Abstract
Irritable bowel syndrome (IBS) is the most common functional gastrointestinal disorder referred to gastroenterologists. Although the pathophysiology remains unclear, accumulating evidence points to the presence of low-level immune activation both in the gut and systemically. Circulating polyunsaturated fatty acids (PUFA) have recently attracted attention as being altered in a variety of disease states. Arachidonic acid (AA), in particular, has been implicated in the development of a pro-inflammatory profile in a number of immune-related disorders. AA is the precursor of a number of important immunomodulatory eicosanoids, including prostaglandin E2 (PGE2) and leukotriene B4 (LTB4). We investigated the hypothesis that elevated plasma AA concentrations in plasma contribute to the proposed pro-inflammatory profile in IBS. Plasma AA and related PUFA were quantified by gas chromatography analysis in IBS patients and controls. Both PGE2 and LTB4 were measured in serum using commercially available ELISA assays. AA concentrations were elevated in our patient cohort compared with healthy controls. Moreover, we demonstrated that this disturbance in plasma AA concentrations leads to downstream elevations in eicosanoids. Together, our data identifies a novel proinflammatory mechanism in irritable bowel syndrome and also suggests that elevated arachidonic acid levels in plasma may serve as putative biological markers in this condition.
Keywords: arachidonic acid, biological marker, eicosanoid, gas chromatography, IBS, pro-inflammatory profile
Irritable bowel syndrome (IBS) is a common and potentially disabling, though nonfatal, medical disorder that can affect up to 20% of the population. It is the most common functional gastrointestinal disorder referred to gastroenterologists (1). Although it has evolved over the years in terms of nomenclature (2, 3), classification (4–6), and appreciation of its frequency (7), a poor understanding of disease pathophysiology has remained a constant. According to the Rome III criteria, a symptom-based classification system, the disease-defining symptom profile encompasses abdominal pain and an altered bowel habit, with distension, bloating, and a variety of disturbances in defecatory function being additional features (8).
The disorder is increasingly viewed as a disorder of the brain-gut axis, a construct describing a bidirectional interaction between the gastrointestinal tract (GIT), incorporating the intestinal epithelial barrier, the mucosa-associated lymphoid tissue (MALT), gut muscle and the enteric nervous system (ENS), and the central nervous system (CNS) (9, 10). Most recently, the proposal that low-grade inflammation, as evidenced by the release of mast cell mediators and activation of lymphocytes in the colo-rectal mucosa and by the detection of elevated levels of pro-inflammatory cytokines in serum (8, 11–13), has provided a new dimension to this paradigm. The source of this low-grade inflammation, or immune activation, whether luminal or central, has remained elusive. Polyunsaturated fatty acids (PUFA) and their metabolites have been shown to influence inflammatory processes (14). Moreover, a pro-inflammatory PUFA profile has recently been reported in the maternal separation rodent model, an animal model of IBS (15). However, it has not been extensively studied in this regard in the clinical setting even though it may provide some new insights into disease pathophysiology.
The fatty acid composition of the body is largely determined by dietary intake (16). Western society has a high ratio of ω-6 PUFA compared with ω-3 PUFA largely due to a high consumption of ω-6–rich vegetable oils in comparison to a low consumption of ω-3–rich foods such as oily fish (17). The dietary dominance of the ω-6 fatty acids favors the elaboration of pro-inflammatory mediators produced along ω-6 metabolic pathways over less inflammatory ω-3 pathway metabolites. This imbalance, with a shift in a pro-inflammatory direction, has been implicated in a number of diseases, including cardiovascular disease (18), depression (19, 20) and a variety of inflammatory states (21). Moreover, the biological significance of this ratio in these disease states is confirmed by studies showing that dietary manipulations aimed at reducing ω-6 dominance can result in favorable disease outcomes with, for example, a reduction of the ω-6:ω-3 ratio to less than 4:1 being credited with reduced mortality in cardiovascular disease (17). This focus on the pro-inflammatory potential of the ω-6 fatty acids has, in particular, been directed toward the role of arachidonic acid (AA) and its metabolites as inflammatory mediators (22).
AA is a 20-carbon PUFA that is derived from ω-6 fatty acids and subsequently enters a biochemical cascade to give rise to potent immunomodulatory eicosanoids including prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) (23). Given the dietary factors outlined above, AA is the major substrate for synthesis of these eicosanoids, produced by the action of cylcooxygenase and lipoxygenase enzymes, respectively, in Western societies (22). These prostaglandins and leukotrienes can be biologically active even at very low concentrations, and thus, minor alterations in AA status can induce profound downstream consequences (23). Interestingly, PGE2 has been shown to induce IL-6 synthesis in macrophages (24). Elevated IL-6 levels have, to date, represented one of the most robust findings supportive of immune activation in IBS (11, 25, 26). Despite the importance of the AA cascade, the limited data available on PUFA in IBS have not focused on either the parent molecule or the immunomodulatory metabolites produced along the pathway (27).
In this study we hypothesized that the inflammatory signature in IBS is derived from an increased dominance of ω-6 PUFA over their ω-3 counterparts, leading to an increased input to the AA cascade. To test this theory, we measured the plasma PUFA profile in IBS patients and healthy controls and also examined possible downstream alterations in eicosanoid production. The analysis endpoints for this study were total plasma ω-6 content, total plasma ω-3 content, the ω-6:ω-3 ratio, plasma AA concentrations, serum PGE2 concentrations, and serum LTB4 concentrations.
MATERIALS AND METHODS
Subjects
Female patients were recruited from a university database of IBS patients. The database consisted of people who had either attended gastroenterology clinics at Cork University Hospital or had responded to direct advertisement on the university campus or local newspaper regarding participation in IBS research. Individuals aged between 18 and 65 years who satisfied Rome II criteria for IBS and in whom organic gastrointestinal diseases and clinically significant systemic diseases had been excluded were considered for inclusion in the study. Pregnant women, individuals with known lactose intolerance or immunodeficiency, or individuals who had any recent transient illness (i.e., within 2 weeks of participation in the study), such as viral illnesses or chest infections, were excluded.
