Abstract
Cytochromes P450 (CYPs) metabolize polyunsaturated long-chain fatty acids (PUFA-LC) to several classes of oxygenated metabolites. Through use of human recombinant CYPs, we recently showed that CYP1A1, -2C19, -2D6, -2E1, and -3A4 are mainly hydroxylases, whereas CYP1A2, -2C8, -2C9, and -2J2 are mainly epoxygenases of arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), respectively. It is worth noting that the last double bond of these PUFAs, i.e., ω6 in AA or ω3 in EPA and DHA, respectively, was preferentially epoxidized. In this study, we have characterized the stereoselectivity of this epoxidation reaction by comparison with the PUFA-LC epoxide stereoisomers obtained from the enantioselective bacterial CYP102A1 F87V. The stereoselectivity of the epoxidation of the last olefin of AA (ω6), EPA (ω3), or DHA (ω3) differed between the CYP isoforms but was similar for EPA and DHA. These data give additional insight into the PUFA-LC epoxide enantiomers generated by the hepatic CYPs.
Keywords: regioselectivity, arachidonic acid, eicospentaenoic acid, docosahexaenoic acid, CYP102A1 F87V
Cytochromes P450 (CYPs) belong to a protein superfamily among which some members metabolize polyunsaturated long-chain fatty acids (PUFA-LC) to several classes of oxygenated metabolites. The product profiles depend on the involved CYP isoforms and may consist of a series of regio- and stereo-isomeric epoxides and hydroxylated compounds (1–3). In humans, CYP isoforms from family 1 to 3 are mainly epoxygenases, whereas CYP isoforms from family 4 are mainly ω-hydroxylases (4, 5).
CYP epoxygenases convert arachidonic acid (AA) to four epoxyeicosatrienoic acid regioisomers (5,6-, 8,9-, 11,12-, and 14,15-EET) that function as lipid mediators. EETs produce vascular relaxation, have antiinflammatory effects on blood vessels and in the kidney, promote angiogenesis, and protect ischemic myocardium and brain [for review, see (6–8)]. CYP epoxygenases also convert the ω3-PUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to epoxy-derivatives (9), which are potent dilators of coronary arterioles (10–12) or pulmonary artery (13) and inhibit platelet aggregation (14). The EPA-derived epoxides account for five epoxyeicosatetraenoic acids (5,6-, 8,9-, 11,12-, 14,15-, and 17,18-EETeTr), whereas the DHA-derived epoxides account for six epoxydocosapentaenoic acids (4,5-, 7,8-, 10,11-, 13,14-, 16,17-, and 19,20-EDP). A high regio- and stereoselectivity has been observed when testing the effects of chemically synthesized epoxides from AA and EPA. For example, in the rat, renal arteries were dilated by 11(R),12(S)-EET but not by 11(S),12(R)-EET or 14,15-EET enantiomers (15). Also in rats, among all EETeTr enantiomers, only the 17(R),18(S)-enantiomer, not 17(S),18(R), was effective on calcium-activated potassium (BK) channels in isolated cerebral arteries (11). However, in porcine coronary microvessels, all regioisomeric EETeTrs had similar vasodilatory potencies (16). Thus, the regio- and stereochemical features of the CYP-derived metabolites govern their biological activity and/or potency and functional significance. The biological activity of DHA epoxides regioisomers and enantiomers is still largely unknown as are the identity and reaction specificity of the individual CYP isoforms involved in EPA and DHA metabolisms (17).
According to previous studies, CYP isoforms display different regioselectivities for PUFA hydroxylation and epoxidation. For example, CYP1A1 is mainly an hydroxylase toward AA, whereas it acts primarily as an epoxygenase toward EPA as substrate (18). CYP102A1 (Bacillus Megaterium BM-3), a bacterial CYP that closely resembles eukaryotic microsomal P450s (19), is mainly an ω-hydroxylase for AA and a highly specific epoxygenase for EPA. Replacement of phenylalanine 87 (F87) with valine (V) converts CYP102A1 into a highly specific AA epoxygenase (20).
Through use of human recombinant CYPs, we recently evidenced that CYP1A1, -2C19, -2D6, -2E1, and -3A4 are mainly hydroxylases of AA, EPA, and DHA, whereas CYP2C8, -2C9, -2J2, and -1A2 are mainly epoxygenases (21). Epoxygenase regioselectivity depends on the nature of the unsaturated fatty acid of concern, because four, five, or six double bonds can be epoxidized. The number of epoxygenase products increases with the number of unsaturations, and the last double bond is preferentially oxidized, i.e., ω6 in AA or ω3 in EPA and DHA, respectively. However, to our knowledge, the studies about the stereoselectivity of this epoxidation have only dealt with AA or EPA and a few CYPs (18, 20, 22–29); none of them has been focused on DHA. Therefore, we have systematically investigated the stereochemistry of the epoxidation reaction of the last double bond of AA, EPA, and DHA by nine recombinant CYPs, with special emphasis on DHA epoxide enantiomers. The CYP102A1 F87V mutant was thus used as a model because of its high enantiofacial selectivity; indeed, it is known to epoxidize the si,re face of the last olefin of AA or EPA. This allowed us to identify, by comparison, the PUFA epoxide enantiomers generated by the human recombinant CYPs.
EXPERIMENTAL PROCEDURES
Chemicals
All chemicals of analytical grade were from Sigma-Aldrich (L'Isle d'Abeau, France). HPLC-purity grade solvents were from Carlo Erba (Val de Reuil, France). The counter-ion for HPLC, n-dibutylamine in its acetate concentrated form and m-chloroperbenzoic acid (m-CPBA), were both from Fluka (Buchs, Switzerland). The following PUFAs were from Cayman Chemicals (Spi-Bio, Montigny le Bretonneux, France): 5Z,8Z,11Z,14Z-eicosatetraenoic acid (AA C20:4), 5Z,8Z,11Z,14Z,17Z-eicosapentaenoic acid (EPA C20:5), 4Z,7Z,10Z,13Z,16Z,19Z-DHA C22:6. [1-14C]AA (2.07 GBq/mol) was from Amersham Biosciences (UK), [1-14C]EPA (1.92 GBq/mol) was from MP Biomedicals (Irvine, CA), and [1-14C]DHA (1.92 GBq/mol) was from Moravek Biomedicals (Brea, CA).
