol (prop) 5 min. Values shown represent means of [Ca2+]i stimulation over basal (s/b) ± SEM of at least three independent experiments. Statistical comparison of multiple groups was performed using one-way ANOVA followed by Bonferroni's multiple comparison test (**P < 0.01, vs. control).
DISCUSSION
Here we report that in dU937 cells and in primary HM a series of inflammatory mediators activating Gi-coupled receptors (FPR1, BLT1, and P2Y4) desensitize CysLT1R response in a unidirectional way through activation of PKC and that a hierarchy becomes evident in the desensitization process. Conversely, PAF-R, exclusively coupled to Gq in these cellular systems, not only transregulates CysLT1R without the apparent involvement of a second messenger–activated kinase but also is cross-desensitized by the CysLT1R. Interestingly, Gs-coupled receptors (β2AR, H1/2R, and EP2/4R) are able to desensitize CysLT1R response through activation of PKA. As we already suggested for extracellular nucleotides, heterologous desensitization seems to affect mostly the Gi-mediated signaling of the promiscuous (Gq/Gi) CysLT1R.
The human promonocytic leukemia U937 cell line, known to constitutively express only CysLT1Rs (16, 29, 30), has been selected to study the regulation of this important inflammatory receptor in a cellular system that is generally believed to contribute to the innate immune surveillance in many pathological states. As we and others have previously demonstrated (29, 30, 33, 34), one of the principal CysLT1R-induced responses in these cells is the increase of [Ca2+]i. Classically, Gq-coupled receptors are the ones responsible for inositol phosphate (IP)3 and [Ca2+]i generation. However, it is now known that PLCβ2 and PLCβ3 are also activated by the βγ subunit of the Gi family of G proteins, as is the case for CysLT1R (16, 30).
We have previously demonstrated that CysLT1 receptor is heterologously desensitized by P2Y2- and P2Y6 receptors for ATP and UDP, respectively, in a unidirectional way (16). Here we expand those observations to different classes of receptors constitutively expressed in dU937 cells and human monocytes but differently coupled to calcium homeostasis through the activation of Gi or Gq heterotrimeric G proteins. We first examined the effect of the activation of two “pure” Gi coupled receptors, such as those activated by fMLF and LTB4, and of a promiscuously coupled receptor (Gi and Gq, at least in leukocytes), such as P2Y4, preferentially activated by UTP (data not shown). The CysLT1R desensitization had an extent similar to that previously published for ATP and UDP (16) and was almost completely prevented by the PKC inhibitor GFX, confirming the ability of this second messenger–activated kinase to heterologously regulate CysLT1R functionality. In agreement with our previous work, none of the [Ca2+]i responses induced by these stimuli could be affected by a first challenge with LTD4 (unidirectional regulation). This was not totally unexpected, at least for fMLF, because it is known that FPR1 is resistant to this process because of the lack of PKC phosphorylation sites (35).
Surprisingly, in examining the ability of PAF-R to modulate CysLT1R response, we found that PAF-induced desensitization could not be prevented by preincubation of cells not only with GFX but also with inhibitors of PKA, PKG or CaMKII, alone or in combination with GFX. Although we cannot rule out the possibility that another kinase might be involved, these data point to additional mechanism(s) of transregulation besides phosphorylation (36), either at the receptor level or distal to receptor/G protein coupling. Unfortunately, despite several attempts to study CysLT1R phosphorylation in dU937 cells, we could not demonstrate even an agonist-induced phosphorylation (data not shown), likely because of the insufficient level of endogenous expression to permit detection of 32P incorporation in immunoprecipitated receptors, as already reported by other groups (37).
At this stage we can only speculate about a number of possible phosphorylation-independent mechanisms (38). One possibility is a reduced activation of PLC (39) or an IP3-receptor downregulation (40). Another possible mechanism is a phosphorylation-independent GRK2-mediated sequestration of Gαq (41). Alternatively, because members of the Gαq subfamily are known have a slow intrinsic GTPase activity in vitro (42), their rate is increased dramatically by the so-called regulators of G protein signaling (RGS) proteins. It has been demonstrated that RGS4 is able to inhibit [Ca2+]i mobilization induced by PAF-R, as well as PAF- and PMA-induced PAF-R phosphorylation; it also blocked cross-phosphorylation by fMLF (43). Furthermore, LTD4 also cross-desensitized the PAF-R, albeit partially.