Trial protocol
A total of 67 subjects, 41 patients with IBS and 26 healthy, sex-matched controls of comparable age and BMI, gave fully informed consent to take part in this study, which had local ethics committee approval. Each potentially eligible patient was evaluated by a review of clinical history, performance of a physical examination, and measurement of full blood count and serum biochemistry, with any clinically significant abnormalities leading to exclusion. The age (mean ± SD) of the patients was 45 ± 11.74 years, and the age of the comparison group was 39.04 ± 12.78. All patients and healthy comparison subjects were drug free, including anti-inflammatory medications. The study was powered to detect differences in fatty acid concentrations at the P < 0.05 level between controls and IBS patients but not within patient subgroups.
Assessments
On arrival at the clinical investigation laboratory at 08.30 h, each subject completed the self report patient health questionnaire (PHQ) to assess the presence of major depression. This is a reliable and valid instrument that was developed as a diagnostic tool to be used in primary care (28). It tests for the presence of major depression using diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). The responses on the depression subscale of the questionnaire can also be used as a dimensional tool to rate the severity of depression (29). In addition to the PHQ, clinical severity of IBS was evaluated using self report ordinal scales in accordance with a previously published method (30, 31). This involved subjects rating the severity of their IBS symptoms on a four-point ordinal scale (0-–3) with regard to each abdominal complaint, interference with daily activities, and avoidance behavior as a result of complaints. A summarizing severity score for each patient was determined by taking the sum of the individual scores.
Sample collection
Whole blood was collected at 09.00 h in tubes that contained ethylenediametetraacetic acid (EDTA). Samples were centrifuged immediately and the plasma frozen at −80°C until ready for analysis. Serum, where applicable, was generated similarly except that the collection tubes did not contain any anticoagulant.
Reagents
HPLC-grade methanol and chloroform were obtained from Alkem/Reagecon (Cork, Ireland). All other reagents were obtained from Sigma (Germany) unless otherwise stated.
Fatty acid analysis
Lipids from 1 ml of blood plasma were extracted with 25 ml of chloroform:methanol 2:1 (v/v) containing 5 ppm butylated hydroxytoluene as an antioxidant (32), and the solvent was removed via gentle evaporation at 45°C under nitrogen gas. Phospholipids were then separated by solid phase extraction using 500 mg NH2 phase columns (Phenomenex, UK) as described previously (33). Phospholipids were transesterified as previously described (34), extracted with 4 mls of hexane and an aliquot taken for gas chromatography (GC) analysis.
Fatty acids were quantified as fatty acid methyl esters (FAME) by GC analysis using a Varian 3400 gas liquid chromatograph (Varian 3400 capillary GC, Varian, Walnut Creek, CA) fitted with a flame ionization detector. The results were expressed as a percentage of FAME (%, g/100 g FAME). Separation of the FAME was performed on a Chrompack CP Sil 88 column (Chrompack, Middlelburg, The Netherlands) 100 m × 0.25mm ID × 20 μm film thickness). Helium was used as a carrier gas at a pressure of 33.7 psi. The injector temperature was 225°C isothermal with a hold time of 5 min and the detector temperature was 250°C. The column temperature was programmed from an initial temperature of 80°C to a final temperature of 200°C, with an initial delay of 8 min (hold time), at a rate of 8.5°C/min during each analysis. The column was held at the final temperature of 200°C for 7 min (final hold time). Collected data were recorded and analyzed on a Minichrom PC system (VG Data Systems, Manchester, UK). Fatty acids were identified based on the retention time of reference standards (Sigma).
Eicosanoid analysis
PGE2 and LTB4 were measured in serum from a reduced subject group of the trial subjects outlined above. From 25 of the patient group (47 ± 10.63 years) and 19 of the control group (36.21 ± 11.87 years), serum samples in addition to the plasma samples were prepared. Separate Assay Designs EIA assay kits (Cambridge Biosciences, UK) were used to measure the analytes, and the assays were performed as per the manufacturer's instructions.
Data analysis
Data were expressed as mean values ± SEM. Data were analyzed by Student-test, one way ANOVA, ANCOVA, and by Dunnets multiple comparison posthoc tests as appropriate. Correlations were assessed according to the Pearson product moment correlation.
RESULTS
Subject characteristics
DSM-IV major depression was comorbid in 41% (17 out of 41) of IBS patients. None of the control group (n = 26) met criteria for current depression. Twenty-two per cent (9 out of 41) of patients rated their IBS symptoms as mild (a sum score of 3 or less on the severity scale); 41% (17 out of 41) reported symptoms of moderate severity; and 37% (15 out of 41) reported symptoms which were severe in nature (i.e., a score of 6 or greater on the severity scale). Twelve of the IBS cohort were classified as having diarrhea-predominant IBS (D-IBS), 9 as constipation-predominant (C-IBS), and 20 had an alternating bowel habit (A-IBS). In addition, 17 of the IBS group had currently active symptoms (CA), 17 were categorized as recently active (RA), and 7 were in a quiescent disease phase (Q).
PUFA profile in IBS
omega-6 levels.
There was no difference between plasma total ω-6 content in IBS compared with controls (32.76 ± 0.48 versus 32.59 ± 0.61 g/100 g fatty acid methyl esters (FAME); t = 0.22, df = 65, P = 0.83). An ANOVA did not reveal any differences between disease subtype or disease status (Table 1).
TABLE 1.