Recombinant CYPs and CYP102A1 F87V
Microsomes containing the human recombinant CYPs (CYP1A1, -1A2, -2C8, -2C9, -2C19, -2E1, -2D6, -2J2, and -3A4) were from BD-Biosciences Gentest (Supersome Enzymes, Woburn, MA). BD CYP Supersomes are recombinant cDNA-expressed CYP enzymes prepared from the baculovirus-infected insect cell system BTI-TH-5B1-4. Their respective characteristics were as follows (data from the suppliers): CYP1A1: 105 pmol/mg protein, NADPH P450 reductase 2400 nmol/min.mg, and 7-ethoxyresorufin deethylase activity 39 min−1; CYP1A2: 159 pmol/mg protein, NADPH P450 reductase 2600 nmol/min.mg, and phenacetin deethylase activity 56 min−1; CYP2C8: 278 pmol/mg protein, NADPH P450 reductase 170 nmol/min.mg, b5 410 pmol/mg, and paclitaxel 6-α-hydroxylase activity 9.9 min−1; CYP2C9: 370 pmol/mg protein, NADPH P450 reductase 260 nmol/min.mg, b5 560 pmol/mg, and diclofenac 4’-hydroxylase activity 20 min−1; CYP2C19: 192 pmol/mg protein, NADPH P450 reductase 880 nmol/min.mg, b5 260 pmol/mg, and S-mephenytoin 4’-hydroxylase activity 21 min−1; CYP2D6: 126 pmol/mg protein, NADPH P450 reductase 3500 nmol/min.mg, and bufuralol 1’-hydroxylase activity 59 min−1; CYP2E1: 182 pmol/mg protein, NADPH P450 reductase 4100 nmol/min.mg, b5 700 pmol/mg, and p-nitrophenol hydroxylase activity 11 min−1; CYP2J2: 278 pmol/mg protein, NADPH P450 reductase 450 nmol/min.mg, b5 320 pmol/mg, and terfenadine hydroxylase activity 43 min−1; CYP3A4: 870 pmol/mg protein, NADPH P450 reductase 2800 nmol/min.mg, b5 1200 pmol/mg, and testosterone 6-β-hydroxylase activity 150 min−1.
CYP102A1 F87V (P450 BM-3 F87V)
Crude extracts of CYP102A1 F87V were prepared according to (30). In brief, 250 ml of TBamp media, supplemented with 250 µl trace element solution, was inoculated with an LBamp-overnight culture of Escherichia coli DH5a pCWOri P450 BM-3 F87V. After reaching an optical density at 578 nm of 0.8 during cell cultivation (30°C, 250 rpm), D-aminolevulinic acid as precursor of porphyrin synthesis (0.5 mM, final concentration) was added and expression was induced by supplementation with isopropyl-beta-D-thiogalactoside (ITPG) (100 mM in final concentration). After 20 h of expression, E. coli cells were harvested by centrifugation and resuspended in phosphate buffer (25 ml; KH2PO4/K2HPO4, 25 mM, pH 7.5). The E. coli cells were subsequently lysed using a high-pressure homogenizer (1500 bar, three cycles). The lysate was centrifuged and further clarified by filtration through a low protein binding filter (0.45 mm). The resulting crude extract was lyophilized overnight and the powder stored at − 20°C. CYP102A1 F87V concentrations were estimated to be about 20 µM from CO-difference spectra as reported by Omura and Sato(31) using ϵ = 91 mM−1. cm−1. Use of the NADPH-depletion assay according to a well-established protocol led to 2.57 U.mg−1 as activity of this mutant in the crude extract when lauric acid was used as substrate (32).
Chemical synthesis of radiolabeled epoxides
14C-radiolabeled epoxides of PUFA were synthetized with m-CPBA then separated by HPLC and purified. Briefly, 100 nmol of [1-14C]PUFA were incubated with one equivalent of m-CPBA diluted in 0.1 ml of CH2Cl2 for 15 min at room temperature. Samples were then dried under nitrogen, resuspended in ethanol, and separated by HPLC as described below. The radiolabeled monoepoxides, previously identified by LC-MS (9), were collected.
Synthesis of radiolabeled epoxides by CYPs
All of the reaction mixtures were prepared in a final volume of 100 µL and contained 1 nmol of radiolabeled substrates, [1-14C]AA, [1-14C]EPA, and [1-14C]DHA, in 0.12 M phosphate buffer, pH 7.4 containing 5 mM MgCl2 and 10 pmol of recombinant CYP. To obtain the final concentration of 10 µM (CYP2C9, 2C8, CYP2C19, CYP2J2) or 50 µM (CYP1A1, 1A2, 2E1, 3A4), these compounds were supplemented with cold substrate. After a 5 min preincubation at 37°C, the reaction was started by addition of 1 mM NADPH. Different substrate concentrations were used to avoid auto-inhibition previously described (21). After 30 min, the reaction was stopped by addition of 0.1 ml acetonitrile containing 0.2% acetic acid (v/v). Fatty acids and their metabolites were twice extracted by 2 ml ethyl acetate. Samples dried under nitrogen were dissolved in 50 µL ethanol, and the metabolites were resolved by HPLC as described below.
Incubation with CYP 102A1 F87V
The reaction mixture contained 100 µM of AA, EPA, or DHA and radiolabeled substrate, 10 µg of crude extract, 0.12 M phosphate buffer, pH 7.4, and 5 mM MgCl2. After a 2 min preincubation at 30°C, the reaction was started by addition of 1 mM NADPH. After 2 min, the reaction was stopped by addition of 0.1 ml of a solution of acetonitrile with 0.2% acetic acid (v/v). The samples were then processed as above.
HPLC conditions
The monoepoxide or hydroxylated metabolites of fatty acids were separated by a reversed phase ion-pairing LC as previously reported (9). Briefly, the regioisomers were separated isocratically on a RP-Capcell Pak C18, 120 Å, 250 mm × 4.6 mm, particle diameter 5 µm (Shiseido Co Ltd, Japan, distributed by C.I.L, Ste Foy La Grande, France). The mobile phase A consisted of a mixture of water-methanol-acetonitrile (52/8/40; v/v/v) containing 15 mM n-dibutylamine. The composition of this mobile phase was adjusted for each substrate as previously reported (9). Radioactivity was measured with a β-radiometric flow scintillation analyzer Flo-one 500Tr (Perkin-Elmer-Packard Biosciences) with Ultima Flo scintillation cocktail added to the mobile phase at a flow of 1 ml/min. The radiolabeled monoepoxides, previously identified by LC-MS, were collected. The samples acidified by 20 µL of acetic acid were twice extracted by 2 ml ethyl acetate and dried under nitrogen. Then, the methylation reaction was started by addition to the samples of 300 µL of diethylether saturated with diazomethane. After incubation for 15 min at room temperature, the samples were dried under nitrogen and redissolved into hexane prior to analysis by chiral HPLC.
HPLC-MS identification of epoxides
The epoxidized metabolites were separated under the HPLC conditions described above and identified by MS on a mass spectrometer (Navigator model, Thermo-Electron, Les Ulis, France) equipped with an atmospheric pressure chemical ionization (APCI) source and operated in negative-ion mode. The cone voltage was optimized in the range of 30–45 V to enhance molecular fragmentation. The vaporizer temperature for APCI was 350°C and the source was 150°C. The drying nitrogen flow was 352 L/h, the needle of the corona pin was set at 3 kV, the skimmer at 0.9 V, and the quadrupole ion energy at 2.6 V. For epoxide identification, the negative ions were monitored in full scan mode in 1 s from m/z 50 to 600.
