Abstract
Omega-3 fatty acids, e.g., dokosahexaenoic acid (DHA) and eikosapentaenoic acid (EPA), ameliorate inflammatory reactions by various mechanisms, but the role of prostaglandins remains unclear. Our aim was to determine if dietary supplementation with a DHA-rich fish oil influenced the release of PGF2α from peripheral blood mononuclear cells (PBMC). In the OmegAD study, 174 Alzheimer disease patients received either 1.7 g DHA plus 0.6 g EPA or a placebo daily for six months. PBMCs from the 21 (9 on fish oil and 12 on placebo) first-randomized patients were stimulated with either lipopolysaccharide (LPS) or phytohemagglutinin (PHA) before and after 6 months. Our results showed that plasma concentrations of DHA and EPA increased significantly at 6 months in the omega-3 group. PGF2α release from LPS- (but not from PHA-) stimulated PBMC was significantly diminished in this group; no change was noted in the placebo group. PGF2α changes correlated inversely with changes in plasma DHA and EPA. Decreased IL-6 and IL-1β levels correlated with decreased PGF2α levels. The stimulus-specific PGF2α release from PBMC after 6 months of oral supplementation with the DHA-rich fish oil might be one event related to reduced inflammatory reactions associated with omega-3 fatty acid intake.
Keywords: DHA, EPA, LPS, Omega-3 fatty acid, PBMC, PGF, PHA, prostaglandin
Omega-3 fatty acids (ω3 FA), e.g., eikosapentaenoic acid (EPA, 20:5 ω3) and dokosahexaenoic acid (DHA, 22:6 ω3), present in marine oils, modulate inflammatory reactions and ameliorate symptoms of several autoimmune and other inflammatory disorders (1, 2). In addition, EPA and DHA administration reduces cardiovascular morbidity and mortality (3). Recently, high-fish intake or dietary supplementation with ω3 FAs was linked to reductions in the risk of developing Alzheimer's disease (AD) (4–6) and to delay cognitive decline in patients with very mild AD (7).
ω3 FA exert the anti-inflammatory effects on several cellular levels, including modulation of surface receptor, ion pumps, G-proteins, binding to transcription factors (e.g., NFκB), and gene interactions (8–10). One prevalent hypothesis is that ω3 FA, particularly EPA, give rise to prostaglandins and leukotrienes with reduced pro-inflammatory activity compared with corresponding arachidonic acid (AA; 20:4 ω6) derived compounds, because the former contain one additional double bond, changing the 3D structure and, hence, the ability to bind to receptors. Moreover, an abundance of ω3 FAs might reduce generation of ω6 metabolites by, among other things, competing for the same enzyme systems. In addition, EPA and DHA give rise to the highly anti-inflammatory metabolites resolvins and protectins, which interacts with prostaglandin synthesis (11).
One of the ω6-based prostaglandins, PGF2α, is synthesized either directly from AA or via prostaglandin E2 from AA (12, 13). PGF2α is highly active in the reproductive tract (14), but it is also involved in leukocyte migration (15) and promotes atherosclerosis (16). PGE2, synthesized after activation of monocytes, macrophages, and other leukocytes, modulates inflammatory reactions; e.g., it inhibits lymphocyte proliferation (17, 18), NK cell activity (19) and the production of cytokines [e.g., interferon–γ and IL-2 (from TH1 cells)]. It has also a stimulatory effect on generation of IL-4, IL-5 and IL-10 (from TH2 cells) in vitro (20–22) and on hematopoietic stem cell homing, survival, and proliferation (23). Moreover, PGE2 increases IL-23-induced IL-17 production as well as promotes inflammation through TH1 differentiation, phenomena involved in enhancing neutrophil recruitment and migration (24, 25).
The aim of this study was to evaluate the effects of oral supplementation for 6 months of ω3 FA on PGE2 release from peripheral blood mononuclear cells (PBMC) by measuring its stable metabolite PGF2α. We used two different stimuli, one with effects mainly on monocytes [lipopolysaccharide (LPS)], and the second on T-lymphocytes [phytohemagglutinin (PHA)]. The study is a part of a trial, the OmegAD study, where a product rich in DHA was given to patients with mild to moderate AD. The goal of the OmegAD study was, among other things, to see if this ω3 FA preparation would reduce the cognitive deterioration in AD (7).