PUFA concentrations (g/100 g FAME), ω-6:ω-3 ratio, and eicosanoid concentrations (pg/ml) in IBS according to disease subtype and status.
| Disease Subtype |
Disease Status |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | D-IBS | C-IBS | A-IBS | F, (df), P | CA | RA | Q | F, (df), P |
| Total ω-6 | 33.16 ± 0.86 | 32.39 ± 0.94 | 32.69 ± 0.76 | 0.13, (3,63), 0.95 | 32.47 ± 0.49 | 32.05 ± 0.87 | 35.19 ± 1.19 | 1.88, (3,63), 0.14 |
| Total ω-3 | 4.49 ± 0.30 | 5.36 ± 0.78 | 5.02 ± 0.56 | 1.98, (3,63), 0.13 | 5.04 ± 0.43 | 5.20 ± 0.66 | 4.07 ± 0.38 | 2.22, (3,63), 0.09 |
| ω-6:ω-3 | 7.89 ± 0.71 | 7.06 ± 1.56 | 8.27 ± 1.07 | 1.10, (3,63), 0.36 | 7.51 ± 0.88 | 8.81 ± 1.23 | 9.34 ± 0.94 | 1.24, (3,63), 0.30 |
| AA | 8.39 ± 0.41 | 8.05 ± 0.54 | 8.81 ± 0.42 | 2.03, (3,63), 0.12 | 9.09 ± 0.54 | 7.90 ± 0.60 | 8.63 ± 0.54 | 3.02, (3,63), 0.04a |
| PGE2 | 1835 ± 362.8 | 1115 ± 202.4 | 1500 ± 187.5 | 3.42, (3, 40), 0.03a | 1531 ± 364 | 1431 ± 142.6 | 1655 ± 527.6 | 2.41 (3, 40), 0.08 |
| LTB4 | 339 ± 83.57 | 331.2 ± 66.57 | 331 ± 31.33 | 1.53, (3, 33), 0.22 | 361 ± 78.51 | 319.7 ± 38.34 | 324.3 ± 65.99 | 1.65 (3, 33), 0.2 |
P < 0.05, one way ANOVA.
Abbreviations: AA, arachidonic acid; A-IBS, alternating IBS; C-IBS, constipation-predominant IBS; CA, currently active; D-IBS, diarrhea-predominant IBS; FAME, fatty acid methyl esters; LTB4, leukotriene B4; PGE2, prostaglandin E2; PUFA, polyunsaturated fatty acid; Q, quiescent; RA, recently active.
omega-3 levels.
Plasma total ω-3 content was significantly elevated in the IBS cohort compared with controls (4.94 ± 0.33 versus 3.88 ± 0.30 g/100 g FAME; t = 2.21, df = 65, P = 0.03). An ANOVA analysis did not reveal any differences between disease subtypes or status (Table 1).
omega-6:omega-3 ratio.
There was a trend toward a decreased ω-6:ω-3 ratio in the IBS patients relative to control subjects (8.03 ± 0.64 versus 10.25 ± 1.17; t = 1.81, df = 65, P = 0.07). An ANOVA analysis did not highlight any differences between disease subtypes or disease status (Table 1).
Arachidonic acid levels
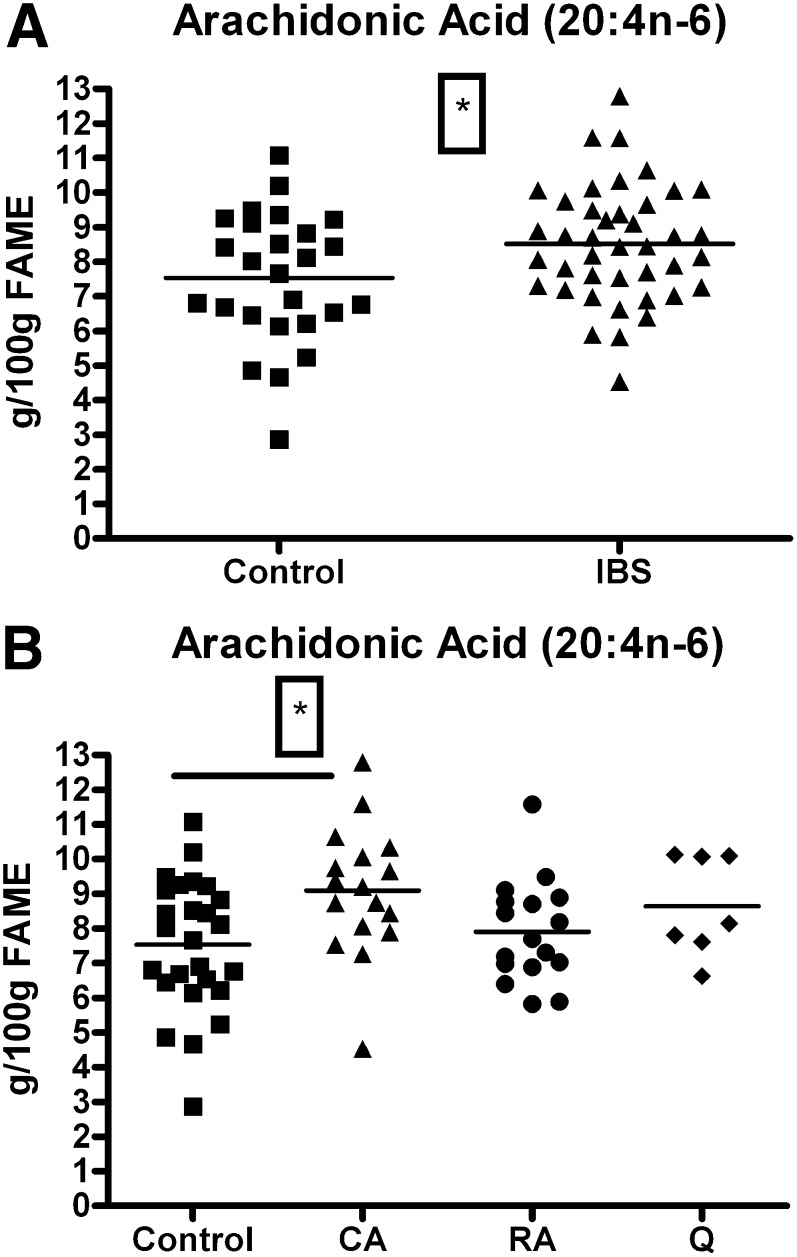
Plasma AA concentrations were significantly elevated in IBS patients compared with controls (8.52 ± 0.26 versus 7.53 ± 0.37 g/100 g FAME; t = 2.22, df = 65, P = 0.029) (Fig. 1A). An ANOVA analysis revealed that the increase observed could not be assigned to a particular disease subtype (Table 1). An ANOVA analysis followed by a Dunnets multiple comparison posthoc test revealed that the currently active subgroup had significantly elevated plasma AA concentrations compared with control levels (9.09 ± 0.54 versus 7.53 ± 0.37 g/100 g FAME; q = 2.86, df = [3, 63], P < 0.05) (Fig. 1B). An ANCOVA analysis revealed no influence of smoking habits on these results (F = 1.60, df = [1, 63], P = 0.21).