HPLC chiral analysis
For chiral analysis, the standard reactions of incubation were repeated 3- to 7-fold. The products collected from the HPLC runs, i.e., 14,15-EETs, 17,18-EETeTr, or 19,20-EDP, were pooled and derivatized with diazomethane to methylesters that were subsequently analyzed by chiral-phase HPLC on a Chiralcel OB column (250 × 4.6 mm; Daicel, Interchim, Montluçon, F). The solvent was hexane with 0.3% isopropyl alcohol and 0.05% (v/v) acetic acid and was delivered at a flow rate of 1 ml/min. The enantiomers were detected with a β-radiometric flow scintillation analyzer Flo-one 500Tr (Perkin-Elmer-Packard Biosciences). The enantiomers were identified by comparison with known products generated by CYP102A1 F87V catalyst.
GC-MS identification of epoxides
The epoxidized samples were analyzed by GC-MS on an Agilent GC 6890 coupled to a 5973 MS Detector (Agilent, Les Ulis, France) and equipped with a DB-5MS column (low-bleeding, 5% phenyl/95% dimethylpolysiloxane phase) 30 m × 0.25 mm inner diameter × 0.25 µm film thickness (J and W Scientific, Agilent). The temperatures of the injection port and interface were 250 and 280°C, respectively; those of the ion source and MS analyzer were set at 150 and 106°C, respectively. The samples were injected in splitless mode. The oven temperature was first set at 60°C for 5 min, elevated to 230°C at the rate of 50°C/min and then to 290°C at 1°C/min. The compounds were ionized by electron impact at 70 eV energy. The carboxylic group of DHA monoepoxides was converted to methyl ester as previously described. The reaction solvent was dried under a nitrogen stream and redissolved in hexane prior to injection. Analytes were detected by total ion current from m/z 50 to 600.
RESULTS
Regioselectivity of CYP isoforms and CYP 102A1 F87V on the epoxidation of AA, EPA, and DHA
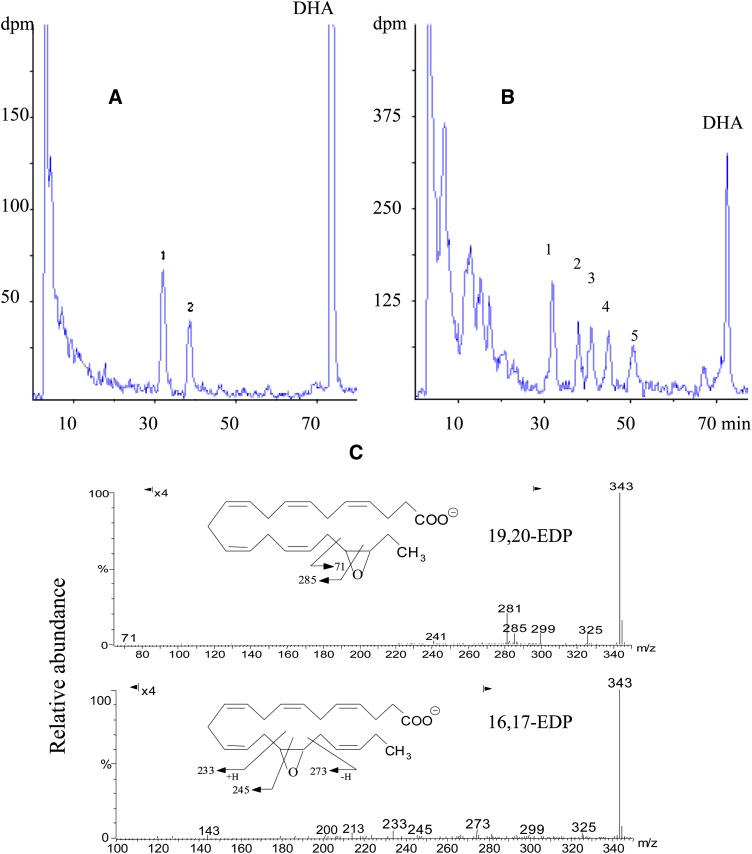
Incubation of AA, EPA, or DHA with recombinant CYP1A1, -1A2, -2C8, -2C9, -2C19, -2D6, -2E1, -2J2, and -3A4 resulted in the preferential epoxidation of the last double bond of the alkyl chain and generated the 14,15-EET, 17,18- EETeTr, and 19,20-EDP, respectively (Table 1). A high regioselectivity was observed with CYP1A1, -2D6, and -2E1 as epoxidation took place mainly on the last and the penultimate double bonds. Such a regioselectivity had been already reported with CYP102A1 or its mutants toward AA and EPA (20, 24). However, to our knowledge, no description of DHA metabolism by CYP102A1 or its F87V mutant is available in the literature. Thus, this ω3-PUFA was incubated with the CYP102A1 F87V crude extract to generate the corresponding epoxides (EDPs). After separation by reversed-phase ion-pair HPLC, the main metabolites appeared to be two monoepoxides in the ratio 37:63 and identified as the 16,17- and the 19,20-EDP (Fig. 1A). Their characterization relied on their HPLC retention times identical to those of synthetic 16,17- and 19,20-EDP (Fig. 1B) and on their mass spectra analysis by LC-MS-APCI (Fig. 1C) or GC-MS-electron ionization after derivatization as methyl esters. LC-MS-APCI analysis showed the presence of major negative fragment ions at m/z 343 [base peak, M-H]−. The fragmentation of regioisomers was governed by the epoxide ring located in the alkyl chain and involved either a cleavage in position α with respect to the epoxide moiety or a cleavage of the oxirane ring itself at two positions. This led to three characteristic ions from 16,17-EDP, namely m/z=233 and 273 (fragmentation in the α position) and m/z=245 (fragmentation in the oxirane ring). Two characteristic ions were obtained from 19,20-EDP, namely m/z=71 (fragmentation in the α position) and m/z=285 (fragmentation in the oxirane ring). Other ions, i.e., m/z 325[M-H2O]−, m/z 299 [M-CO2]−, and m/z 281 [M-H2O-CO2]−, were issued from fragmentation of the carboxylic group and were common for 16,17- or 19,20-EDP regioisomers. Mass spectra analysis by GC-MS-electron ionization after derivatization of the two monoepoxides as methyl esters showed major fragment ions at m/z 358 (base peak), m/z 289 and 247 (fragmentation in the α position), and m/z 260 (fragmentation of oxirane ring) for 16,17-EDP. Characteristic ions for 19,20-EDP were at m/z 287 and 329 (fragmentation in the α position) and m/z 300 (fragmentation of oxirane ring). These mass spectra were identical to those generated under similar conditions by synthetic 16,17- or 19,20-EDP regioisomers (data not shown).
