Abstract
We previously established an analysis method for determining the cholesterol levels of five major lipoprotein classes [HDL, LDL, intermediate density lipoprotein (IDL), VLDL, and chylomicrons] in serum by an anion-exchange (AEX)-HPLC method, but lipoprotein(a) [Lp(a)], a well-known risk factor for atherosclerotic diseases, was not determinable. Therefore, we established new AEX-HPLC separation conditions for analyzing the cholesterol levels of six lipoprotein classes, including Lp(a). Serum lipoproteins were separated by HPLC with a diethylaminoethyl-ligand nonporous polymer-based column by elution with a stepwise gradient of the sodium perchlorate concentration. In this improved method, HDL, LDL, IDL, VLDL, chylomicrons, and Lp(a) were each eluted from the column. The cholesterol levels of the eluted lipoproteins were measured enzymatically by a postcolumn reaction. The within-day assay and between-day assay coefficients of variation for the lipoprotein cholesterol levels were in the ranges of 0.29–11.86% and 0.57–11.99%, respectively. The Lp(a) cholesterol levels determined by AEX-HPLC were significantly correlated with the amounts of Lp(a) protein measured by an immunoturbidimetry method available commercially (r = 0.9503, P < 0.0001). Taken together, this AEX-HPLC method may be effectively applied to the analysis of serum lipoproteins with high levels of Lp(a).
Keywords: atherosclerotic risk factor, high-performance liquid chromatography, lipoprotein (a)
Lipoprotein (a) [Lp(a)] is a particular LDL particle in which apolipoprotein B-100 (apoB-100) is linked by a single disulfide bridge to a unique glycoprotein, apo(a) (1). Apo(a) has a hydrophilic carbohydrate-rich structure, which is not present in other apolipoproteins (1). Moreover, the molecular masses of apo(a) are highly varied, ranging from 300–800 kDa, because of the heterogenous gene structure (1–3). Previous reports have shown that the elevated Lp(a) level and the smaller-molecular mass of apo(a) are risk factors for atherosclerotic deceases, i.e., cardiovascular disease, and peripheral arterial disease (4–11).
ELISA or immunoturbidimetric assay for measurement of the Lp(a) protein levels and Western blot analysis for the molecular weight of apo(a) have been widely used (4–11). The separation of Lp(a) by agarose gel electrophoresis (12, 13), gel-permeation chromatography (14), and ultracentrifugation (15, 16) have been reported, though Lp(a) was overlapped with VLDL on agarose gel electrophoresis and gel permeation chromatography, and the fraction of Lp(a) obtained by ultracentrifugation was of the density 1.060–1.125 g/ml and contained dense LDL and HDL2. Therefore, Lp(a) is isolated from the ultracentrifugated Lp(a) fraction containing dense LDL and HDL2 by using gel-permeation chromatography or immunoaffinity chromatography (15, 16).
In a previous article, we showed that HDL, LDL, intermediate density lipoprotein (IDL), VLDL, and chylomicrons were effectively separated by HPLC with an anion-exchange column, and the cholesterol levels of the lipoprotein classes obtained by the HPLC method were highly correlated with those measured by an ultracentrifugation method (17). However, the eluted time of Lp(a) from the column in the HPLC method was not defined. Therefore, we attempted to separate and estimate Lp(a) with another separation condition of anion-exchange (AEX)-HPLC, and six lipoprotein classes, including Lp(a), were separated and analyzed. This article reports an improved AEX-HPLC method for separating and determining HDL, LDL, IDL, VLDL, chylomicrons, and Lp(a), a method that provides precise data on the cholesterol levels of these lipoproteins and a good correlation of the Lp(a) data between the AEX-HPLC method and the immunoturbidimetric method. This AEX-HPLC method was verified to be suitable in the determination of the cholesterol levels of six lipoprotein classes in human serum.
MATERIALS AND METHODS
Materials and chemicals
Total cholesterol and triglyceride concentrations were measured enzymatically using commercially available kits (Sekisui Medical Co., Tokyo, Japan). Lp(a) protein levels were determined by immunoturbidimetry with commercial kits (Sekisui Medical Co.). The enzymatic cholesterol reagent for HPLC was the commercially available TCHO-CL (Serotec Co., Hokkaido, Japan).
Chromatography
The HPLC system reported in the previous article (17) was modified and then used for determining the cholesterol levels of lipoprotein classes [HDL, LDL, IDL, VLDL, chylomicrons, and Lp(a)] in serum. The AEX column, which contained 2.5 μm of nonporous polymer-based gel with diethylaminoethyl ligands, was 3.0 mm inner diameter × 25 mm in size. A serum sample (4 µl) was injected into the column, and lipoprotein classes in the sample were separated using an ordered gradient of perchlorate ion concentrations. The system obtained three pumps (DP-8020; Tosoh, Tokyo, Japan) for the two eluents and one enzymatic reagent. The two eluents used to separate the lipoproteins were eluent A (50 mM Tris-HCl + 1 mM EDTA, disodium salt, and dihydrate, pH 7.5) and eluent B (50 mM Tris-HCl + 500 mM sodium perchlorate + 1 mM EDTA, disodium salt, and dihydrate, pH 7.5). The two eluents used for the gradient elution, which were delivered through pumps, were mixed on line, and the flow rate was held constant at 0.5 ml/min. The gradient patterns for separation of the lipoprotein classes were 20.0% eluent B for 0.0–3.5 min, 24.0% eluent B for 3.5–8.5 min, 27.0% eluent B for 8.5–11.0 min, 32.0% eluent B for 11.0–14.5 min, 36.0% eluent B for 14.5–17.5 min, 36.0–100.0% linear gradient of eluent B for 17.5–18.5 min, 100.0% eluent B for 18.5–21.5 min, 100.0–20.0% linear gradient of eluent B for 21.5.0–22.5 min, and 20.0% eluent B for 22.5–26.0 min. Therefore, it took 26 min to complete the assay of one sample. The eluate from the column containing the separated lipoprotein classes was mixed with an enzymatic reagent (flow rate, 0.2 ml/min). The mixed solution was reacted at 37°C and 2.1 min and was monitored at 600 nm.