SUBJECTS AND METHODS
This study included 25 patients. They were the first to be randomized in the OmegAD study, described in detail in Freund-Levi, et al. (7). In summary, the double-blind, placebo controlled OmegAD study included a total of 204 patients (73 +/− 9 y; 52% woman) with mild to moderated AD. Patients were randomized to 6 months of nutritional supplementation with a ω3 fish oil rich in DHA or to placebo. Patients were treated daily with either 1.7 g DHA plus 0.6 g EPA (EPAX 1050TG (Pronova Biocare A/S Lysaker, Norway) or with an isocaloric placebo oil (1 g corn oil, including 0.6 g linoleic acid). EPAX 1050TG is a 60% ω3 FA concentrate in triacylglycerol form, produced according to good manufacturing practices. DHA and EPA comprise appr. 67% of the fatty acid content. Four milligrams of vitamin E (tocopherol) was added to each EPAX 1050TG and placebo capsule. A total of 174 patients concluded the OmegAD study. Plasma fatty acid profiles and cognition and behavioral data have been published (7, 26, 27). Based on pretrial power calculation concerning cytokine profiles with a statistical significance level of P < 0.05 and 80% power, a minimum of 20 patients was required to detect a difference of 30% between the ω3 FAs and placebo groups through use of cytokine assays.
Blood samples for preparation of PBMCs or plasma for the present study were obtained from 23 patients before and after 6 months of treatment (2 of the 25 patients did not complete the OmegAD trial). Samples from 2 patients had to be excluded because of technical laboratory failure. Thus, 9 (57–82 y; median 75 y; 3 women) of the remaining patients received the ω3 FA preparation and 12 (58–79 y; median 71 y; 4 women) the placebo capsules.
No change in peripheral blood neutrophil, monocyte, and lymphocyte cell counts were recorded after 6 months of ω3 FAs supplementation. Patients were not given specific advice on food intake or time points for ω-3 capsule intake during the study. Food intake in the AD subjects will be reported separately. The two groups did not differ with regard to age, Mini Mental Test Scores (i.e., degree of cognitive deterioration), serum C-reactive protein levels, plasma DHA or EPA levels, blood pressure, body weight, or intake of aspirin.
The study was approved by the ethical committee of the Karolinska Institutet (7). The ω3 FA treatment was safe and well tolerated.
Blood sampling
PBMCs were isolated form EDTA anticoagulated venous blood by means of Lymphoprep (Nycomed Pharma, Oslo, Norway) gradient centrifugation. The cell preparations obtained before and after treatment with ω3 FAs, contained on average, 15 ± 5% monocytes and 85 ± 5% lymphocytes on both occasions (means and SD values). Corresponding figures for the placebo group were 15 ± 5% and 85 ± 5%, respectively. The cell viability in both groups was 96%, as assessed by trypan blue staining.
Laboratory methods
One million PBMCs were suspended in 1 ml Hank's balanced salt solution (HBSS) with CaCl2 and MgCl2, supplemented with penicillin and streptomycin; Hepes 0.0149 mol/l (GIBCO, Paisley, Scotland, UK) and 2% inactivated pooled AB serum.
The PBMCs were stimulated with LPS from E. coli 055:B5, L440 at 10 ng/ml (Sigma, St. Louis, MO) and purified PHA (HA-16) at 10 µg/ml (Murex Biotech Ltd, Dartford, Kent, England). Controls were treated in HBSS alone. Samples were incubated overnight (22 h) in 37°C humidified 5% CO2 atmosphere. Subsequently, cells were centrifuged, and supernatants were collected and stored in −80°C before cytokine determinations (28).
PGF2α release was measured using an enzyme immunoassay kit (Correlate-EIA, Assay Designs, Inc., Ann Arbor, MI) and is expressed in ng/ml. The lower limit for detection of PGF2α was annotated to be 3 pg/ml.
Plasma fatty acid analyses
Plasma fatty acids were analyzed by gas chromatography (THERMO TR-Fame column (30 m × 0.32 mm ID × 0,25 µm film; Thermo Electron Corp., Waltham, MA) and results are given as the relative abundance of individual fatty acids (29). Data for all 174 patients in the OmegAD study have been given previously (7). Likewise, data for the present 21 patients have been given (28).
Statistical analyses
We used the Wilcoxon signed rank test for analyses of dependent data. For comparison of differences in responses between groups over time, we used a Mann-Whitney U test for independent data. For correlation analyses, the Spearman's rank correlation test was applied. P < 0.05 were considered significant. We used median values surrounded by the values for the 25th and 75th percentiles.
RESULTS
Plasma fatty acids
As reported previously (28), at study entry DHA and EPA concentration in plasma were not significantly different between the ω3 FA and the placebo group. In the ω3 FA group, plasma values for DHA as well as for EPA were significantly higher at 6 months compared with pretrial values (Table 1). The placebo group displayed no significant changes of DHA or EPA in plasma compared with pretrial values (Table 1). The rise of DHA levels was larger than that of EPA in the ω3 FA group (+3.7 percentage units and +2.7 percentage units, respectively), suggesting that some conversion of DHA to EPA had taken place as discussed in (28).