TABLE 1.
Percentage of each epoxide formed from three PUFAs by CYPs
| Epoxides |
|||||||
|---|---|---|---|---|---|---|---|
| Last double bond: | Penultimate bond: | Ante-Penultimate bond: | |||||
| 14,15- EET | 11,12-EET | 8,9-EET | 5,6-EET | ||||
| 17,18-EETeTr | 14,15-EETeTr | 11,12-EETeTr | 8,9-EETeTr | 5,6-EETeTr | |||
| CYP | PUFA | 19,20-EDP | 16,17-EDP | 13,14-EDP | 10,11-EDP | 7,8-EDP | 4,5-EDP |
| 1A1 | AA | 100 | nd | nd | nd | — | — |
| EPA | 100 | nd | nd | nd | nd | — | |
| DHA | 100 | nd | nd | nd | nd | nd | |
| 1A2 | AA | 26 | 43 | 10 | 21 | — | — |
| EPA | 76 | 9 | 9 | 6 | nd | — | |
| DHA | 54 | 22 | 8 | 10 | 6 | nd | |
| 2C8 | AA | 54 | 46 | nd | nd | — | — |
| EPA | 41 | 40 | 19 | nd | nd | — | |
| DHA | 47 | 18 | 15 | 20 | nd | nd | |
| 2C9 | AA | 56 | 31 | 13 | nd | — | — |
| EPA | 35 | 25 | 17 | 15 | 8 | — | |
| DHA | 15 | 25 | 12 | 42 | 6 | nd | |
| 2C19 | AA | 37 | 12 | 40 | 11 | — | — |
| EPA | 56 | 17 | 8 | 14 | nd | — | |
| DHA | 27 | 15 | 12 | 32 | 14 | nd | |
| 2D6 | AA | 47 | 18 | 25 | 10 | — | — |
| EPA | 100 | nd | nd | nd | nd | — | |
| DHA | 100 | nd | nd | nd | nd | nd | |
| 2E1 | AA | 100 | nd | nd | nd | — | — |
| EPA | 74 | 15 | 10 | 7 | nd | — | |
| DHA | 93 | 7 | nd | nd | nd | nd | |
| 2J2 | AA | 60 a | 40 a | nd | nd | — | — |
| EPA | 50 | 16 | 15 | 15 | 3 | — | |
| DHA | 77 | nd | 8 | 8 | 7 | nd | |
| 3A4 | AA | 29 | 37 | 21 | 13 | — | — |
| EPA | 41 | 18 | 20 | 7 | 4 | — | |
| DHA | 41 | 18 | 18 | 16 | 7 | nd | |
| CYP102A1 (F87V) | AA | 99 b | <1 | nd | nd | — | — |
| EPA | 73 c | 27 b | nd | nd | nd | — | |
| DHA | 63 | 37 | nd | nd | nd | nd | |
Fig. 1.
DHA metabolism by CYP102A1 F87V. 1A: RP-HPLC Chromatographic resolution of the products generated by CYP102A1 F87V during the metabolism of DHA; Peak 1: 19,20-EDP; Peak 2: 16,17-EDP. A total of 100 µM of [1-14C] DHA was incubated with10 µg of crude extract CYP102A1 F87V. The HPLC analysis was carried out as described in Experimental Procedures. 1B: RP-HPLC chromatographic resolution of the products generated by chemical epoxidation of DHA; Peak 1: 19,20-EDP; Peak 2: 16,17-EDP; Peak 3: 13,14-EDP; Peak 4: 10,11-EDP; Peak 5: 7,8-EDP. These peaks were previously identified by LC-MS (9). 1C: LC-MS of 16,17- and 19,20-EDP generated by incubation of DHA with CYP102A1 F87V as described in Experimental Procedures. Only significant fragments of the epoxide position in the alkyl chain were indicated.
However, the stereoselectivity of the epoxidation reaction by CYP isoforms is poorly known, especially when DHA is a substrate. The lack of commercially available enantiomeric standards for EDP prevents their easy characterization. This led us here to identify these enantiomers from the well-known stereoselectivity of CYP102A1 F87V (24).
Stereoselective epoxidation of the last double bond of AA and EPA by CYP102A1 F87V and recombinant human CYPs
According to previous results (24), incubation of CYP102A1 with AA generates 18(R)-OH-AA and 14(S),15(R)-EET in a 4:1 molar ratio, whereas EPA is metabolized to 17(S)18(R)-EETeTr with 97% optical purity as the sole reaction product. Conversely, the metabolism of PUFAs by the CYP102A1 F87V mutant generates 14(S),15(R)-EET (99% of total products) from AA and 14(S),15(R)- and 17(S),18(R)-EETeTr, 26 and 69% of total, respectively, from EPA (20). In this study, the crude extract of CYP102A1 F87V yielded the same main regioisomers as those obtained with purified CYP102A1 F87V protein, i.e., 14,15-EET for metabolism of AA and a mixture of 14,15- and 17,18-EETeTr for EPA (not shown). These regioisomers were known to be the S,R enantiomers (20). Their use as standards enabled us to identify unambiguously the optical isomers formed by the epoxidation of the last double bond of AA (ω6) and EPA (ω3) by the human recombinant CYPs.