Samples
The serum with a low Lp(a) level [Subject 1, male, age 42, total cholesterol (TC) = 5.87 mmol/l, triglyceride (TG) = 0.56 mmol/l, Lp(a) protein = 0.02 mg/ml] and two sera with a high Lp(a) level [Subject 2, male, age 58, TC = 4.71 mmol/l, TG = 2.01 mmol/l, Lp(a) protein = 0.84 mg/ml; Subject 3, male, age 62, TC = 7.68 mmol/l, TG = 0.81 mmol/l, Lp(a) protein = 0.90 mg/ml] were used for the separation of the six lipoprotein fractions by centrifugation.
The sera from 17 healthy subjects [male/female = 12/5, age = 44.8 ± 16.2, TC = 5.30 ± 0.69 mmol/l, TG = 1.14 ± 0.44 mmol/l, Lp(a) protein = 0.49 ± 0.60 mg/ml] and 16 dyslipidemic patients [male/female = 11/5, age = 49.1 ± 11.3, TC = 6.75 ± 1.04 mmol/l, TG = 1.49 ± 0.88 mmol/l, Lp(a) protein = 0.58 ± 0.42 mg/ml] were used for the correlation between the Lp(a) cholesterol levels obtained by the HPLC method and the Lp(a) protein levels. The 33 sera were obtained from venous blood samples drawn after a 12 h fast. Five hundred microliters of serum sample were mixed with 250 µl of the stock solution (760 g/l sucrose and 1.5 g/l EDTA2K) and stored at −40°C until use. All subjects gave informed consent to participate in this study.
Ultracentrifugation
Sequential ultracentrifugation of serum lipoproteins was performed by the method reported previously (15, 16, 18, 19). The flotation rates of chylomicrons and VLDL were set at >400, and 20–400, respectively, in a solution of 1.745 mol/l sodium chloride (d = 1.063 g/ml). The densities of IDL, LDL, HDL2 + dense LDL + Lp(a), and HDL3 were set as follows: 1.006 < d < 1.019 g/ml, 1.019 < d < 1.060 g/ml, 1.060 < d < 1.125 g/ml, and 1.125 g/ml < d, respectively. An SCP70H2 ultracentrifuge and RP55T angle rotor (both from the Hitachi Koki Co., Tokyo, Japan) were used.
Western blot analysis
The sera from three subjects (Subjects 1 to 3) were used for Western blot analysis. The three sera were injected into the column, and the six fractions containing the separated lipoproteins eluted from the column were obtained (Peak 1 fraction, 2.5–4.5 min; Peak 2 fraction, 6.5–9.5 min; Peak 3 fraction, 12.0–13.5 min; Peak 4 fraction, 14.5–16.0 min; Peak 5 fraction, 17.5–19.5 min; Peak 6 fraction, 21.0–23.0 min). The serum and the collected lipoprotein fractions were analyzed by Western blot analysis withSDS-PAGE with 2-mercaptoethanol. Two goat polyclonal antibodies against apoA-1 and apoB (Rockland), a goat polyclonal antibody against apoE (Chemicon), a goat polyclonal antibody against apo(a) (Cortex Biochem), an alkaline-phosphatase-conjugated rabbit polyclonal antibody against anti-goat antibody (Chemicon), and Western lighting chemiluminescent reagent (CDP-Star for AP-based assays; Perkin-Elmer) were used for Western blot analysis. Lp(a) isoform standard (Technoclone, Vienna, Austria) was used for the determination of the Lp(a) molecular mass in sera.
Linearity, precision, and recovery test
To test for linearity, a serum with a high Lp(a) level [Subject 4, female, age = 65, TC = 5.68 mmol/l, TG = 0.71 mmol/l, Lp(a) protein = 1.21 mg/ml] was used. The samples were diluted serially with 5% BSA solution, and 8 µl aliquots were injected. For within-day and between-day assay precision tests, the two sera [Subject 5, male, age = 46, TC = 5.90 mmol/l, TG = 0.50 mmol/l, Lp(a) protein = 0.02 mg/ml; Subject 6, female, age = 56, TC = 8.93 mmol/l, TG = 0.63 mmol/l, Lp(a) protein = 1.19 mg/ml] were used. The two sera (Subjects 5 and 6) and the purified Lp(a) from human sera (Biomedical Technologies) were used for the recovery test. The Lp(a) was purified with ultracentrifugation and immunoaffinity chromatography. The samples were stored at 4°C until use. The injected volume was 4 µl.
Correlation test
The Lp(a) cholesterol levels measured by HPLC method were compared with the Lp(a) protein levels measured by immunoturbidimetry with commercial kits (Sekisui Medical Co.). The correlation data were estimated in terms of Pearson product- moment correlation coefficients. A value of P < 0.05 was considered statistically significant.