Fig. 1.
Arachidonic acid levels (g/100 g FAME) in healthy female controls and female IBS patients. (1A) Elevation in AA levels in IBS patients (n = 41) compared with control levels (n = 26) (*P < 0.05, t-test). (1B) AA levels in IBS patients according to disease status showing elevated AA levels in the currently active subdivision compared with control values (*P < 0.05, control versus currently active, ANOVA + Dunnetts posthoc test). AA, arachidonic acid; CA, currently active; FAME, fatty acid methyl esters; IBS, irritable bowel syndrome; RA, recently active; Q, quiescent.
Eicosanoid analysis
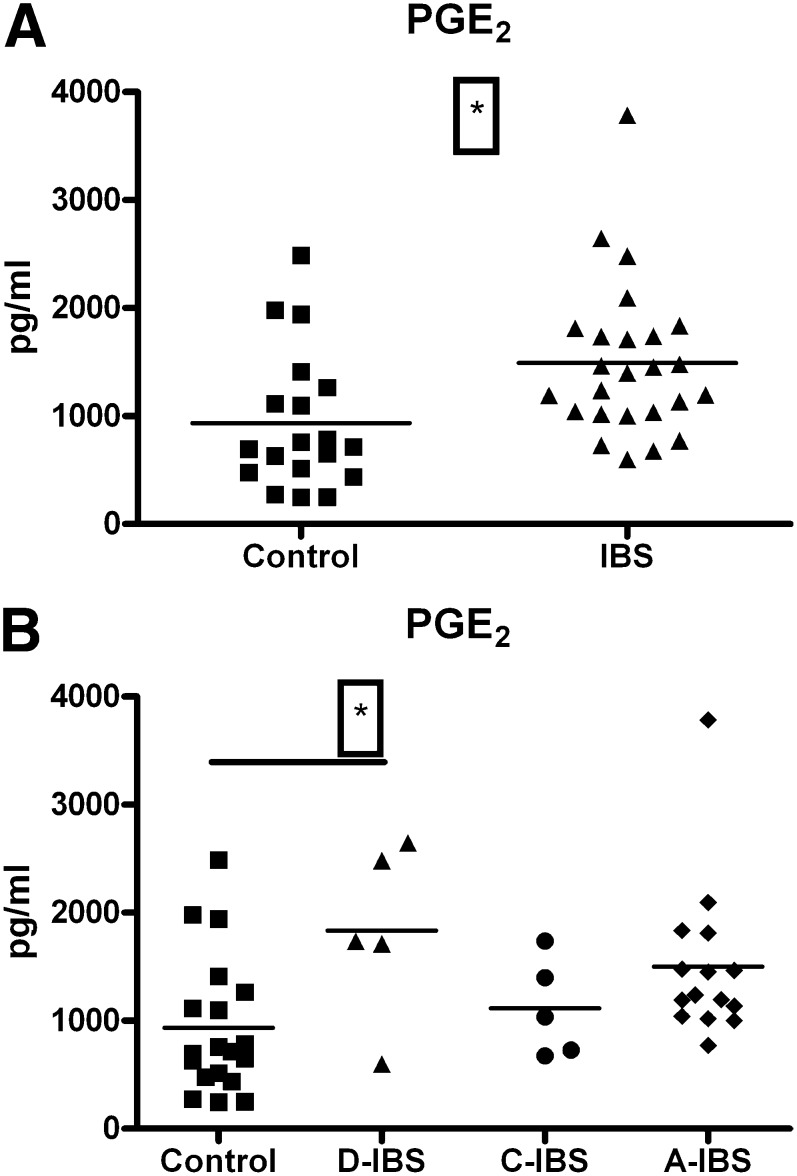
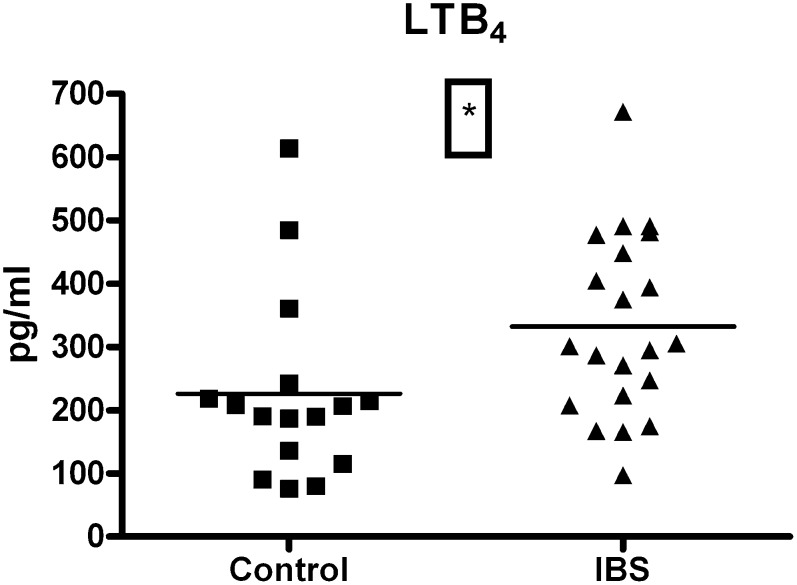
PGE2 serum concentrations were significantly elevated in the IBS group compared with controls (1490 ± 142.3 versus 934.2 ± 145.7 pg/ml; t = 2.69, df = 42, P = 0.01) (Fig. 2A). An ANOVA analysis followed by Dunnets multiple comparison posthoc test revealed that the D-IBS subtype had significantly elevated PGE2 levels compared with controls (1835 ± 362.8 versus 934.2 ± 145.7 pg/ml; q = 2.663, df = [3, 40], P < 0.05) (Fig. 2B). A similar analysis across disease status subgroups revealed a nonsignificant trend toward increased PGE2 levels in all categories (Table 1). Technical reasons prevented the measurement of LTB4 levels in two of the control samples and four of the patient samples. LTB4 serum concentrations were significantly elevated in the IBS cohort compared with controls (332.2 ± 31.33 versus 226.0 ± 36.70 pg/ml; t = 2.21, df = 35, P = 0.03) (Fig. 3). An ANOVA analysis did not reveal any differences between IBS subtypes or status (Table 1). An ANCOVA analysis revealed no influence of smoking habits (PGE2: F = 0.85, df = [1, 40], P = 0.36; LTB4: F = 0.66, df = [1, 33], P = 0.42) or age (PGE2: F = 0.01, df = [1, 40], P = 0.9; LTB4: F = 0.66, df = [1, 33], P = 0.42) on these results.