Because U937 cells are a leukemia-derived cell line, we confirmed our observation repeating some key experiments in primary HM that also have been reported to express mainly the CysLT1R transcript (44). We confirmed that the LTD4-induced [Ca2+]i transient was sensitive to montelukast and PTX (data not shown), indicating that this response is because of a CysLT1R activation (44) similar to the one observed in dU937 cells (16, 29, 30). Basically, all the data observed in dU937 cells have been confirmed in primary HM, validating our observations and demonstrating that our model accurately represents the spectrum of receptors expressed in monocytes.
All these observations suggest a different regulation of the CysLT1R in U937 cells and in primary HM based on the peculiar signaling pathway used by the different receptors to regulate [Ca2+]i homeostasis. Confirmation of these data also comes from experiments performed in a recombinant system, namely, HEK293 cells transiently transfected with the CysLT1R, where we and others (45) have demonstrated that this receptor is exclusively coupled to Gq. In this system we found a complete cross-desensitization between the CysLT1R and P2Y2-R that was not reverted by GFX (data not shown). Thus, changing the coupling from a promiscuous Gq/Gi (dU937) or pure Gi (HM) to a pure Gq (HEK293) cellular system produces a change also in the regulation of receptor functionality. This observation, therefore, should be a warning about the extrapolation of physiological data from results obtained in recombinant systems.
As we previously suggested, CysLT1R heterologous desensitization seems to affect mostly the Gi-coupled pathway of this promiscuous receptor (16). In this work, by dose-dependently inhibiting the Gq-coupled pathway of the CysLT1R (without affecting the PAF-R response), we were able to demonstrate an increase in receptor desensitization induced by PAF. These data suggest a predominant desensitization of the Gi-coupled pathway of the CysLT1R in our system.
In an in vitro model of asthma, we have recently demonstrated the potential role of CysLT1R in the desensitization of the β2AR, highlighting another possible network of cross-talk in inflammation (46). Here we demonstrate that not only Gi- and Gq-coupled receptors but also receptors coupled to Gs (i.e., β2AR, H1/2, and EP2/4) can desensitize the CysLT1R in an heterologous way. Elevated cAMP levels through the activation of PKA are known to cause phosphorylation of either receptors or PLCβ isoforms blocking Gβγ-mediated activation (47). Thus, isoproterenol-, PGE2-, and histamine-mediated cAMP increase in dU937 cells desensitized CysLT1R either because PKA directly phosphorylates the receptor or because it phosphorylates PLCβ. The fact that the PKA inhibitor H89 only partially prevented Gs-dependent CysLT1R desensitization might depend on the limits of pharmacological inhibition or on the fact that additional mechanisms might be involved. As far as we know, this is the first demonstration that PKA might regulate the activity of CysLT1R either in a constitutive or recombinant system.
Lipid mediators were historically considered inflammatory mediators, causing symptoms such as fever, pain, and inflammation. However, recent studies using gene knockout mice (for both biosynthetic enzymes and receptors) have revealed that lipid mediators play a much more fundamental role in normal physiological processes and in disease. The fact that most of the stimuli tested desensitize the CysLT1R in a unidirectional way seems to suggest a hierarchy in the desensitization process. This hierarchy in the circuit of inflammation might reflect either a temporal- or a concentration-dependent control of inflammatory events, thus regulating the amplification of the inflammatory process.
It is becoming clear that receptor crosstalk is the rule and not the exception in cellular signaling and that an integration and communication network presumably tunes the strength and duration of the signals, thereby leading to fine regulation of the ultimate physiological responses (48, 49). Very recently GPR17, an orphan GPCR recently proposed to respond to both cysLTs and extracellular nucleotides (8), also seems to negatively regulate CysLT1R (50). Another group has also demonstrated intracellular localization of a functional CysLT1R, which adds complexity and opens new potential pathways for receptor signaling and transregulation (51).
Furthermore, comparison of the ability of fMLF, C5a, and IL-8 to desensitize one another has led to the observation of hierarchy in receptor desensitization between these chemoattractant receptors. This hierarchy may be important for leukocyte signal processing when multiple inflammatory mediators are present at sites of inflammation. Under these conditions, the “end-target mediators,” such as fMLF, that are more capable of activating terminal functions of neutrophils seem to prevail over “intermediary mediators,” such as LTD4 and other chemokines present at the site of inflammation (52).