RESULTS
Chromatogram of HDL3, HDL2 + dense LDL + Lp(a), LDL, IDL, VLDL, and chylomicron fractions separated by ultracentrifugation
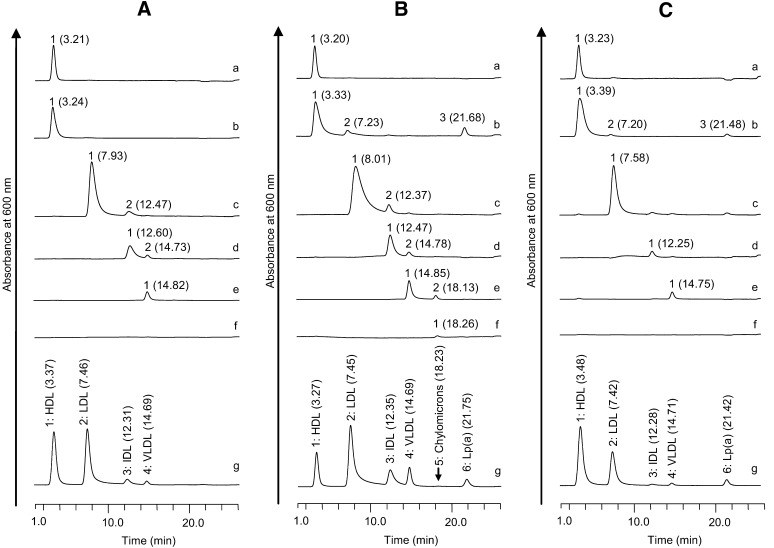
Four, six, and five peaks were identified in the chromatogram of the three sera from Subjects 1, 2, and 3, respectively (Fig. 1Ag, Bg, and Cg). These chromatogram peaks, named Peaks 1–6 in order, were eluted at 20.0, 24.0, 27.0, 32.0, 36.0, and 100% eluent B, respectively (Fig. 1Ag, Bg, and Cg).
Fig. 1.
Chromatograms of lipoproteins separated from three sera by ultracentrifugation. The chromatograms a, b, c, d, e, f, and g are the fractions of HDL3 (1.125 g/ml < d), HDL2 + dense LDL + Lp(a) (1.060 < d < 1.125g/ml), LDL (1.019 < d < 1.060g/ml), IDL (1.006 < d < 1.019g/ml), VLDL (d < 1.019 g/ml, and flotation rate 20–400), and chylomicrons (d < 1.019g/ml and flotation rate >400) separated from the sera by the ultracentrifugation method and the serum, respectively. A–C show the chromatograms of Subjects 1–3, respectively. The numbers in the parentheses are the retention time of each peak.
The HDL3, HDL2 + dense LDL + Lp(a), LDL, IDL, VLDL, and chylomicron fractions obtained by ultracentrifugation of the three sera were analyzed by HPLC to identify Peaks 1–6. The HDL3 peaks derived from sera of Subjects 1, 2, and 3 were eluted at 3.21, 3.20, and 3.23 min, respectively (Fig. 1Aa, Ba, and Ca). The peaks of the HDL2 + dense LDL + Lp(a) samples derived from the two sera of Subjects 2 and 3 were eluted at 3.33, 7.23, and 21.68 min and 3.39, 7.20, and 21.48 min, respectively (Fig. 1Bb, Cb). The peaks eluted at 7.23 and 7.20 min were probably dense LDL because the retention times were nearly the same as those of the major LDL peaks derived from the two sera of Subjects 2 and 3 (Fig. 1Bc, Cc). In the HDL2 + dense LDL + Lp(a) in Subject 1, one peak was observed at 3.24 min (Fig. 1Ab). The protein level of Lp(a) in the sera of Subject 1 was much lower than in the two other sera [Subject 1, Lp(a) = 0.02 mg/ml; Subject 2, Lp(a) = 0.84 mg/ml; Subject 3, Lp(a) = 0.90 mg/ml]. Therefore, Peaks 1 and 6 in sera were likely to indicate HDL and Lp(a), respectively, as major components (Fig. 1Ag, Bg, and Cg).
The major LDL peaks derived from the two sera of Subjects 1 and 2 were eluted at 7.93 and 8.01 min, respectively, and the LDL peak derived from the serum of Subject 3 was eluted at 7.58 min (Fig. 1Ac, Bc, and Cc). In Fig. 1Ac and Bc, minor peaks were eluted at 12.47 and 12.37 min, respectively. The major IDL peaks derived from the two sera samples of Subjects 1 and 2 were eluted at 12.60 and 12.47 min, respectively, and the IDL peak derived from the serum of Subject 3 was eluted at 12.25 min (Fig. 1Ad, Bd, and Cd). In Fig. 1Ad and Bd, minor peaks were eluted at 14.73 and 14.78 min, respectively. The VLDL peaks derived from the two sera of Subjects 1 and 3 were eluted at 14.82 and 14.75 min, respectively (Fig. 1Ae, Ce). The major and minor VLDL peaks derived from the serum of Subject 2 were eluted at 14.85 and 18.13 min, respectively (Fig. 1Bd). Therefore, the major components of Peaks 2, 3, and 4 were LDL, IDL, and VLDL, respectively. The chylomicron peak derived from the serum of Subject 2 was eluted at 18.26 min, and chylomicron peaks were not detected in the other sera of Subjects 1 and 3 (Fig. 1Af, Bf, and Cf). Peak 5 was detected in only the serum of Subject 2 (Fig. 1Ag, Bg, and Cg). The result indicated that Peak 5 contained predominantly chylomicrons.