Fig. 2.
Serum PGE2 levels (pg/ml) in healthy female controls and female IBS patients. (2A) Elevation in PGE2 levels in IBS patients (n = 25) compared with control levels (n = 19) (P < 0.05, t-test). (2B) Elevation in PGE2 levels in IBS patients categorized according to disease subtype compared with control levels (*P < 0.05, control versus D-IBS, ANOVA + Dunnetts). A-IBS, alternating IBS; C-IBS, constipation-predominant IBS; D-IBS, diarrhea-predominant IBS; IBS, irritable bowel syndrome; PGE2, prostaglandin E2.
Fig. 3.
Serum LTB4 levels (pg/ml) in healthy female controls (n = 19) and female IBS patients (n = 25) (P < 0.05, t-test). IBS, irritable bowel syndrome; LTB4, leukotriene B4.
Correlation analysis
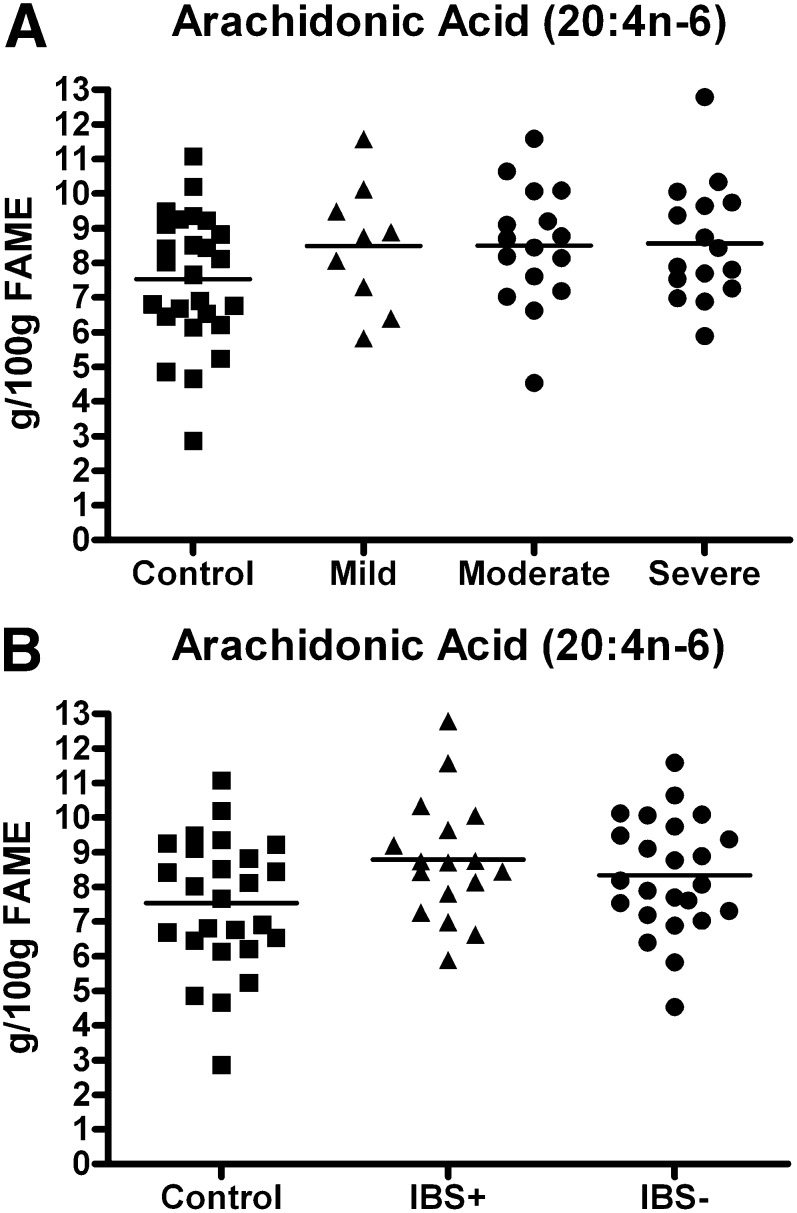
There was no correlation between IBS symptom severity and plasma AA levels (Pearson product moment correlation, r = 0.16, df = 39, P = 0.33). Neither was there a correlation between depression scores and plasma AA levels (Pearson product moment correlation, r = 0.17, df = 39, P = 0.30). Plasma AA levels were nonsignificantly elevated regardless of whether they are classified according to IBS severity (8.49 ± 0.61 g/100 g FAME in the mild group, 8.49 ± 0.43 in the moderate group, and 8.57 ± 0.63 in the severe group; F = 1.60, df = [3, 63], P = 0.20) (Fig. 4A) or ± depressive comorbidity (8.78 ± 0.42 in the depressed subgroup and 8.33 ± 0.34 in the nondepressed subgroup; F = 2.78, df = [2, 64], P = 0.07) (Fig. 4B). Additional statistical analysis revealed no correlation between depression scores and either serum PGE2 or LTB4 concentrations. Nor was there a correlation between IBS symptom severity and serum PGE2 or LTB4 concentrations (unpublished observations).