Considering that monocytes/macrophages are likely to be the major source of cysteinyl-LT in many immunological and inflammatory processes, shedding light on how their receptors are regulated will certainly help to better understand the role of these cells in orchestrating the complex network of integrated signals.
Footnotes
Abbreviations:
- fMLF
- formyl-methionyl-leucyl phenylalanine
- LTD4
- leukotriene D4
- PAF
- platelet activating factor
- PGE2
- prostaglandin E2
- PTX
- pertussis toxin
- UTP
- uracil triphosphate
This work was supported in part by grants from EC FP6 (LSHM-CT-2004-005033) to G.E.R. This publication reflects only the author's views. The European Commission is not liable for any use that may be made of information herein. The work was also partially supported by Grant Fondazione CARIPLO 2006/0882 “Genomics and Proteomics of G Protein-Coupled Receptors: Novel Targets for the Diagnosis and Prevention of Human Diseases” to G.E.R.
REFERENCES
- 1.Samuelsson B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 220: 568–575. [DOI] [PubMed] [Google Scholar]
- 2.Brink C., Dahlen S. E., Drazen J., Evans J. F., Hay D. W., Nicosia S., Serhan C. N., Shimizu T., Yokomizo T. 2003. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 55: 195–227. [DOI] [PubMed] [Google Scholar]
- 3.Capra V., Thompson M. D., Sala A., Cole D. E., Folco G., Rovati G. E. 2007. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med. Res. Rev. 27: 469–527. [DOI] [PubMed] [Google Scholar]
- 4.Funk C. D. 2005. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat. Rev. Drug Discov. 4: 664–672. [DOI] [PubMed] [Google Scholar]
- 5.Capra V., Ambrosio M., Riccioni G., Rovati G. E. 2006. Cysteinyl-leukotriene receptor antagonists: present situation and future opportunities. Curr. Med. Chem. 13: 3213–3226. [DOI] [PubMed] [Google Scholar]
- 6.Riccioni G., Capra V., D'Orazio N., Bucciarelli T., Bazzano L. A. 2008. Leukotriene modifiers in the treatment of cardiovascular diseases. J. Leukoc. Biol. 84: 1374–1378. [DOI] [PubMed] [Google Scholar]
- 7.Fredriksson R., Lagerstrom M. C., Lundin L. G., Schioth H. B. 2003. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63: 1256–1272. [DOI] [PubMed] [Google Scholar]
- 8.Ciana P., Fumagalli M., Trincavelli M. L., Verderio C., Rosa P., Lecca D., Ferrario S., Parravicini C., Capra V., Gelosa P., et al. 2006. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 25: 4615–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austen K. F., Maekawa A., Kanaoka Y., Boyce J. A. 2009. The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J. Allergy Clin. Immunol. 124: 406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y., Borrelli L. A., Kanaoka Y., Bacskai B. J., Boyce J. A. 2007. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene-dependent mitogenic responses of mast cells. Blood. 110: 3263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selbie L. A., Hill S. J. 1998. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol. Sci. 19: 87–93. [DOI] [PubMed] [Google Scholar]
- 12.Werry T. D., Wilkinson G. F., Willars G. B. 2003. Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem. J. 374: 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali H., Richardson R. M., Haribabu B., Snyderman R. 1999. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 274: 6027–6030. [DOI] [PubMed] [Google Scholar]
- 14.Walsh M. T., Foley J. F., Kinsella B. T. 2000. The alpha, but not the beta, isoform of the human thromboxane A2 receptor is a target for prostacyclin-mediated desensitization. J. Biol. Chem. 275: 20412–20423. [DOI] [PubMed] [Google Scholar]
- 15.Steele A. D., Szabo I., Bednar F., Rogers T. J. 2002. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 13: 209–222. [DOI] [PubMed] [Google Scholar]
- 16.Capra V., Ravasi S., Accomazzo M. R., Citro S., Grimoldi M., Abbracchio M. P., Rovati G. E. 2005. CysLT1 receptor is a target for extracellular nucleotide-induced heterologous desensitization: a possible feedback mechanism in inflammation. J. Cell Sci. 118: 5625–5636. [DOI] [PubMed] [Google Scholar]