In the LDL and IDL fractions of sera from Subjects 1 and 2 and a VLDL fraction of serum from Subject 2, minor peaks were found (Peak 2 in Fig. 1Ac, Bc, Ad, Bd, and Be), suggesting the molecular heterogeneity of LDL, IDL, and VLDL (20–26).
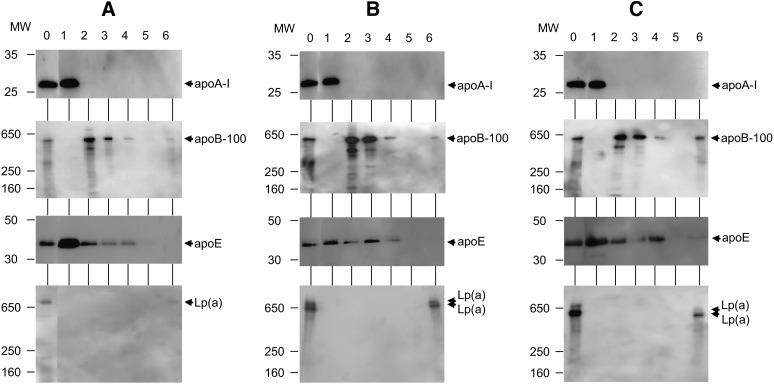
Western blot analysis of Peaks 1–6 fractionated by the HPLC method
In the chromatograms of the fractions separated by ultracentrifugation, Peaks 1–6 in sera were identified as HDL, LDL, IDL, VLDL, chylomicron, and Lp(a), respectively (Fig. 1Ag, Bg, and Cg). The HPLC samples of Peaks 1–6 in the separate sera of Subjects 1, 2, and 3 were analyzed through Western blotting using four goat polyclonal antibodies against apoA-1, B, and E, and apo(a) to confirm the apolipoproteins contained in the six peaks.
In the sera of Subjects 1, 2, and 3 (Fig. 2A0, B0, and C0), apoA-1, apoB-100, and apoE were detected at 27, 500, and 35 kDa, respectively. On these lanes (Fig. 2A0, B0, and C0), apo(a) proteins were detected at 730, 710, and 640 kDa, and 660 and 540 kDa, respectively. In the Peak 1 (HDL) fraction of Subjects 1, 2, and 3 (Fig. 2A1, B1, and C1), apoA-1 and E were detected, and apoB-100 and apo(a) were not detected. In the Peak 2 (LDL) fraction, Peak 3 (IDL) fraction, and Peak 4 (VLDL) fraction of Subjects 1, 2, and 3 (Fig. 2A2–4, B2–4, and C2–4), apoB-100 and E were detected, and apoA-1 and apo(a) were not. In the Peak 5 (chylomicron) fraction of Subjects 1, 2, and 3 (Fig. 2A5, B5, and C5), apoA-1, B-48, E, and apo(a) were not detected because of the trivial amounts of chylomicrons in sera (Fig. 1Ab, Bg, and Cg). In the Peak 6 [Lp(a)] fraction of Subjects 1, 2, and 3 (Fig. 2A6, B6, and C6), apo(a) was detected as one band (730 kDa), two bands (710 and 640 kDa), and two bands (660 and 540 kDa), respectively. In the Peak 6 [Lp(a)] fraction of Subjects 1, 2, and 3 (Fig. 2A6, B6, and C6), apoB-100 was also detected.
Fig. 2.
Western blot analysis for Peaks 1–6 fractionated by the HPLC method. Samples of the fractionated Peaks 1–6 were analyzed using Western blot analysis with SDS-PAGE. Lanes 0–6 are the serum sample and Peaks 1–6, respectively. Four gels and goat polyclonal antibodies were used against apoA-1, apoB, apoE, and apo(a) to detect the apolipoproteins in the serum and six fractionated samples. For analysis of the apoA-1 and apoE, and apoB-100 and apo(a), 5–20% gradient gel and 5% gel were used, respectively. A: The serum of Subject 1 and the fractionated samples were used. The 27 kDa bands (apoA-1) were detected in lanes 0 and 1 of the first gel. The 500 kDa bands (apoB-100) were detected in lanes 0, 2, 3, 4, and 6 of the second gel. The 35 kDa bands (apoE) were detected in lanes 0–4 of the third gel. A 730 kDa band [apo(a)] was detected in lanes 0 and 6 of the fourth gel. B: The serum of Subject 2 and the fractionated samples were used. The 27 kDa bands (apoA-1) were detected in lanes 0 and 1 of the first gel. The 500 kDa bands (apoB-100) were detected in lanes 0, 2, 3, 4, and 6 of the second gel. The 35 kDa bands (apoE) were detected in lanes 0–4 of the third gel. The 710 and 640 kDa bands [apo(a)] were detected in lanes 0 and 6 of the fourth gel. C: The serum of Subject 3 and the fractionated samples were used. The 27 kDa bands (apoA-1) were detected in lanes 0 and 1 of the first gel. The 500 kDa bands (apoB-100) were detected in lanes 0, 2, 3, 4, and 6 of the second gel. The 35 kDa bands (apoE) were detected in lanes 0–4 of the third gel. The 660 and 540 kDa bands [apo(a)] were detected in lanes 0 and 6 of the fourth gel.