TABLE 1.
Plasma EPA and DHA, LPS-induced cytokine, and PHA-induced PGF2α
| ω3 FA Group |
Placebo Group |
|||||
|---|---|---|---|---|---|---|
| At Baseline | After 6 Mo | P | At Baseline | After 6 Mo | P | |
| Fatty acid | ||||||
| EPA, % | 1.99 (1.17–2.35) | 4.73 (3.59–5.09) | 0.008 | 1.71 (1.33–2.74) | 1.87 (1.16–2.37) | NS |
| DHA, % | 3.02 (2.47–3.76) | 6.74 (6.00–7.35) | 0.008 | 4.17 (2.60–6.02) | 3.29 (3.00–4.89) | NS |
| LPS stimulation | ||||||
| IL-6, ng/ml | 53.8 (30.9–60.4) | 28.9 (16.6–35.2) | 0.008 | 38.4 (26.9–54.1) | 29.5 (23.2–36.8) | 0.028 |
| IL-1β, ng/ml | 1.49 (1.34–2.68) | 1.22 (0.89–1.68) | 0.008 | 1.83 (1.45–2.26) | 1.58 (1.14–1.78) | NS |
| G-CSF, ng/ml | 1.28 (1.05–2.82) | 0.98 (0.49–1.80) | 0.02 | 1.77 (1.63–2.34) | 1.18 (0.88–1.71) | NS |
| TNF-α, ng/ml | 6.85 (3.83–8.32) | 5.52 (4.64–6.64) | NS | 7.85 (6.04–8.65) | 5.26 (3.28–6.89) | NS |
| PHA stimulation | ||||||
| PGF2α, ng /ml | 0.15 (0.04–0.93) | 0.18 (0.12–0.73) | NS | 0.18 (0.07–0.43) | 0.44 (0.12–0.86) | NS |
Levels in supernatants of stimulated PBMC in the 21 OmegAD study subjects. Values are medians; the 25th–75th percentile values are given in parentheses. P values are given for changes between baseline and 6-mo values. The decrease in IL-6 was significantly larger after 6 mo in the ω3 FA group than in the placebo group (P = 0.039). DHA, dokosahexaenoic acid; EPA, eikosapentaenoic acid; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; LPS, lipopolysaccharide; NS, not significant; PGF2α, prostaglandin F2α; PHA, phytohemagglutinin; TNF, tumor necrosis factor.
PGF2α synthesis in cell supernatants
Quiescent PBMCs released only minute amounts of PGF2α. At baseline, the two groups did not differ significantly as to PGF2α release from stimulated PBMCs induced by LPS or PHA.
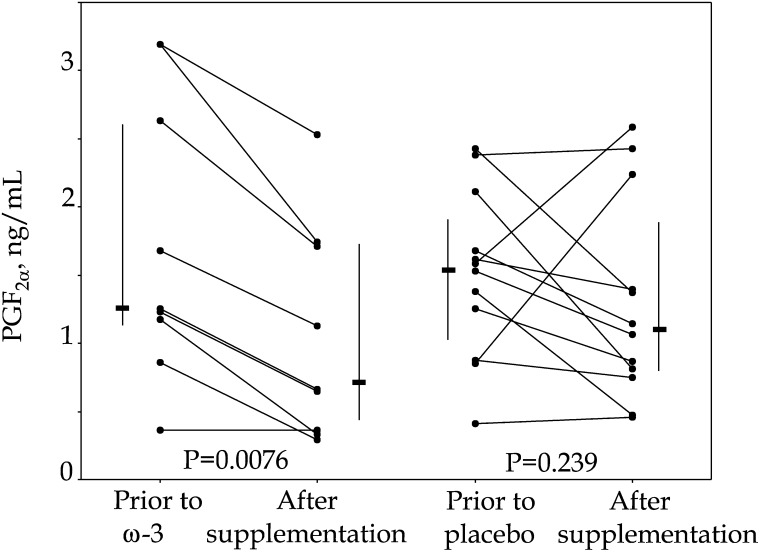
LPS. LPS conferred a 100-fold rise of the PGF2α concentration in supernatants. At 6 months of treatment, mean values for PGF2α release from LPS-stimulated PBMCs from the 9 AD patients given the ω3 FAs preparation were significantly lower than baseline (P = 0.0076) (Fig. 1). In contrast, mean values for PGF2α releases for the placebo-treated AD patients were not significantly lower at 6 months compared with pretrial values (P > 0.05) (Fig. 1). The reduction of PGF2α in the ω3 FA group for LPS values between baseline and 6 months was trendwise significant for the difference from the corresponding values for the placebo group (P = 0.06).