- 17.Chuang T. T., Iacovelli L., Sallese M., De Blasi A. 1996. G protein-coupled receptors: heterologous regulation of homologous desensitization and its implications. Trends Pharmacol. Sci. 17: 416–421. [DOI] [PubMed] [Google Scholar]
- 18.Freedman N. J., Lefkowitz R. J. 1996. Desensitization of G protein-coupled receptors. Recent Prog. Horm. Res. 51: 319–351; discussion 352–353. [PubMed] [Google Scholar]
- 19.Stout R. D., Suttles J. 2004. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 76: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 21.Woszczek G., Pawliczak R., Qi H. Y., Nagineni S., Alsaaty S., Logun C., Shelhamer J. H. 2005. Functional characterization of human cysteinyl leukotriene 1 receptor gene structure. J. Immunol. 175: 5152–5159. [DOI] [PubMed] [Google Scholar]
- 22.Ichiyama T., Hasegawa M., Ueno Y., Makata H., Matsubara T., Furukawa S. 2005. Cysteinyl leukotrienes induce monocyte chemoattractant protein 1 in human monocytes/macrophages. Clin. Exp. Allergy. 35: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury R. P., Lee J. M., Greaves D. R. 2005. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat. Clin. Pract. Cardiovasc. Med. 2: 309–315. [DOI] [PubMed] [Google Scholar]
- 24.Mueller C. F., Wassmann K., Widder J. D., Wassmann S., Chen C. H., Keuler B., Kudin A., Kunz W. S., Nickenig G. 2008. Multidrug resistance protein-1 affects oxidative stress, endothelial dysfunction, and atherogenesis via leukotriene C4 export. Circulation. 117: 2912–2918. [DOI] [PubMed] [Google Scholar]
- 25.Kilfeather S. 2002. 5-lipoxygenase inhibitors for the treatment of COPD. Chest. 121: 197S–200S. [DOI] [PubMed] [Google Scholar]
- 26.Biswas S. K., Sica A., Lewis C. E. 2008. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J. Immunol. 180: 2011–2017. [DOI] [PubMed] [Google Scholar]
- 27.Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. 2009. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61: 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomhave E. D., Richardson R. M., Didsbury J. R., Menard L., Snyderman R., Ali H. 1994. Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J. Immunol. 153: 3267–3275. [PubMed] [Google Scholar]
- 29.Capra V., Accomazzo M. R., Ravasi S., Parenti M., Macchia M., Nicosia S., Rovati G. E. 2003. Involvement of prenylated proteins in calcium signaling induced by LTD4 in differentiated U937 cells. Prostaglandins Other Lipid Mediat. 71: 235–251. [DOI] [PubMed] [Google Scholar]
- 30.Capra V., Ravasi S., Accomazzo M. R., Parenti M., Rovati G. E. 2004. CysLT1 signal transduction in differentiated U937 cells involves the activation of the small GTP-binding protein Ras. Biochem. Pharmacol. 67: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 31.DeLean A., Munson P. J., Rodbard D. 1978. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 235: E97–E102. [DOI] [PubMed] [Google Scholar]
- 32.Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F., et al. 1991. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266: 15771–15781. [PubMed] [Google Scholar]
- 33.Sakano T., Fujie A., Hamasaki T., Harada Y., Taniguchi H., Ueda K. 1988. Intracellular Ca2+ mobilization in immature and more mature U937 induced to differentiate by dimethyl sulfoxide or phorbol myristate acetate. Cell. Immunol. 111: 390–397. [DOI] [PubMed] [Google Scholar]
- 34.Pollock K., Creba J. 1990. Leukotriene D4 induced calcium changes in U937 cells may utilize mechanisms additional to inositol phosphate production that are pertussis toxin insensitive but are blocked by phorbol myristate acetate. Cell. Signal. 2: 563–568. [DOI] [PubMed] [Google Scholar]