Linearity, precision, and recovery tests
The serum with a high Lp(a) level [Subject 4, Lp(a) protein = 1.21 mg/ml] and the two sera with a low Lp(a) level [Subject 5, Lp(a) protein = 0.02 mg/ml] and high Lp(a) level [Subject 6, Lp(a) protein = 1.19 mg/ml] were subjected to linearity, precision, and recovery tests, respectively, as follows.
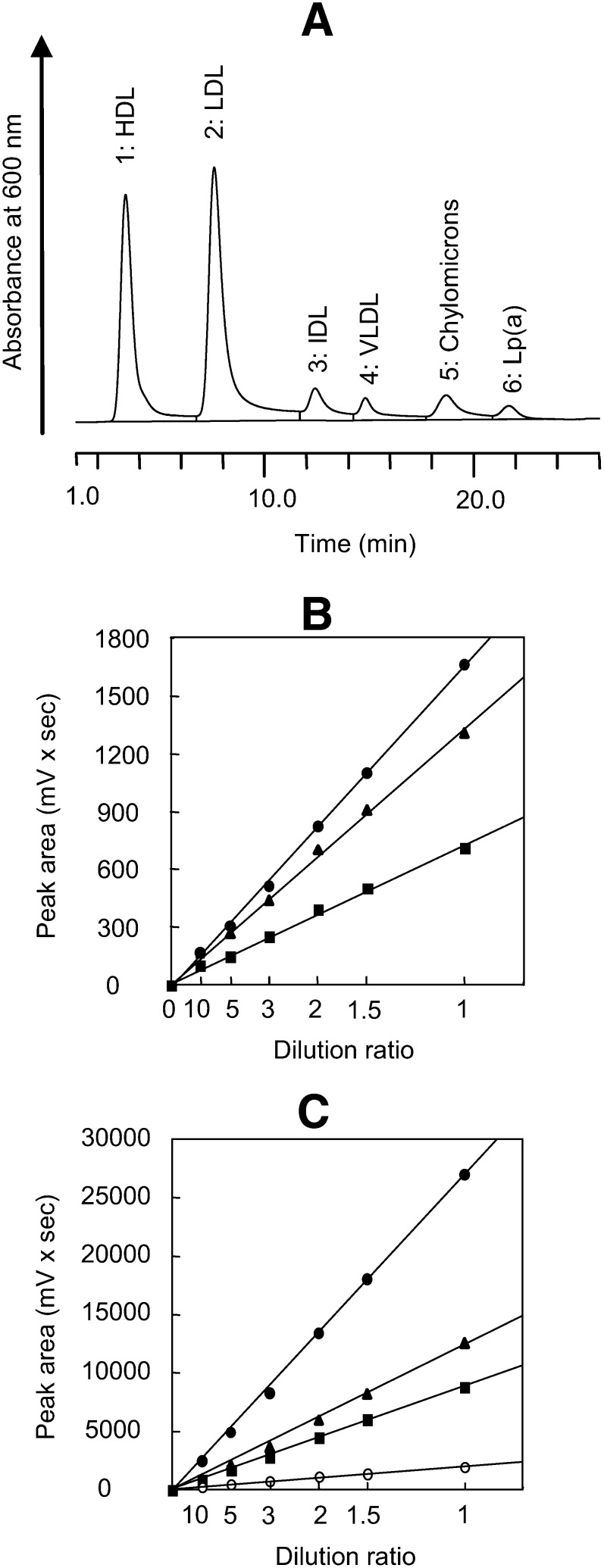
Figure 3A shows a chromatogram of the serum from Subject 4. Linear relationships were found between the peak area of each lipoprotein class (Peaks 1–6) and the total peak area and dilution ratio in a range of up to 10 times (Fig. 3B, C). Tables 1 and 2 show the precision data of the sera of Subjects 5 and 6 applied to the AEX-HPLC method. The values of within-assay and between-assay coefficients of variation of the cholesterol concentration of each lipoprotein class were 0.29–11.86% and 0.57–11.99%, respectively (Table 1). The values of within-assay and between-assay coefficients of the variation in retention time of each lipoprotein class were 0.021–0.22% and 0.22–1.04%, respectively (Table 2). The replicability was satisfactory.
Fig. 3.
Linearity of the peak areas of each lipoprotein. A: The serum with a high Lp(a) level [Subject 4, Lp(a) protein = 1.21 mg/ml] was analyzed. The retention times of Peaks 1–6 were 3.35, 7.58, 12.38, 14.80, 18.67, and 21.68, respectively. The sample that was diluted two times with 5% BSA solution was analyzed using an injected volume of 8 µl. B: These data are the areas of Peaks 4 (triangle), 5 (circle), and 6 (square) from 8 µl samples diluted up to 10 times. C: These data are the areas of Peaks 1(square), 2 (triangle), 3 (open circle), and total peaks (closed circle) from 8 µl samples diluted up to 10 times.
TABLE 1.