Fig. 1.
Changes in PGF2α release from PBMC, stimulated with 10 ng LPS/ml. PBMC were obtained from patients with mild to moderate Alzheimer's disease, before and after 6 months of ω3 FAs or placebo oil supplementation. Individual values are flanked by median and 25th–75th percentile values. LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell; PGF2α, prostaglandin F2α.
PHA. PHA conferred a 30-fold rise of the PGF2α concentration in supernatants. PGF2α release from PBMCs stimulated with 10 µg PHA /ml from ω3 FA treated patients (n = 9) or the placebo oil (n = 10, due to insufficient amounts of donor cells from 2 patients) was not changed after 6 months of treatment (Table 1).
TNF-α, IL-1β, IL-6, and G-CSF release
As described previously (28), supplementation with ω3 FAs was associated with significant reductions of the release of IL-1β, IL-6, and G-CSF after 6 months compared with pretrial values when PBMCs were stimulated with 10 ng LPS/ml. The placebo group displayed no or minor changes (Table 1). In contrast, the TNF-α release did not change in any of the treatment groups (28).
Correlation analyses
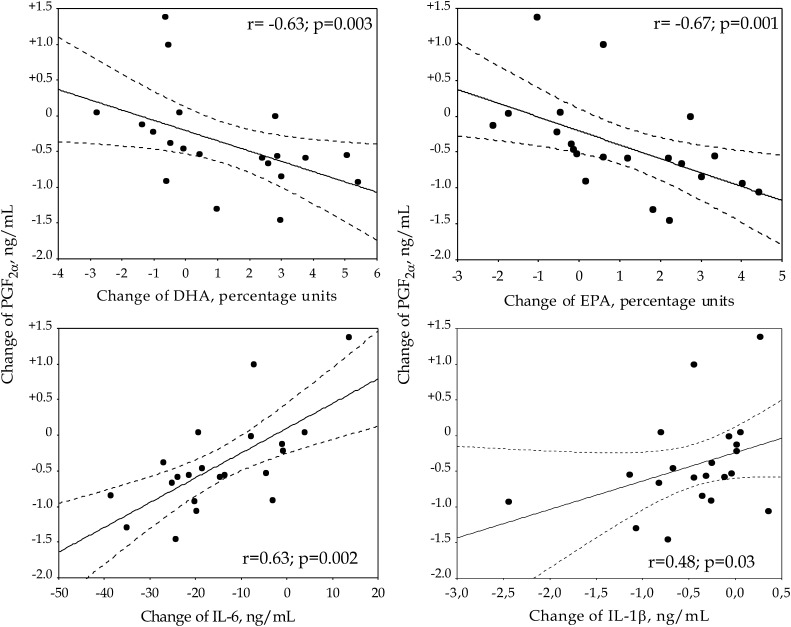
When relating values for the LPS-induced release of PGF2α to plasma concentrations of DHA and EPA, we found that changes in DHA and EPA for all 21 subjects correlated significantly to changes in PGF2α release (r = −0.6271; P = 0.003 for DHA, and r = −0.6662; P = 0.001 for EPA) (Fig. 2). Thus, the more DHA or EPA increased, the lower was the PGF2α release.
Fig. 2.
Correlations between changes of PGF2α and DHA (top left), EPA (top right), IL-6 (bottom left) or IL-1β (bottom right). Values for PGF2α, IL-6 and Il-1β refer to releases from LPS stimulated PBMC, while values for EPA and DHA refer to plasma concentrations (28). Solid lines = regression line. Dotted lines = the 95% confidence interval for the regression line. DHA, dokosahexaenoic acid; EPA, eikosapentaenoic acid; IL, interleukin; LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell; PGF2α, prostaglandin F2α.
Changes in PGF2α release induced by LPS were also significantly related to changes in IL-6 (r = 0.6338; P = 0.002) and IL-1β (r = 0.4792; P = 0.028) for all subjects, respectively (Fig. 2). Thus, the more PGF2α decreased, the lower the release of IL-6 and IL-1β.
There was no correlation of changes in PGF2α and the releases of TNF-α or G-CSF (r = 0.0012; P = 0.99, r = 0.3844; P = 0.085, respectively).