Fig. 4.
Evaluation of the AA levels and IBS symptom severity according to the presence or absence of psychiatric comorbidity. (4A) AA levels in mild, moderate, and severe IBS. (4B) AA levels in IBS patients with and without psychiatric comorbidity. AA, arachidonic acid; FAME, fatty acid methyl esters; IBS, irritable bowel syndrome; IBS+, IBS with psychiatric comorbidity; IBS−, IBS without psychiatric comorbidity.
DISCUSSION
Here we show, what is to our knowledge, the first demonstration of altered circulating PUFA metabolites in IBS. The principal finding in this study is that plasma AA concentrations were significantly elevated in the IBS cohort compared with controls. Although the study was not powered to detect IBS subgroup differences, an analysis of the data according to disease status revealed that the data was robust enough to indicate that it was the currently active IBS cohort that made the largest contribution to the elevated plasma AA concentrations. Although a further analysis according to disease subtype (C-IBS, A-IBS, or D-IBS) did not yield any statistically significant results, there appeared to be a uniform increase across all disease subtypes. Interestingly, a previous study that examined plasma fatty acid profiles in a mixed gender IBS cohort did not report any alterations (27). However, although that study did report AA concentrations, it was not one of its statistical endpoint measures nor did it examine the inflammatory mediators produced along the AA cascade. Moreover, it did not take account of possible gender differences in PUFA profiles that have been previously reported (35).
Correlation analyses revealed that there was no relationship between plasma AA concentrations and IBS symptom severity, suggesting that elevated AA is not a direct cause of IBS. Additionally, if the data is grouped according to those with mild, moderate, or severe IBS, the increase is evident in all subgroups, albeit at a nonsignificant level. Given the complexity of AA metabolism (23), it is not surprising that our correlation analysis did not yield a simple linear relationship between levels of this fatty acid and symptom severity in our IBS patients. It has previously been shown that altered fatty acid profiles can have multiple downstream effects, confirming the challenges that confront attempts at correlating elevated AA levels directly with IBS symptom scores (36). Because of the recent association between AA and depression (19), we also examined whether those patients with a depressive comorbidity made a greater contribution to the altered AA concentrations. There was no difference between these patients and the cohort that did not meet the criteria for depression.
We have also demonstrated that the consequences of the elevated AA levels include significant elevations in both PGE2 and LTB4 in our IBS cohort. Of further interest is that the elevation in serum PGE2 concentrations is largely due to the D-IBS subtype, whereas the trend toward elevated LTB4 levels is equally apparent across all disease subtypes and status. Our laboratory and other research groups have recently reported elevated IL-6 levels in IBS (11, 26). The source of such alterations has heretofore not been identified, but it is possible that the findings reported here might shed some light on the matter. It has previously been reported that PGE2 can elevate IL-6 levels (24). Furthermore, it has been shown that PGE2 itself can activate COX-2, the inducible enzyme responsible for the formation of its immediate, unstable precursor prostaglandin H2 (PGH2) (37). It is certainly plausible that the altered biological cascade described here could foster the disturbed basal IL-6 profile reported in IBS due to a self-sustaining, but low level, chronic increase in PGE2 production. Interestingly, although PGE2 does possess anti-inflammatory properties, they do not appear to extend to IL-6 (38).
Of course, these alterations in PUFA metabolites may have additional functional consequences. AA itself has a role in intestinal barrier function (39), and the increases described here are likely to have an impact in that regard. A role for elevated PGE2 levels in altered GIT muscle activity is also possible (23). Of further note is that circulating PGE2 can readily cross the blood brain barrier and thus impact the CNS component of the brain-gut axis, as well as the ENS (40). An increased number of GIT immune cells has been proposed as an indicator of immune activation in IBS (41, 42). Interestingly, LTB4 is regarded as a neutrophil chemoattractant and a promoter of both leukocyte adhesion and infiltration (22, 43). Moreover, the elevated LTB4 we report here may have relevance to the morphological and functional changes that have been detected in mast cells in IBS (44), as this leukotriene is both an activation product of mast cells and a chemoattractant for their progenitors (45). It is also known that alterations in basal prostaglandin levels can impact on the brain-gut axis through the effects they exert on HPA-axis secretory activity (40). In short, these eicosanoids and their PUFA precursor are critical agents in the normal functioning of the GIT, and the perturbations in the system we have described here could directly or indirectly impact motility, secretion, nociception, cytoprotection, and the immunological milieu of the gut (46, 47). Moreover, we cannot exclude that there are other AA metabolites produced along this complex metabolic cascade that contribute to the physiological and behavioral symptoms associated with IBS.
Contrary to our expectations, the increases in AA levels occurred despite an apparent shift toward the ω-3 PUFA arm of the metabolic cascade. We found that total ω-3 levels were significantly elevated in our IBS cohort compared with controls without any alteration in total ω-6 levels. The factors behind this shift toward the ω-3 fatty acids are difficult to explain from the current dataset and are not in line with reports from other disorders that have been associated with elevated immune parameters such as depression (19) and Crohn's disease (48). Of note is that in the latter study, the elevated ω-6:ω-3 ratio was related to specific cytokine genotypes. We did not record subject dietary habits in this study and consequently cannot rule out an altered dietary consumption of fatty acids in the IBS cohort. Despite this limitation, data from previous studies suggests that such an alteration would have an anti-inflammatory outcome (49). It is noteworthy that an apparent trend toward a reduction in the ω-6:ω-3 ratio did not reach statistical significance in this study and that the magnitude of the ω-3 shift described may not be large enough to be of immunological importance. It is also interesting that the IBS is not thought to be more common in countries with traditionally high fish consumption rates, like Japan, than in Western societies (5). However more detailed epidemiological studies are required to probe the potential associations that have been demonstrated for other disorders with an inflammatory component such as depression and cardiovascular diseases (18, 19). The results presented here suggest that even if dietary factors come into play, they are insufficient to counteract the sequalae of events leading to the increased input to the AA cascade. While the precise mechanism behind these alterations is unclear at the moment, the activity of phospholipase A2 (PLA2), the enzyme responsible for the release of arachidonic acid from the cell membranes, may be worth investigating in future studies (50).