Precision data for cholesterol levels of assayed sera
| Cholesterol Concentrations |
|||||||
|---|---|---|---|---|---|---|---|
| Between Assay (n = 10) |
Within Assay (n = 10) |
||||||
| Lipoproteins | Mean (mmol/l) | SD (mmol/l) | CV (%) | Mean (mmol/l) | SD (mmol/l) | CV (%) | |
| Subject 5 | HDL | 2.07 | 0.012 | 0.57 | 2.08 | 0.006 | 0.29 |
| LDL | 2.88 | 0.038 | 1.31 | 2.90 | 0.018 | 0.64 | |
| IDL | 0.33 | 0.019 | 5.77 | 0.31 | 0.011 | 3.47 | |
| VLDL | 0.14 | 0.004 | 2.87 | 0.14 | 0.002 | 1.42 | |
| Chylomicrons | 0.00 | — | — | 0.00 | — | — | |
| Lp(a) | 0.02 | 0.002 | 8.85 | 0.02 | 0.001 | 6.40 | |
| Total | 5.45 | 0.032 | 0.59 | 5.45 | 0.012 | 0.22 | |
| Subject 6 | HDL | 2.82 | 0.122 | 4.32 | 2.87 | 0.022 | 0.75 |
| LDL | 4.90 | 0.194 | 3.96 | 4.93 | 0.061 | 1.24 | |
| IDL | 0.44 | 0.053 | 11.99 | 0.51 | 0.021 | 4.11 | |
| VLDL | 0.22 | 0.021 | 9.73 | 0.23 | 0.028 | 11.86 | |
| Chylomicrons | 0.03 | 0.003 | 9.68 | 0.03 | 0.002 | 8.25 | |
| Lp(a) | 0.60 | 0.027 | 4.38 | 0.62 | 0.009 | 1.50 | |
| Total | 9.02 | 0.361 | 4.00 | 9.20 | 0.106 | 1.16 | |
TABLE 2.
Precision data for retention times of lipoprotein peaks
| Retention Times |
|||||||
|---|---|---|---|---|---|---|---|
| Between Assay (n = 10) |
Within Assay (n = 10) |
||||||
| Lipoproteins | Mean (min) | SD (min) | CV (%) | Mean (min) | SD (min) | CV (%) | |
| Subject 5 | HDL | 3.41 | 0.032 | 0.93 | 3.40 | 0.0032 | 0.093 |
| LDL | 7.41 | 0.032 | 0.43 | 7.40 | 0.0032 | 0.043 | |
| IDL | 12.31 | 0.032 | 0.26 | 12.30 | 0.0032 | 0.026 | |
| VLDL | 14.71 | 0.032 | 0.22 | 14.70 | 0.0032 | 0.021 | |
| Chylomicrons | — | — | — | — | — | — | |
| Lp(a) | 21.99 | 0.057 | 0.26 | 21.97 | 0.048 | 0.22 | |
| Subject 6 | HDL | 3.49 | 0.032 | 0.91 | 3.50 | 0.032 | 0.090 |
| LDL | 7.62 | 0.079 | 1.04 | 7.70 | 0.016 | 0.21 | |
| IDL | 12.38 | 0.042 | 0.34 | 12.40 | 0.017 | 0.13 | |
| VLDL | 14.77 | 0.048 | 0.33 | 14.80 | 0.0032 | 0.021 | |
| Chylomicrons | 18.40 | 0.067 | 0.36 | 18.42 | 0.035 | 0.19 | |
| Lp(a) | 21.46 | 0.052 | 0.24 | 21.49 | 0.032 | 0.15 | |
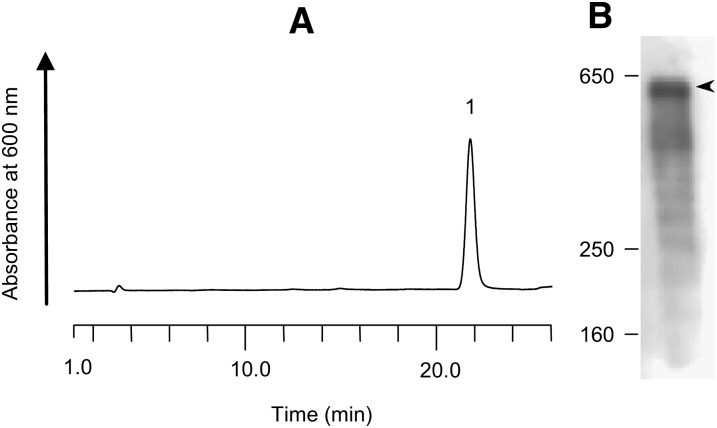
Fig. 4A and B shows a chromatogram of the purified Lp(a) and a Western blot with a goat polyclonal antibody against apo(a). The peak of Lp(a) appeared at 21.60 min (Fig. 4A). The apo(a) protein was detected at 580 kDa (Fig. 4B). The purified Lp(a) was added to the sera of Subjects 5 and 6, and these samples were analyzed by the AEX-HPLC method for estimation of recovery. The recovery rates of Lp(a) were 95–113% (Table 3).
Fig. 4.
Chromatogram and Western blotting pattern of the purified Lp(a) sample for the recovery test. A: The Lp(a) sample was analyzed by HPLC. The retention time was 21.60 min. B: The Lp(a) sample was analyzed by Western blot analysis using a goat polyclonal antibody against apo(a) with a 5% gel of SDS-PAGE. One band of 580 kDa was detected.
TABLE 3.
Recovery data of Lp(a)
| Original Concentration (mmol/l) | Added Amount (mmol/l) | Amount Found (mmol/l) | Recovery Rate (%) | |
|---|---|---|---|---|
| Subject 5 | 0.02 | 0.10 | 0.09 | 95 |
| 0.20 | 0.20 | 100 | ||
| 0.40 | 0.44 | 110 | ||
| Subject 6 | 0.59 | 0.13 | 0.14 | 109 |
| 0.25 | 0.29 | 113 | ||
| 0.51 | 0.55 | 109 |
These results indicate that the cholesterol levels of the six lipoprotein classes can be accurately determined by the AEX-HPLC method.