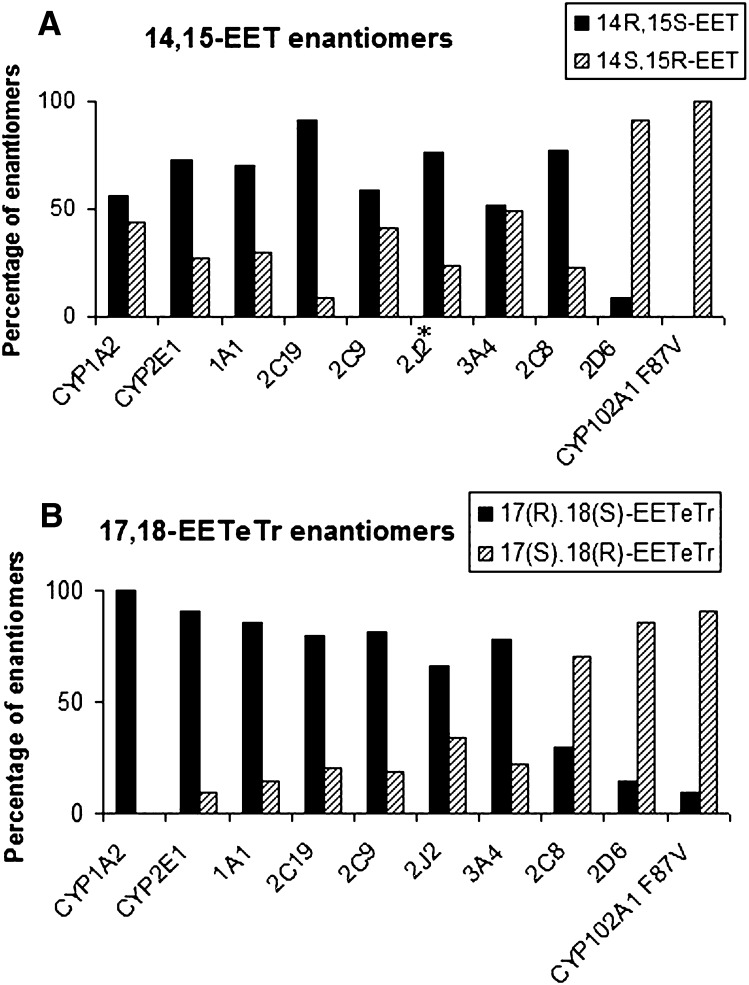
As shown in Fig. 2A, CYP1A1, -1A2, -2E1, -2C9, -2J2, and -3A4 displayed a nearly racemic epoxidation of the last double bond of AA (ω6) compared with an absolute stereoselectivity for CYP2C19, -2D6, and CYP102A1 F87V, which was in favor of the 14(R),15(S)-EET for CYP2C19 and of its enantiomer for CYP2D6 and CYP102A1 F87V. By contrast, epoxidation of the last double bond of EPA (ω3) (Fig. 2B) by most of the recombinant CYPs generated an enantiomeric excess of 17(R),18(S)-EETeTr, while CYP2D6, -2C8, and CYP102A1 F87V displayed an opposite stereoselectivity compared with the other CYPs. The epoxidation stereoselectivity of the EPA ω3 olefin by CYP1A2, -2E1, and CYP102A1 F87V (although opposite) was nearly absolute. As already described (22), CYP2C8 showed an opposite stereoselectivity of epoxidation of the AA ω6- and EPA ω3-olefins, as the R,S enantiomer was mainly generated from AA and S,R from EPA.
Fig. 2.
Stereochemistry of epoxidation of the last double bond of AA and EPA by CYPs. Results are expressed as percentages of the two enantiomers generated by incubation of AA (A) and EPA (B) with CYP102A1 F87V and human recombinant CYPs. Enantiomers were identified by comparison with the S,R enantiomer generated by CYP102A1 F87V. * CYP2J2 data were previously reported (42).
Stereoselective epoxidation of the last double bond of DHA by CYP102A1 F87V and human recombinant CYPs
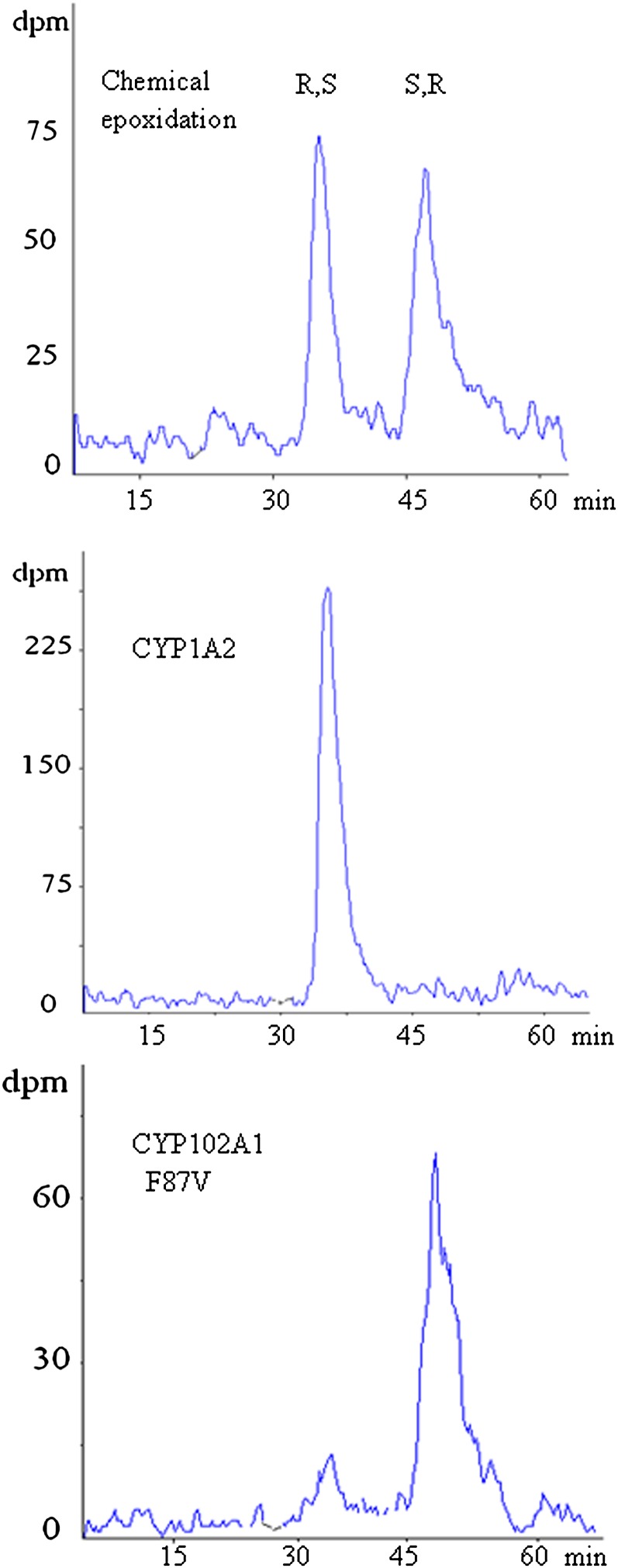
As expected, the biosynthesis of 19,20-EDP by CYP102A1 F87V was highly asymmetric and presumably generated the 19(S),20(R)-EDP enantiomer (87% optical purity) (Fig. 3).
Fig. 3.
HPLC chiral analysis of 19,20 EDP. Representative HPLC chiral analysis illustrating the stereoselectivity of epoxidation of CYP102A1 F87V and CYP1A2 during the 19,20-double bond epoxidation of DHA versus the racemisation of this olefin by chemical epoxidation.