CONCLUSION
We have comprehensively characterized the PUFA profile in a female IBS population. Our results indicate that AA levels are increased in the clinical setting. We have demonstrated a clear and robust increase in pro-inflammatory markers downstream of AA in our IBS cohort that may have relevance for previously described alterations in pro-inflammatory markers such as IL-6. Although further studies are required to elucidate the mechanism behind this phenomenon, the alterations described here may represent a novel biomarker candidate panel in IBS that is especially needed given the current reliance on a symptom-based diagnostic scheme.
Acknowledgments
The authors would like to acknowledge Dr. Sandra Barry, Ms. Neasa O'Leary, Mr. Srinivas Suda, and Mr. David Russell for their contributions to the study.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- CNS
- central nervous system
- FAME
- fatty acid methyl esters
- GIT
- gastrointestinal tract
- IBS
- irritable bowel syndrome
- LTB4
- leukotriene B4
- MALT
- mucosa-associated lymphoid tissue
- PGE2
- prostaglandin E2
- PHQ
- Public Health Questionnaire
The authors are supported in part by the Science Foundation Ireland in the form of a centre grant (Alimentary Pharmabiotic Centre), by GlaxoSmithKline, by the Health Research Board (HRB) of Ireland, and the Higher Education Authority (HEA) of Ireland. A.A.H. is in receipt of a Teagasc Walsh Fellowship.
REFERENCES
- 1.Ersryd A., Posserud I., Abrahamsson H., Simren M. 2007. Subtyping the irritable bowel syndrome by predominant bowel habit: Rome II versus Rome III. Aliment. Pharmacol. Ther. 26: 953–961. [DOI] [PubMed] [Google Scholar]
- 2.Quigley E. M. 2006. Changing face of irritable bowel syndrome. World J. Gastroenterol. 12: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiller R. C. 2004. Irritable bowel syndrome. Br. Med. Bull. 72: 15–29. [DOI] [PubMed] [Google Scholar]
- 4.Boyce P. M., Koloski N. A., Talley N. J. 2000. Irritable bowel syndrome according to varying diagnostic criteria: are the new Rome II criteria unnecessarily restrictive for research and practice? Am. J. Gastroenterol. 95: 3176–3183. [DOI] [PubMed] [Google Scholar]
- 5.Drossman D. A., Camilleri M., Mayer E. A., Whitehead W. E. 2002. AGA technical review on irritable bowel syndrome. Gastroenterology. 123: 2108–2131. [DOI] [PubMed] [Google Scholar]
- 6.Fass R., Longstreth G. F., Pimentel M., Fullerton S., Russak S. M., Chiou C. F., Reyes E., Crane P., Eisen G., McCarberg B., et al. 2001. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch. Intern. Med. 161: 2081–2088. [DOI] [PubMed] [Google Scholar]
- 7.Gilkin R. J., Jr 2005. The spectrum of irritable bowel syndrome: a clinical review. Clin. Ther. 27: 1696–1709. [DOI] [PubMed] [Google Scholar]
- 8.Clarke G., Quigley E. M., Cryan J. F., Dinan T. G. 2009. Irritable bowel syndrome: towards biomarker identification. Trends Mol. Med. 15: 478–89. [DOI] [PubMed] [Google Scholar]
- 9.Ringel Y., Drossman D. A. 2002. Irritable bowel syndrome: classification and conceptualization. J. Clin. Gastroenterol. 35(Suppl. 1): S7–S10. [DOI] [PubMed] [Google Scholar]
- 10.Van Oudenhove L., Demyttenaere K., Tack J., Aziz Q. 2004. Central nervous system involvement in functional gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 18: 663–80. [DOI] [PubMed] [Google Scholar]
- 11.Dinan T. G., Quigley E. M., Ahmed S. M., Scully P., O'Brien S., O'Mahony L., O'Mahony S., Shanahan F., Keeling P. W. 2006. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 130: 304–311. [DOI] [PubMed] [Google Scholar]
- 12.Spiller R. 2008. Irritable bowel syndrome–the new inflammatory bowel disease? Clin. Med. 8: 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macsharry J., O'Mahony L., Fanning A., Bairead E., Sherlock G., Tiesman J., Fulmer A., Kiely B., Dinan T. G., Shanahan F., et al. 2008. Mucosal cytokine imbalance in irritable bowel syndrome. Scand. J. Gastroenterol. 43: 1467–1476. [DOI] [PubMed] [Google Scholar]
- 14.Calder P. C. 2005. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 33: 423–427. [DOI] [PubMed] [Google Scholar]
- 15.Clarke G., O'Mahony S. M., Hennessy A. A., Ross P., Stanton C., Cryan J. F., Dinan T. G. 2009. Chain reactions: early-life stress alters the metabolic profile of plasma polyunsaturated fatty acids in adulthood. Behavioural Brain Res. 205: 319–321. [DOI] [PubMed] [Google Scholar]
- 16.Flower R. J., Perretti M. 2005. Controlling inflammation: a fat chance? J. Exp. Med. 201: 671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills S. C., Windsor A. C., Knight S. C. 2005. The potential interactions between polyunsaturated fatty acids and colonic inflammatory processes. Clin. Exp. Immunol. 142: 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das U. N. 2000. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot. Essent. Fatty Acids. 63: 351–362. [DOI] [PubMed] [Google Scholar]
- 19.Dinan T., Siggins L., Scully P., O'Brien S., Ross P., Stanton C. 2009. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J. Psychiatr. Res. 43: 471–476. [DOI] [PubMed] [Google Scholar]
- 20.Su K. P., Huang S. Y., Chiu T. H., Huang K. C., Huang C. L., Chang H. C., Pariante C. M. 2008. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry. 69: 644–651. [DOI] [PubMed] [Google Scholar]
- 21.Simopoulos A. P. 1991. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 54: 438–463. [DOI] [PubMed] [Google Scholar]