DISCUSSION
This study has shown that oral supplementation with a DHA-enriched ω3 marine FAs preparation reduced the release of the stable arachidonate metabolite PGF2α from ex vivo LPS-stimulated PBMC. This reduction correlated significantly with the rise of plasma EPA and DHA, as well as to reductions of IL-6 and IL-1β, simultaneously released from these same LPS-stimulated PBMC. These results point to interactions between the eikosanoid, FA, and cytokine systems and may be part of the anti-inflammatory reactions associated with ω3 FA treatment.
In the presently employed assay for PGF2α, the manufacturer (Invitrogen, Inc.) declares that the cross-reactivity with the ω3 based PGF3α is 21%. Thus, we cannot determine how much of the assayed PGF2α actually originated from AA or from EPA (hence being PGF3α). Nonetheless, even if one assumes that the proportion of PGF3α increased after 6 months of ω3 supplementation, the total outcome of all isoforms of PGF was a reduction. It is also reasonable to assume that the biological activity of the PGF mixture might be lower than if all PGF originated from AA. The same reasoning is valid for PGE2 and PGE3.
PGF2α is considered to be a major and stable metabolite of prostaglandin E2 (PGE2) (12, 13). The generation of PGE and PGF proceeds from AA and the common precursor prostaglandin H. By means of an enzyme, 9KPGR, PGE can be converted to PGF (12). Various factors can alter the generation of either eikosanoid, such as mutations in generating enzymes, stimuli, and environment (30–33). Thus, interactions of EPA, DHA, LPS, and PHA might be of significance for generation of one or the other eikosanoid. Moreover, PGE and PGF can interact on the receptor level (34). Therefore, we decided to measure a common end product, PGF, which would reflect turnover of more than one single eikosanoid.
Given the effects of PGE and PGF to influence cytokine production (20–22, 24, 25), one might hypothesize that PGE2 (as well as PGE3), generated and released rapidly in and from the LPS-stimulated PBMC, might have interacted with the relatively slower signaling systems for generation of the aforementioned cytokines. In our study, the release of PGF2α into the culture medium corresponds to a concentration of approx. 1.5 nM (being similar to previous data on PGE2 by Harizi et al. (35). The question then arises if this concentration is of biological significance. Previous studies have shown that much higher concentrations of PGE2 are needed for blockade of, for example, PHA-mediated release of pro-inflammatory cytokines and lymphocyte proliferation (18, 36). Although previous studies usually have focused on inhibitory effects of prostaglandins on generation of cytokines such as TNF-α and IL-1β, recent data have emphasized that PGE2 might also enhance immune and inflammatory reactions (37, 38, Vedin unpublished data). Thus, PGE2 induced pro-stimulatory molecules of the TNF/TNF receptor superfamily (39) enhanced hematopoietic stem cell homing, survival, and proliferation (23) and increased IL-23-induced IL-17 production, a phenomenon involved in neutrophil recruitment and migration (24). As well, PGE2 and PGF2α may promote vascular inflammation (16, 25). Consequently, one might ask about the mechanisms for the simultaneous reductions of PGF2α and several cytokines, as well as the statistically significant correlation between changes in PGF2α, IL-6, IL-1 observed in our study. Do all reactions depend on the effects of ω3 FA on common mechanisms for generation of these molecules? Or do reduced release of prostaglandins directly influence generation and release of IL-6, IL-1, G-CSF (but not TNF)? Our study and current literature cannot resolve these issues but might serve as a starting point for research on pro- and anti-inflammatory effects of prostaglandins. Moreover, whether the effect of ω3 FA is on CD14, TLR4 or other parts of the receptor system for LPS or downstream remains to be settled (40).
The PGF2α results obtained here after stimulation with the alternate stimulus PHA strongly suggest that the structures and cells targeted by PHA (lectins on most T-lymphocytes) were not affected in this capacity by the increase of ω3 FAs. This is in accordance with studies by Wasserman et al. showing that PGF2α had no effect on PHA-mediated lymphocyte proliferation (18). In contrast, T-lymphocytes might be influenced by ω3 FAs, as shown by Trebble et al. (41) when using concanavalin A, a different T-cell mitogen, as the stimulus and cell proliferation as the read-out system. These considerations point to a rather specific effect of ω3 FAs on certain but not all signaling pathways. However, we have no data here to further dissect the various signaling pathways for LPS and PHA.
Our PGF2α data compare well with previous results for PGE2 generation from LPS-stimulated PBMC obtained from healthy subjects after oral supplementation with ω3 FAs. Thus, two studies using varying doses of EPA-rich fish oil preparations reported decreases of PGE2 in a dose-dependent way (41, 42). Trebble et al. (41) also found a negative correlation between generated PGE2 and plasma EPA but did not report if there also was a relation to DHA. Rees et al. (42) observed no changes of released TNF-α, IL-1β, or IL-6, which differs from our results. As discussed previously (28), effects of EPA and DHA differ in a number of respects, such as binding to PPARγ or to the RX receptor, and for membrane fluidity (9). Our PGF2α data also compare well with previous in vitro results for PGE2 and PGF2α generation from various cells after ω3 FA treatment (43).