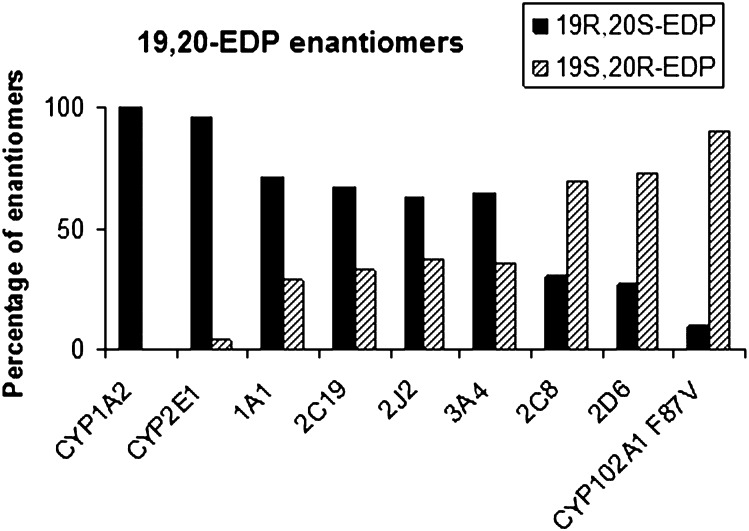
Identification of this enantiomer was based on the previously described C-H acceptor chemistry and active site-binding coordinates of CYP102A1 F87V known to preferentially deliver oxygen to the si,re-face of the last olefinic bond for both AA (ω6) and EPA (ω3) (24). If the enantiomer generated from DHA by CYP102A1 F87V is the 19(S),20(R)-EDP, the main product issued from epoxidation of the last double bond ω3 of DHA by CYP1A1, -1A2, -2E1, -2C19, -2J2, and -3A4 should be the 19(R),20(S)-EDP. Figure 4 shows that all the CYPs under study generated an enantiomeric excess in favor of the 19(R),20(S)-EDP for CYP1A1, -1A2, -2E1, -2J2, and -3A4 and of its optical antipode for CYP2D6, -2C8, and CYP102A1 F87V. One should note that the stereoselectivity displayed by CYP1A2, -2E1, and CYP102A1 F87V was nearly absolute and the major EDP enantiomer generated by CYP2C8 was the S,R enantiomer. The same observations being valid for EPA suggests that the stereoselectivity of epoxidation of the last olefinic bond ω3 of EPA and DHA by CYPs is very similar.
Fig. 4.
Stereochemistry of epoxidation of the last double bond of DHA by CYPs. Results are expressed as percentages of the two enantiomers generated by incubation of DHA with CYP102A1 F87V and human recombinant CYPs. Enantiomers were identified by comparison with the S,R enantiomer generated by CYP102A1 F87V.
DISCUSSION
Little is known about the identity and reaction specificity of the individual CYP isoforms involved in EPA and DHA metabolisms (17). The use of human recombinant CYPs allowed us to recently demonstrate that the epoxygenase-hydroxylase activity ratio is dependent upon the nature of PUFAs and CYP isoforms (21). However, with EPA and DHA, epoxygenase products were more abundant than with AA. Furthermore, the last double bond of the alkyl chain of AA (ω6), EPA (ω3), or DHA (ω3) was preferentially epoxidized. After a reassessment of the regiospecificity of AA-, EPA-, and DHA- epoxidation of the last olefinic bond by human CYPs of families 1–3, we have characterized the stereochemistry of this reaction with special emphasis on DHA-epoxide enantiomers.
Regioselectivity of epoxidation
Depending on the nature of the PUFAs, four, five, or six double bonds may be concerned by the regioselectivity of the epoxidation reaction. In this study, the preferentially oxidized double bond by CYP1A1, -1A2, -2D6, -2J2, and -2E1 was always the last one, i.e., 14,15 (AA), 17,18 (EPA), or 19,20 (DHA). This high regioselectivity involves a strict position of PUFAs in their active sites with the last double bond close to the ferryl-oxo complex of CYPs. However, many other double bonds have been shown to be epoxidized, particularly by CYP2C enzymes (33). Recently published crystal structures of CYP2C8/2C9 (34) have evidenced a great flexibility in the conformation of the active site cavity, which allows the binding of ligands in multiple positions. Furthermore, thanks to their high flexibility, fatty acids would move in the close vicinity of the ferryl-oxo complex to be epoxidized in many positions.
A comparison of the catalytic specificities of CYP102A1 and human CYP1A1 (24) also allowed some interesting parallels. Much of what we know regarding CYP structure-function relationships derives from studies with soluble bacterial CYPs, especially CYP102A1 (P450 BM-3). The crystal structure of the substrate-free heme domain revealed an open access channel from the protein surface deep into the heme active site (35). The phenylalanine F87 is located over the heme surface in a position to anchor the hydrocarbon tail of substrates. Replacement of the aromatic ring of F87 by a valine in the F87V mutant allowed further displacement of the fatty acid molecule along the longitudinal axis of the access channel (20). Thus, the metabolism of AA by CYP102A1 leads to the production of 18-OH-AA (main product) and 14,15-EET, whereas the F87V mutant generates 14,15-EET as the sole reaction product. In our study, metabolism of EPA or DHA by CYP102A1 F87V generated a mixture of two regioisomers, namely the 14,15- and 17,18- EETeTr for EPA, as previously described (20), or the 16,17- and 19,20- EDPs for DHA. Thus, it appeared that the regioselectivity of the last olefin epoxidation was very similar for EPA and DHA, as the ω3 and ω6 olefins were epoxidized in each case. Furthermore, it must be underlined that the preferred site of AA and EPA for catalyzed oxygen insertion by CYP102A1 is the (ω-2) carbon atom leading to 18-OH-AA and 17,18-EETeTr, respectively. This indicates that both fatty acids likely occupy more or less similar spatial coordinates in the enzyme active site.
Modeling experiments provide evidence for lowest interaction energies of the C20 fatty acid-CYP1A1 complexes when the substrates are docked in orientations leading to hydroxylation at C19 or electron abstraction at C18 and C17 (18). Such energetic differences may explain the regioselectivity of CYP-mediated reactions (36). It appears that flexible AA and EPA are stabilized in the active site mainly by hydrophobic interactions, thus governing the regiospecificity of substrate oxidation.
Stereoselectivity of epoxidation
The stereoselectivity of PUFA epoxidation was studied using CYP102A1 F87V as a model because of its high enantioselectivity. Regardless of the fatty acid, the si,re faces of the last double bond, 14,15 or 17,18-olefin for AA and EPA, respectively, were always selected for epoxidation (20). We assumed that epoxidation would also take place on the si,re face of the 19,20-olefin of DHA, given the similar regioselectivity established between EPA and DHA.
The absolute configurations of the regioisomers were assigned by chromatographic comparisons with enantiomerically pure methylated fatty acid epoxides prepared by total chemical synthesis (racemic mixture) and enzymatic epoxidation from the enantioselective CYP102A1 F87V. Furthermore, the chiral analysis by HPLC on Chiralcel OB showed that the S,R enantiomer epoxide of the last double bond of AA and EPA was eluted after its optical antipode, as was the case in all previous studies (18, 22, 23, 33, 37). These results were verified with DHA epoxide enantiomers, namely the 19,20-EDP. It is worth underlining that the elution order was the opposite on Chiralcel OD (38) or Chiralcel OJ (28).