- 22.Calder P. C. 2008. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 52: 885–897. [DOI] [PubMed] [Google Scholar]
- 23.Eberhart C. E., Dubois R. N. 1995. Eicosanoids and the gastrointestinal tract. Gastroenterology. 109: 285–301. [DOI] [PubMed] [Google Scholar]
- 24.Bagga D., Wang L., Farias-Eisner R., Glaspy J. A., Reddy S. T. 2003. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA. 100: 1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinan T. G., Clarke G., Quigley E. M., Scott L. V., Shanahan F., Cryan J., Cooney J., Keeling P. W. 2008. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am. J. Gastroenterol. 103: 2570–2576. [DOI] [PubMed] [Google Scholar]
- 26.Liebregts T., Adam B., Bredack C., Roth A., Heinzel S., Lester S., Downie-Doyle S., Smith E., Drew P., Talley N. J., et al. 2007. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 132: 913–920. [DOI] [PubMed] [Google Scholar]
- 27.Kilkens T. O., Honig A., Maes M., Lousberg R., Brummer R. J. 2004. Fatty acid profile and affective dysregulation in irritable bowel syndrome. Lipids. 39: 425–431. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer R. L., Kroenke K., Williams J. B. 1999. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 282: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K., Spitzer R. L., Williams J. B. 2001. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleijenberg G., Fennis J. F. 1989. Anamnestic and psychological features in diagnosis and prognosis of functional abdominal complaints: a prospective study. Gut. 30: 1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Horst H. E., van Dulmen A. M., Schellevis F. G., van Eijk J. T., Fennis J. F., Bleijenberg G. 1997. Do patients with irritable bowel syndrome in primary care really differ from outpatients with irritable bowel syndrome? Gut. 41: 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 33.Bondia-Pons I., Morera-Pons S., Castellote A. I., Lopez-Sabater M. C. 2006.. Determination of phospholipid fatty acids in biological samples by solid-phase extraction and fast gas chromatography. J. Chromatography. 1116: 204–208. [DOI] [PubMed] [Google Scholar]
- 34.Park P., Goins R. 1994. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J. Food Sci. 59: 1262–1266. [Google Scholar]
- 35.Childs C. E., Romeu-Nadal M., Burdge G. C., Calder P. C. 2008. Gender differences in the n-3 fatty acid content of tissues. Proc. Nutr. Soc. 67: 19–27. [DOI] [PubMed] [Google Scholar]
- 36.Grimble R. F., Tappia P. S. 1998. Modulation of pro-inflammatory cytokine biology by unsaturated fatty acids. Z. Ernahrungswiss. 37(Suppl 1): 57–65. [PubMed] [Google Scholar]
- 37.Kobayashi N., Barnard R. J., Henning S. M., Elashoff D., Reddy S. T., Cohen P., Leung P., Hong-Gonzalez J., Freedland S. J., Said J., et al. 2006. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin. Cancer Res. 12: 4662–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miles E. A., Allen E., Calder P. C. 2002. In vitro effects of eicosanoids derived from different 20-carbon fatty acids on production of monocyte-derived cytokines in human whole blood cultures. Cytokine. 20: 215–223. [DOI] [PubMed] [Google Scholar]
- 39.Willemsen L. E., Koetsier M. A., Balvers M., Beermann C., Stahl B., van Tol E. A. 2008. Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur. J. Nutr. 47: 183–191. [DOI] [PubMed] [Google Scholar]
- 40.Turnbull A. V., Rivier C. L. 1999. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 79: 1–71. [DOI] [PubMed] [Google Scholar]
- 41.Spiller R. C. 2007. Role of infection in irritable bowel syndrome. J. Gastroenterol. 42(Suppl 17): 41–47. [DOI] [PubMed] [Google Scholar]
- 42.Spiller R. C., Jenkins D., Thornley J. P., Hebden J. M., Wright T., Skinner M., Neal K. R. 2000. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 47: 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funk C. D. 2001.. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 44.Barbara G., Stanghellini V., De Giorgio R., Cremon C., Cottrell G. S., Santini D., Pasquinelli G., Morselli-Labate A. M., Grady E. F., Bunnett N. W., et al. 2004. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 126: 693–702. [DOI] [PubMed] [Google Scholar]
- 45.Weller C. L., Collington S. J., Brown J. K., Miller H. R., Al-Kashi A., Clark P., Jose P. J., Hartnell A., Williams T. J. 2005. Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors. J. Exp. Med. 201: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dajani E. Z., Shahwan T. G., Dajani N. E. 2003. Prostaglandins and brain-gut axis. J. Physiol. Pharmacol. 54(Suppl 4): 155–164. [PubMed] [Google Scholar]
- 47.Miller S. B. 2006. Prostaglandins in health and disease: an overview. Semin. Arthritis Rheum. 36: 37–49. [DOI] [PubMed] [Google Scholar]
- 48.Guerreiro C. S., Ferreira P., Tavares L., Santos P. M., Neves M., Brito M., Cravo M. 2009. Fatty acids, IL6, and TNFalpha polymorphisms: an example of nutrigenetics in Crohn's disease. Am. J. Gastroenterol. 104: 2241–2249. [DOI] [PubMed] [Google Scholar]
- 49.Ferrucci L., Cherubini A., Bandinelli S., Bartali B., Corsi A., Lauretani F., Martin A., Andres-Lacueva C., Senin U., Guralnik J. M. 2006. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 91: 439–446. [DOI] [PubMed] [Google Scholar]
- 50.Yedgar S., Cohen Y., Shoseyov D. 2006. Control of phospholipase A2 activities for the treatment of inflammatory conditions. Biochim. Biophys. Acta. 1761: 1373–1382. [DOI] [PubMed] [Google Scholar]