The relation of prostaglandins to the AD brain pathology has attracted attention over the years. There are recent data suggesting that PGE2 stimulates production of amyloid-β peptides (which can result in the AD typical plaques) (44) and that these peptides can further stimulate PGE2 generation (45). Hence, reducing PGE2 (and thus PGF2α) might be of significance for progression of AD. However, therapy with blockers of cyclooxygenase activity (impairing generation of prostaglandins) have not been successful in reducing cognitive decline in AD.
In conclusion, this study shows that release from PBMC of PGF2α, generated directly from AA or via PGE2, decreased in the DHA-enriched ω3 FA supplemented group compared with the placebo group in a stimulus-specific way. This novel finding agrees with and adds to previous data on effects of EPA supplementation, suggesting that EPA and DHA effects are rather similar in this particular respect, although differences are noted for other effector variables. In this context, it may be speculated that DHA (and EPA) gives rise to anti-inflammatory and neuroprotective lipid mediators, which appears to be part of the resolution phase of inflammation (33, 46).
Acknowledgments
The authors thank Bengt Vessby and Siv Tengblad for fatty acid analyses; A-C Tysén- Bäckström and Andreas Svensson for patient management; and Lilian Walther Jallow, for support with the Bioplex 100 Systemreader. The authors thank Jesper Haeggström, Karolinska Institutet, for valuable review of prostaglandin metabolism.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AD
- Alzheimer's disease
- DHA
- dokosahexaenoic acid
- EPA
- eikosapentaenoic acid
- G-CSF
- granulocyte colony-stimulating factor
- IL
- interleuikin
- LPS
- lipopolysaccharide
- PBMC
- peripheral blood mononuclear cell
- PGE2
- prostaglandin E2
- PGF2α
- prostaglandin F2α
- PHA
- phytohemagglutinin
- TNF
- tumor necrosis factor
This study was supported by Swedish Medical Research Council Grants 19X-05991 and 71XS-13135. This study was also supported by funding from the Swedish Association against Rheumatism; Karolinska Institutet; Huddinge University Hospital; the Funds of Å Wiberg; King Gustaf V:s 80-year; Uggla; N Svartz; Vårdal; Swedish Cancer Society; Cancer Society of Stockholm; Swedish Alzheimer Foundation; and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet. Clinical Trials. Gov Identifier: NCT00211159.
REFERENCES
- 1.James M. J., Proudman S. M., Cleland L. G. 2003. Dietary n-3 fats as adjunctive therapy in a prototypic inflammatory disease: issues and obstacles for use in rheumatoid arthritis. Prostaglandins Leukot. Essent. Fatty Acids. 68: 399–405. [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos A. P. 2002. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21: 495–505. [DOI] [PubMed] [Google Scholar]
- 3.Leon H., Shibata M. C., Sivakumaran S., Dorgan M., Chatterley T., Tsuyuki R. T. 2008. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 337: a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barberger-Gateau P., Letenneur L., Deschamps V., Peres K., Dartigues J. F., Renaud S. 2002. Fish, meat, and risk of dementia: cohort study. BMJ. 325: 932–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris M. C., Evans D. A., Bienias J. L., Tangney C. C., Bennett D. A., Wilson R. S., Aggarwal N., Schneider J. 2003. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 60: 940–946. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer E. J., Bongard V., Beiser A. S., Lamon-Fava S., Robins S. J., Au R., Tucker K. L., Kyle D. J., Wilson P. W., Wolf P. A. 2006. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch. Neurol. 63: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 7.Freund-Levi Y., Eriksdotter-Jonhagen M., Cederholm T., Basun H., Faxen-Irving G., Garlind A., Vedin I., Vessby B., Wahlund L. O., Palmblad J. 2006. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 63: 1402–1408. [DOI] [PubMed] [Google Scholar]
- 8.Calder P. C. 2006. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 75: 197–202. [DOI] [PubMed] [Google Scholar]
- 9.de Urquiza A. M., Liu S., Sjoberg M., Zetterstrom R. H., Griffiths W., Sjovall J., Perlmann T. 2000. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 290: 2140–2144. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G., Etherton T. D., Martin K. R., Vanden Heuvel J. P., Gillies P. J., West S. G., Kris-Etherton P. M. 2005. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 336: 909–917. [DOI] [PubMed] [Google Scholar]