Comparison of the Lp(a) cholesterol levels estimated by the AEX-HPLC method with Lp(a) protein levels measured by immunoturbidimetric assay
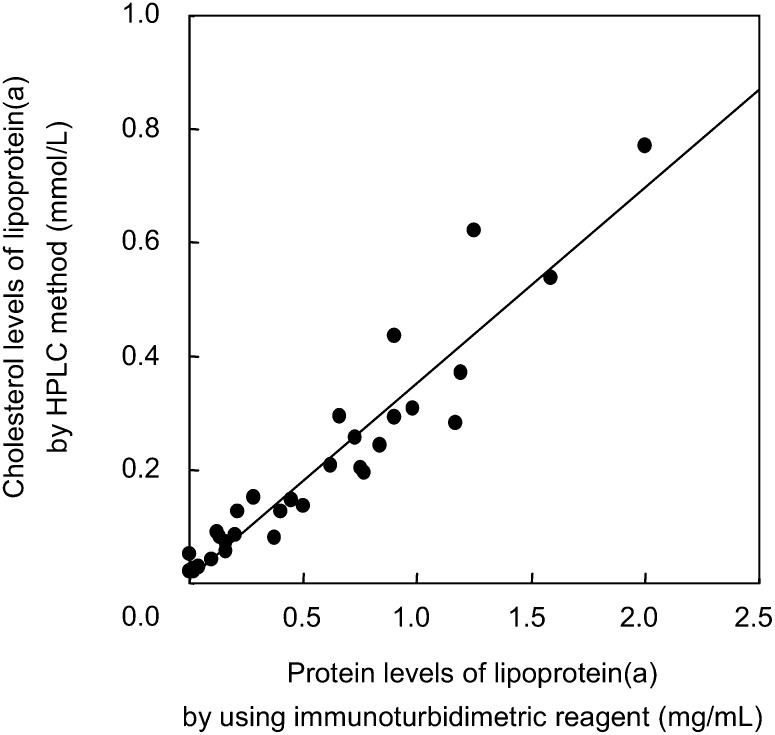
The correlation between the Lp(a) cholesterol levels estimated by the AEX-HPLC method and the Lp(a) protein levels measured by an immunoturbidimetric assay in 17 healthy subjects and 16 dyslipidemic patients are shown in Fig. 5. The correlation coefficient was 0.9503 (P < 0.0001, n = 33). This satisfactory correlation supports the usefulness of the AEX-HPLC method for the determination of cholesterol levels in Lp(a).
Fig. 5.
Correlation of Lp(a) cholesterol values obtained by the HPLC method with the Lp(a) protein values obtained by immunoturbidimetric reagent. Aliquots of 4 µl of 17 healthy sera and 16 dyslipidemic sera were analyzed by the HPLC method, and the cholesterol concentrations of Lp(a) were determined. Lp(a) protein values in the sera were determined using a commercial immunoturbidimetry kit (Sekisui Medical Co.). The linear regression equation, correlation coefficient, and P value were y = 0.3427×+0.0112, r = 0.9503, and P < 0.0001 respectively.
DISCUSSION
In our previously established HPLC method (17) for determining the cholesterol levels of HDL, LDL, IDL, VLDL, and chylomicrons, the inquiry focused on how Lp(a) is eluted and whether Lp(a) can be measured. Therefore, we established another AEX-HPLC method for the determination of cholesterol levels in six major lipoprotein classes, including Lp(a). This report shows a satisfactory separation of the six lipoprotein classes [HDL, LDL, IDL, VLDL, chylomicrons, and Lp(a)] using the AEX-HPLC and good correlation of the Lp(a) cholesterol levels between this HPLC method and commercially available immunoassay (Fig. 5).
The apo(a) protein is heterogeneous in molecular mass because of the different gene sizes. Utermann et al. (2) have shown that apo(a) has six phenotypes with molecular masses ranging from 400–700 kDa: F, B, S1, S2, S3, and S4. Another study reported 11 apo(a) phenotypes with molecular masses ranging from 419–838 kDa (3). Moreover, these previous reports indicated that one or two phenotypes of the apo(a) protein in a single subject can be detected (2, 3). In this article, the apo(a) in the three sera from Subjects 1, 2, and 3 was found to have one phenotype (730 kDa), two phenotypes (710 and 640 kDa), and two phenotypes (660 and 540 kDa), respectively (Fig. 2A1, B1, and C1). All of the apo(a) were found in the Peak 6 [Lp(a)] fraction separated by the AEX-HPLC method (Fig. 2A7, B7, and C7). These results indicate that all of the Lp(a) isoforms containing the different phenotypes of apo(a) may be eluted from the AEX column. The Lp(a) peaks (Peak 6) in Subjects 2 and 3 had slightly different retention times, at 21.75 and 21.42 min, respectively (Fig. 1Bg, Cg). This difference in the retention time may be attributed to the different molecular masses of apo(a) contained in the Lp(a) of Subjects 2 and 3, but this remains to be determined.