- 35.Prossnitz E. R., Kim C. M., Benovic J. L., Ye R. D. 1995. Phosphorylation of the N-formyl peptide receptor carboxyl terminus by the G protein-coupled receptor kinase, GRK2. J. Biol. Chem. 270: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 36.Willars G. B., Muller-Esterl W., Nahorski S. R. 1999. Receptor phosphorylation does not mediate cross talk between muscarinic M(3) and bradykinin B(2) receptors. Am. J. Physiol. 277: C859–C869. [DOI] [PubMed] [Google Scholar]
- 37.Naik S., Billington C. K., Pascual R. M., Deshpande D. A., Stefano F. P., Kohout T. A., Eckman D. M., Benovic J. L., Penn R. B. 2005. Regulation of cysteinyl leukotriene type 1 receptor internalization and signaling. J. Biol. Chem. 280: 8722–8732. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson S. S. 2007. Phosphorylation-independent attenuation of GPCR signalling. Trends Pharmacol. Sci. 28: 173–179. [DOI] [PubMed] [Google Scholar]
- 39.Richardson R. M., Ali H., Tomhave E. D., Haribabu B., Snyderman R. 1995. Cross-desensitization of chemoattractant receptors occurs at multiple levels. Evidence for a role for inhibition of phospholipase C activity. J. Biol. Chem. 270: 27829–27833. [DOI] [PubMed] [Google Scholar]
- 40.Honda Z., Takano T., Hirose N., Suzuki T., Muto A., Kume S., Mikoshiba K., Itoh K., Shimizu T. 1995. Gq pathway desensitizes chemotactic receptor-induced calcium signaling via inositol trisphosphate receptor down-regulation. J. Biol. Chem. 270: 4840–4844. [DOI] [PubMed] [Google Scholar]
- 41.Sallese M., Mariggio S., D'Urbano E., Iacovelli L., De Blasi A. 2000. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: direct interaction of kinase N terminus with activated galphaq. Mol. Pharmacol. 57: 826–831. [PubMed] [Google Scholar]
- 42.Fields T. A., Casey P. J. 1997. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem. J. 321: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson R. M., Marjoram R. J., Barr A. J., Snyderman R. 2001. RGS4 inhibits platelet-activating factor receptor phosphorylation and cellular responses. Biochemistry. 40: 3583–3588. [DOI] [PubMed] [Google Scholar]
- 44.Woszczek G., Chen L. Y., Nagineni S., Kern S., Barb J., Munson P. J., Logun C., Danner R. L., Shelhamer J. H. 2008. Leukotriene D(4) induces gene expression in human monocytes through cysteinyl leukotriene type I receptor. J. Allergy Clin. Immunol. 121: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch K. R., O'Neill G. P., Liu Q., Im D. S., Sawyer N., Metters K. M., Coulombe N., Abramovitz M., Figueroa D. J., Zeng Z., et al. 1999. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 399: 789–793. [DOI] [PubMed] [Google Scholar]
- 46.Rovati G. E., Baroffio M., Citro S., Brichetto L., Ravasi S., Milanese M., Crimi E., Brusasco V. 2006. Cysteinyl-leukotrienes in the regulation of beta2-adrenoceptor function: an in vitro model of asthma. Respir. Res. 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali H., Sozzani S., Fisher I., Barr A. J., Richardson R. M., Haribabu B., Snyderman R. 1998. Differential regulation of formyl peptide and platelet-activating factor receptors. Role of phospholipase Cbeta3 phosphorylation by protein kinase A. J. Biol. Chem. 273: 11012–11016. [DOI] [PubMed] [Google Scholar]
- 48.Gudermann T., Schoneberg T., Schultz G. 1997. Functional and structural complexity of signal transduction via G-protein-coupled receptors. Annu. Rev. Neurosci. 20: 399–427. [DOI] [PubMed] [Google Scholar]
- 49.Hur E. M., Kim K. T. 2002. G protein-coupled receptor signalling and cross-talk: achieving rapidity and specificity. Cell. Signal. 14: 397–405. [DOI] [PubMed] [Google Scholar]
- 50.Maekawa A., Balestrieri B., Austen K. F., Kanaoka Y. 2009. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc. Natl. Acad. Sci. USA. 106: 11685–11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen C. K., Campbell J. I., Ohd J. F., Morgelin M., Riesbeck K., Landberg G., Sjolander A. 2005. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 65: 732–742. [PubMed] [Google Scholar]
- 52.Heit B., Tavener S., Raharjo E., Kubes P. 2002. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]