- 11.Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. 2009. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 461: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortier M. A., Krishnaswamy K., Danyod G., Boucher-Kovalik S., Chapdalaine P. 2008. A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems. J. Physiol. Pharmacol. 59(Suppl 1): 65–89. [PubMed] [Google Scholar]
- 13.Samuelsson B., Goldyne M., Granstrom E., Hamberg M., Hammarstrom S., Malmsten C. 1978. Prostaglandins and thromboxanes. Annu. Rev. Biochem. 47: 997–1029. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T., Narumiya S. 2002. Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat. 68–69: 557–573. [DOI] [PubMed] [Google Scholar]
- 15.de Menezes G. B., dos Reis W. G., Santos J. M., Duarte I. D., de Francischi J. N. 2005. Inhibition of prostaglandin F(2alpha) by selective cyclooxygenase 2 inhibitors accounts for reduced rat leukocyte migration. Inflammation. 29: 163–169. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y., Lucitt M. B., Stubbe J., Cheng Y., Friis U. G., Hansen P. B., Jensen B. L., Smyth E. M., FitzGerald G. A. 2009. Prostaglandin F2alpha elevates blood pressure and promotes atherosclerosis. Proc. Natl. Acad. Sci. USA. 106: 7985–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin J. S., Bankhurst A. D., Messner R. P. 1977. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J. Exp. Med. 146: 1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasserman J., Hammarstrom S., Petrini B., Blomgren H., von Stedingk L. V., Vedin I. 1987. Effects of some prostaglandins and leukotrienes on lymphocytes, monocytes and their activity in vitro. Int. Arch. Allergy Appl. Immunol. 83: 39–43. [DOI] [PubMed] [Google Scholar]
- 19.Spaggiari G. M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M. C., Moretta L. 2008. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 111: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 20.Betz M., Fox B. S. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146: 108–113. [PubMed] [Google Scholar]
- 21.Fedyk E. R., Brown D. M., Phipps R. P. 1997. PGE2 regulation of B lymphocytes and T helper 1 and T helper 2 cells: induction of inflammatory versus allergic responses. Adv. Exp. Med. Biol. 407: 237–242. [DOI] [PubMed] [Google Scholar]
- 22.Hilkens C. M., Snijders A., Snijdewint F. G., Wierenga E. A., Kapsenberg M. L. 1996. Modulation of T-cell cytokine secretion by accessory cell-derived products. Eur. Respir. J. Suppl. 22: 90s–94s. [PubMed] [Google Scholar]
- 23.Hoggatt J., Singh P., Sampath J., Pelus L. M. 2009. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 113: 5444–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemos H. P., Grespan R., Vieira S. M., Cunha T. M., Verri W. A., Jr., Fernandes K. S., Souto F. O., McInnes I. B., Ferreira S. H., Liew F. Y., et al. 2009. Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production. Proc. Natl. Acad. Sci. USA. 106: 5954–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., Sugimoto Y., Narumiya S. 2009. Prostaglandin E2–EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat. Med. 15: 633–640. [DOI] [PubMed] [Google Scholar]
- 26.Freund-Levi Y., Basun H., Cederholm T., Faxen-Irving G., Garlind A., Grut M., Vedin I., Palmblad J., Wahlund L. O., Eriksdotter-Jonhagen M. 2008. Omega-3 supplementation in mild to moderate Alzheimer's disease: effects on neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry. 23: 161–169. [DOI] [PubMed] [Google Scholar]
- 27.Irving G. F., Freund-Levi Y., Eriksdotter-Jonhagen M., Basun H., Brismar K., Hjorth E., Palmblad J., Vessby B., Vedin I., Wahlund L. O., et al. 2009. Omega-3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer's disease: the omega-3 Alzheimer's disease study. J. Am. Geriatr. Soc. 57: 11–17. [DOI] [PubMed] [Google Scholar]
- 28.Vedin I., Cederholm T., Freund Levi Y., Basun H., Garlind A., Faxen Irving G., Jonhagen M. E., Vessby B., Wahlund L. O., Palmblad J. 2008. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am. J. Clin. Nutr. 87: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 29.Boberg M., Croon L. B., Gustafsson I. B., Vessby B. 1985. Platelet fatty acid composition in relation to fatty acid composition in plasma and to serum lipoprotein lipids in healthy subjects with special reference to the linoleic acid pathway. Clin. Sci. (Lond.). 68: 581–587. [DOI] [PubMed] [Google Scholar]