Our investigations showed that the epoxidation of the last double bond by most of the hepatic CYPs is more stereoselective with the (ω3) PUFA (EPA or DHA) as substrates than with the (ω6) AA. Interestingly, CYP2C8 showed an opposite stereoselectivity of this reaction between AA and EPA or DHA. CYP2C9 epoxidized EPA with a lower regioselectivity for the last olefin than CYP2C8 as already reported (22).The same was observed with DHA, resulting in a decreased capacity of CYP2C9 to synthesize the 19,20-EDP. This prevented us from characterizing the enantioselectivity of this reaction.
This study confirmed the previously described stereoselectivity of epoxidation catalyzed by the CYP2C (33, 39) and CYP1A1 (18) subfamilies in AA and EPA metabolisms. Whereas CYP102A1 F87V is known to epoxidize the si,re face of the last double bonds of AA, EPA, and DHA, CYP1A1, -1A2, -2E1, -2C19, and -2J2 proved to preferentially oxidize the re,si face during the metabolism of EPA or DHA. This difference suggests that these ω3-PUFAs were strictly positioned in the active site with the re,si face set in the close vicinity of the ferryl-oxo complex of these CYPs. In our opinion, this positioning is governed by hydrophobic interactions but not by the reactivity of the double bond hydrogens. In the case of CYP102A1, it has been suggested that the F87 residue blocks the ω-terminus of fatty acids (18) so that the si,re face is presented near the ferryl-oxo complex. By analogy, the F equivalent residues of CYP1A1, -1A2, -2E1, and -2C19 might play the same role in blocking the ω-methyl terminus but in such a position that the re,si face of the double bond will be preferentially epoxidized.
CONCLUSION
A careful examination of the epoxidation reaction of the last double bonds of the three substrates emphasizes high similarities in the stereoselectivity of EPA and DHA epoxidation by the nine CYPs but noteworthy differences between the CYPs. The similarities are likely attributable to the position of the last double bond in such long-chain fatty acids; indeed, EPA and DHA belong to the ω3 class, whereas AA is a member of the ω6 class. As attempts to get crystallized PUFA-bound human CYPs have been unsuccessful until now, our experimental data were important, because the enantioselective metabolism of PUFAs appears unpredictable from the sole knowledge of active site conformation.
The CYP-dependent metabolism of EPA and DHA is a source of physiologically active compounds that may contribute to the beneficial cardiovascular effects attributed to diets rich in ω3-PUFAs (40). The relative importance of the hepatic CYPs to biologically active metabolites in the vasculature and other tissues remains to be established. Because the epoxidized metabolites of AA are known to be involved in the regulation of blood pressure (41), some functional effects of supplementation with ω3-PUFAs may result from the conversions of EPA and DHA to the corresponding epoxidized metabolites and perhaps to specific enantiomers. Stereoisomers are acknowledged to often display very different physiological properties. While the 17(R),18(S)-EETeTr has been shown to be the active metabolite on the BK channels in rat cerebral arteries (11) and DHA epoxides to inhibit the platelet aggregation (14), it remains to determine which of the R,S or S,R 19,20-EDP is the more active. Much work is needed to take into account the biological activities of these enantiomers.
Acknowledgments
The authors thank Drs. F. Berthou and J-P. Salaün for their helpful contributions to this study.
Footnotes
Abbreviations:
- AA
- arachidonic acid [5,8,11,14-eicosatetraenoic acid or C20:4, (n-6)]
- BK
- calcium-activated potassium
- CYP
- cytochrome P450
- EET
- epoxieicosatrienoic acid
- EPA
- eicosapentaenoic acid [5,8,11,14,17-eicosapentaenoic acid or C20:5, (n-3)]
- EETeTr
- epoxieicosatetraenoic acid
- DHA
- docosahexaenoic acid [4,7, 10,13,16,19-docosahexaenoic acid or C22:6, (n-3)]
- EDP
- epoxidocosapentaenoic acid
- F87
- phenylalanine 87
- PUFA-LC
- polyunsatured long-chain fatty acids
- DBA
- n-dibutylamine
- OR
- cytochrome P450 reductase
- m-CPBA
- m-chloroperbenzoic acid
- LC-MS-APCI
- liquid chromatography-mass spectrometry-atmospheric pressure chemical ionization
- V
- valine
This work was supported by a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche (to S.G.).
REFERENCES
- 1.Capdevila J. H., Harris R. C., Falck J. R. 2002. Microsomal cytochrome P450 and eicosanoid metabolism. Cell. Mol. Life Sci. 59: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroetz D. L., Zeldin D. C. 2002. Cytochrome P450 pathways of arachidonic acid metabolism. Curr. Opin. Lipidol. 13: 273–283. [DOI] [PubMed] [Google Scholar]
- 3.Spector A. A., Fang X., Snyder G. D., Weintraub N. L. 2004. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 43: 55–90. [DOI] [PubMed] [Google Scholar]
- 4.Roman R. J. 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 5.Fer M., Corcos L., Dreano Y., Plee-Gautier E., Salaun J. P., Berthou F., Amet Y. 2008. Cytochromes P450 from family 4 are the main omega hydroxylating enzymes in humans: CYP4F3B is the prominent player in PUFA metabolism. J. Lipid Res. 49: 2379–2389. [DOI] [PubMed] [Google Scholar]
- 6.Capdevila J. H., Falck J. R., Harris R. C. 2000. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 41: 163–181. [PubMed] [Google Scholar]
- 7.Spector A. A., Norris A. W. 2007. Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. Cell Physiol. 292: C996–C1012. [DOI] [PubMed] [Google Scholar]
- 8.Spector A. A. 2009. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 50 (Suppl.): S52–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fer M., Goulitquer S., Dreano Y., Berthou F., Corcos L., Amet Y. 2006. Determination of polyunsaturated fatty acid monoepoxides by high performance liquid chromatography-mass spectrometry. J. Chromatogr. A. 1115: 1–7. [DOI] [PubMed] [Google Scholar]
- 10.Hercule H. C., Salanova B., Essin K., Honeck H., Falck J. R., Sausbier M., Ruth P., Schunck W. H., Luft F. C., Gollasch M. 2007. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp. Physiol. 92: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 11.Lauterbach B., Barbosa-Sicard E., Wang M. H., Honeck H., Kargel E., Theuer J., Schwartzman M. L., Haller H., Luft F. C., Gollasch M., et al. 2002. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 39: 609–613. [DOI] [PubMed] [Google Scholar]
- 12.Ye D., Zhang D., Oltman C., Dellsperger K., Lee H. C., VanRollins M. 2002. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 303: 768–776. [DOI] [PubMed] [Google Scholar]