- 30.Hammarberg T., Hamberg M., Wetterholm A., Hansson H., Samuelsson B., Haeggstrom J. Z. 2009. Mutation of a critical arginine in microsomal prostaglandin E synthase-1 shifts the isomerase activity to a reductase activity that converts prostaglandin H2 into prostaglandin F2alpha. J. Biol. Chem. 284: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mickleborough T. D., Tecklenburg S. L., Montgomery G. S., Lindley M. R. 2009. Eicosapentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin. Nutr. 28: 71–77. [DOI] [PubMed] [Google Scholar]
- 32.Razzak A., Aldrich C., Babcock T. A., Saied A., Espat N. J. 2008. Attenuation of iNOS in an LPS-stimulated macrophage model by omega-3 fatty acids is independent of COX-2 derived PGE2. J. Surg. Res. 145: 244–250. [DOI] [PubMed] [Google Scholar]
- 33.Weldon S. M., Mullen A. C., Loscher C. E., Hurley L. A., Roche H. M. 2007. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 18: 250–258. [DOI] [PubMed] [Google Scholar]
- 34.Fujino H., Vielhauer G. A., Regan J. W. 2004. Prostaglandin E2 selectively antagonizes prostaglandin F2alpha-stimulated T-cell factor/beta-catenin signaling pathway by the FPB prostanoid receptor. J. Biol. Chem. 279: 43386–43391. [DOI] [PubMed] [Google Scholar]
- 35.Harizi H., Norbert G. 2004. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell. Immunol. 228: 99–109. [DOI] [PubMed] [Google Scholar]
- 36.Minakuchi R., Wacholtz M. C., Davis L. S., Lipsky P. E. 1990. Delineation of the mechanism of inhibition of human T cell activation by PGE2. J. Immunol. 145: 2616–2625. [PubMed] [Google Scholar]
- 37.Scales W. E., Chensue S. W., Otterness I., Kunkel S. L. 1989. Regulation of monokine gene expression: prostaglandin E2 suppresses tumor necrosis factor but not interleukin-1 alpha or beta-mRNA and cell-associated bioactivity. J. Leukoc. Biol. 45: 416–421. [PubMed] [Google Scholar]
- 38.Roman A. S., Schreher J., Mackenzie A. P., Nathanielsz P. W. 2006. Omega-3 fatty acids and decidual cell prostaglandin production in response to the inflammatory cytokine IL-1beta. Am. J. Obstet. Gynecol. 195: 1693–1699. [DOI] [PubMed] [Google Scholar]
- 39.Krause P., Bruckner M., Uermosi C., Singer E., Groettrup M., Legler D. F. 2009. Prostaglandin E(2) enhances T-cell proliferation by inducing the costimulatory molecules OX40L, CD70, and 4–1BBL on dendritic cells. Blood. 113: 2451–2460. [DOI] [PubMed] [Google Scholar]
- 40.De Smedt-Peyrusse V., Sargueil F., Moranis A., Harizi H., Mongrand S., Laye S. 2008. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 105: 296–307. [DOI] [PubMed] [Google Scholar]
- 41.Trebble T. M., Wootton S. A., Miles E. A., Mullee M., Arden N. K., Ballinger A. B., Stroud M. A., Burdge G. C., Calder P. C. 2003. Prostaglandin E2 production and T cell function after fish-oil supplementation: response to antioxidant cosupplementation. Am. J. Clin. Nutr. 78: 376–382. [DOI] [PubMed] [Google Scholar]
- 42.Rees D., Miles E. A., Banerjee T., Wells S. J., Roynette C. E., Wahle K. W., Calder P. C. 2006. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am. J. Clin. Nutr. 83: 331–342. [DOI] [PubMed] [Google Scholar]
- 43.Arntzen K. J., Brekke O. L., Vatten L., Austgulen R. 1998. Reduced production of PGE2 and PGF2 alpha from decidual cell cultures supplemented with N-3 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 56: 183–195. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino T., Namba T., Takehara M., Nakaya T., Sugimoto Y., Araki W., Narumiya S., Suzuki T., Mizushima T. 2009. Prostaglandin E2 stimulates the production of amyloid-beta peptides through internalization of the EP4 receptor. J. Biol. Chem. 284: 18493–18502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hull M., Muksch B., Akundi R. S., Waschbisch A., Hoozemans J. J., Veerhuis R., Fiebich B. L. 2006. Amyloid beta peptide (25–35) activates protein kinase C leading to cyclooxygenase-2 induction and prostaglandin E2 release in primary midbrain astrocytes. Neurochem. Int. 48: 663–672. [DOI] [PubMed] [Google Scholar]
- 46.Serhan C. N., Chiang N., Van Dyke T. E. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]