- 13.Morin C., Sirois M., Echave V., Rizcallah E., Rousseau E. 2009. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 296: L130–L139. [DOI] [PubMed] [Google Scholar]
- 14.VanRollins M. 1995. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J. Pharmacol. Exp. Ther. 274: 798–804. [PubMed] [Google Scholar]
- 15.Zou A. P., Fleming J. T., Falck J. R., Jacobs E. R., Gebremedhin D., Harder D. R., Roman R. J. 1996. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am. J. Physiol. 270: F822–F832. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Oltman C. L., Lu T., Lee H. C., Dellsperger K. C., VanRollins M. 2001. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am. J. Physiol. Heart Circ. Physiol. 280: H2430–H2440. [DOI] [PubMed] [Google Scholar]
- 17.Serhan C. N., Chiang N. 2008. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br. J. Pharmacol. 153 (Suppl. 1): S200–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz D., Kisselev P., Ericksen S. S., Szklarz G. D., Chernogolov A., Honeck H., Schunck W. H., Roots I. 2004. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem. Pharmacol. 67: 1445–1457. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Poulos T. L. 1999. Fatty acid metabolism, conformational change, and electron transfer in cytochrome P-450(BM-3). Biochim. Biophys. Acta. 1441: 141–149. [DOI] [PubMed] [Google Scholar]
- 20.Graham-Lorence S., Truan G., Peterson J. A., Falck J. R., Wei S., Helvig C., Capdevila J. H. 1997. An active site substitution, F87V, converts cytochrome P450 BM-3 into a regio- and stereoselective (14S,15R)-arachidonic acid epoxygenase. J. Biol. Chem. 272: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 21.Fer M., Dreano Y., Lucas D., Corcos L., Salaun J. P., Berthou F., Amet Y. 2008. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch. Biochem. Biophys. 471: 116–125. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa-Sicard E., Markovic M., Honeck H., Christ B., Muller D. N., Schunck W. H. 2005. Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem. Biophys. Res. Commun. 329: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 23.Capdevila J. H., Karara A., Waxman D. J., Martin M. V., Falck J. R., Guenguerich F. P. 1990. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J. Biol. Chem. 265: 10865–10871. [PubMed] [Google Scholar]
- 24.Capdevila J. H., Wei S., Helvig C., Falck J. R., Belosludtsev Y., Truan G., Graham-Lorence S. E., Peterson J. A. 1996. The highly stereoselective oxidation of polyunsaturated fatty acids by cytochrome P450BM-3. J. Biol. Chem. 271: 22663–22671. [DOI] [PubMed] [Google Scholar]
- 25.Daikh B. E., Laethem R. M., Koop D. R. 1994. Stereoselective epoxidation of arachidonic acid by cytochrome P-450s 2CAA and 2C2. J. Pharmacol. Exp. Ther. 269: 1130–1135. [PubMed] [Google Scholar]
- 26.Karara A., Dishman E., Jacobson H., Falck J. R., Capdevila J. H. 1990. Arachidonic acid epoxygenase. Stereochemical analysis of the endogenous epoxyeicosatrienoic acids of human kidney cortex. FEBS Lett. 268: 227–230. [DOI] [PubMed] [Google Scholar]
- 27.Kulas J., Schmidt C., Rothe M., Schunck W. H., Menzel R. 2008. Cytochrome P450-dependent metabolism of eicosapentaenoic acid in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 472: 65–75. [DOI] [PubMed] [Google Scholar]
- 28.Wei S., Brittin J. J., Falck J. R., Anjaiah S., Nithipatikom K., Cui L., Campbell W. B., Capdevila J. H. 2006. Chiral resolution of the epoxyeicosatrienoic acids, arachidonic acid epoxygenase metabolites. Anal. Biochem. 352: 129–134. [DOI] [PubMed] [Google Scholar]
- 29.Zeldin D. C., Moomaw C. R., Jesse N., Tomer K. B., Beetham J., Hammock B. D., Wu S. 1996. Biochemical characterization of the human liver cytochrome P450 arachidonic acid epoxygenase pathway. Arch. Biochem. Biophys. 330: 87–96. [DOI] [PubMed] [Google Scholar]
- 30.Nazor J., Dannenmann S., Adjei R. O., Fordjour Y. B., Ghampson I. T., Blanusa M., Roccatano D., Schwaneberg U. 2008. Laboratory evolution of P450 BM3 for mediated electron transfer yielding an activity-improved and reductase-independent variant. Protein Eng. Des. Sel. 21: 29–35. [DOI] [PubMed] [Google Scholar]
- 31.Omura T., Sato R. 1964. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J. Biol. Chem. 239: 2379–2385. [PubMed] [Google Scholar]
- 32.Peters M. W., Meinhold P., Glieder A., Arnold F. H. 2003. Regio- and enantioselective alkane hydroxylation with engineered cytochromes P450 BM-3. J. Am. Chem. Soc. 125: 13442–13450. [DOI] [PubMed] [Google Scholar]
- 33.Daikh B. E., Lasker J. M., Raucy J. L., Koop D. R. 1994. Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J. Pharmacol. Exp. Ther. 271: 1427–1433. [PubMed] [Google Scholar]
- 34.Wester M. R., Yano J. K., Schoch G. A., Yang C., Griffin K. J., Stout C. D., Johnson E. F. 2004. The structure of human cytochrome P450 2C9 complexed with flurbiprofen at 2.0-A resolution. J. Biol. Chem. 279: 35630–35637. [DOI] [PubMed] [Google Scholar]
- 35.Li H., Poulos T. L. 1997. The structure of the cytochrome p450BM-3 haem domain complexed with the fatty acid substrate, palmitoleic acid. Nat. Struct. Biol. 4: 140–146. [DOI] [PubMed] [Google Scholar]
- 36.Higgins L., Korzekwa K. R., Rao S., Shou M., Jones J. P. 2001. An assessment of the reaction energetics for cytochrome P450-mediated reactions. Arch. Biochem. Biophys. 385: 220–230. [DOI] [PubMed] [Google Scholar]
- 37.Hammonds T. D., Blair I. A., Falck J. R., Capdevila J. H. 1989. Resolution of epoxyeicosatrienoate enantiomers by chiral phase chromatography. Anal. Biochem. 182: 300–303. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J. Y., Blair I. A. 1994. Direct resolution of epoxyeicosatrienoic acid enantiomers by chiral-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 657: 23–29. [DOI] [PubMed] [Google Scholar]
- 39.Zeldin D. C., DuBois R. N., Falck J. R., Capdevila J. H. 1995. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch. Biochem. Biophys. 322: 76–86. [DOI] [PubMed] [Google Scholar]
- 40.Hooper L., Thompson R. L., Harrison R. A., Summerbell C. D., Ness A. R., Moore H. J., Worthington H. V., Durrington P. N., Higgins J. P., Capps N. E., et al. 2006. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 332: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGiff J. C., Quilley J. 2001. 20-Hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids and blood pressure. Curr. Opin. Nephrol. Hypertens. 10: 231–237. [DOI] [PubMed] [Google Scholar]
- 42.Wu S., Moomaw C. R., Tomer K. B., Falck J. R., Zeldin D. C. 1996. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J. Biol. Chem. 271: 3460–3468. [DOI] [PubMed] [Google Scholar]