Berneis et al. (20) have reported that LDLs are heterogenous in relation to different subtypes of VLDL and IDL in lipoprotein metabolism. The LDL and IDL fractions in sera obtained from Subjects 1 and 2 contained minor peaks (Fig. 1Ac, Ad, Bc, and Bd). The VLDL fraction in sera obtained from Subject 2 contained minor peaks (Fig. 1Be). This finding may be relevant to the heterogeneity of the LDL, IDL, and VLDL particles.
It is generally established that HDL, LDL, IDL, VLDL, chylomicrons, and Lp(a) are composed of apoA-I, A-II, Cs (I-III), D, and E; apo B-100, C-III, and E; apoB-100, Cs (I-III), and E; apoB-100, Cs (I-III), and E; apoB-48, A-I, and A-II; and apo B-100 and apo (a), respectively (1, 27). ApoA-1 and E, apoB-100 and E, apoB-100 and E, apoB-100 and E, and apoB-100 and apo(a) were detected in the HDL, LDL, IDL, VLDL, and Lp(a) fractions using the AEX-HPLC method, respectively (Fig. 2). In Fig. 2B, apoB-48 and A-1 in the chylomicron fraction of Subject 2 were not detected. The failure in detection might be attributed to the low concentration of the lipoprotein or insufficient sensitivity.
In conclusion, this study shows that six major lipoprotein classes in human sera can be separated using AEX-HPLC, and the cholesterol level of each lipoprotein can be determined. We validated the HPLC method by examining its linearity, precision, and recovery and by the correlation of the values obtained by the HPLC method with data obtained by commercially available immunoassay. These results suggest that this improved HPLC method is suitable for the convenient and accurate evaluation of cholesterol levels of six lipoprotein classes, including Lp(a), in human sera.
Acknowledgments
The authors thank Ms. Mika Kon, Ms. Akiko Isobe, and Ms. Erika Suzuki for their excellent technical assistance.
Footnotes
Abbreviations:
- AEX
- anion-exchange
- apo
- apolipoprotein
- Lp(a)
- lipoprotein(a)
- IDL
- intermediate density lipoprotein
- TC
- total cholesterol
- TG
- triglyceride
REFERENCES
- 1.Berglund L., Ramakrishnan R. 2004. Lipoprotein (a): an elusive cardiovascular risk factor. Arterioscler. Thromb. Vasc. Biol. 24: 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utermann G., Menzel H. J., Kraft H. G., Duba H. C., Kemmler H. G., Seitz C. 1987. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J. Clin. Invest. 80: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaubatz J. W., Ghanem K. I., Guevara J., Jr., Nava M. L., Patsch W., Morrisett J. D. 1990. Polymorphic forms of human apolipoprotein(a): inheritance and relationship of their molecular weight to plasma levels of lipoprotein(a). J. Lipid Res. 31: 603–613. [PubMed] [Google Scholar]
- 4.Suk Danik J., Rifai N., Buring J. E., Ridker P. M. 2006. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 296: 1363–1370. [DOI] [PubMed] [Google Scholar]
- 5.Ariyo A. A., Thach C., Tracy R. 2003. Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N. Engl. J. Med. 349: 2108–2115. [DOI] [PubMed] [Google Scholar]
- 6.Jones G. T., van Rij A. M., Cole J., Williams M. J. A., Bateman E. H., Marcovina S. M., Deng M., McCormick S. P. A. 2007. Plasma lipoprotein(a) indicates risk for 4 distinct form of vascular disease. Clin. Chem. 53: 679–685. [DOI] [PubMed] [Google Scholar]
- 7.Wild S. H., Fortmann S. P., Marcovina S. M. 1997. A prospective case-control study of lipoprotein(a) levels and apo(a) size and risk of coronary heart disease in Stanford Five-City Project participants. Arterioscler. Thromb. Vasc. Biol. 17: 239–245. [DOI] [PubMed] [Google Scholar]
- 8.Longenecker J. C., Klag M. J., Marcovina S. M., Powe N. R., Fink N. E., Giaculli F., Coresh J. 2002. Small apolipoprotein(a) size predicts mortality in end stage renal disease: the CHOICE study. Circulation. 106: 2812–2818. [DOI] [PubMed] [Google Scholar]
- 9.Dieplinger B., Lingenhel A., Baumgartner N., Poelz W., Displinger H., Haltmayer M., Kronenberg F., Mueller T. 2007. Increased serum lipoprotein(a) concentrations and low molecular weight phenotypes of apolipoprotein(a) are associated with symptomatic peripheral arterial disease. Clin. Chem. 53: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 10.Longenecker J. C., Klag M. J., Marcovina S. M., Lui Y-M., Jaar B. G., Powe N. R., Fink N. E., Levey A. S., Coresh J. 2005. High lipoprotein(a) levels and small apolipoprotein(a) size prospectively predict cardiovascular events in dialysis patients. J. Am. Soc. Nephrol. 16: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 11.Jurgens G., Taddei-Peters W. C., Koltringer P., Petek W., Chen Q., Greilberger J., Macomber P. F., Butman B. T., Stead A. G., Ransom J. H. 1995. Lipoprotein(a) serum concentration and apolipoprotein(a) phenotype correlate with severity and presence of ischemic cerebrovascular disease. Stroke. 26: 1841–1848. [DOI] [PubMed] [Google Scholar]
- 12.Nauck M., Winker K., Marz W., Wieland H. 1995. Quantitative determination of high-, low-, and very-low-density lipoprotein and lipoprotein(a) by agarose gel electrophoresis and enzymatic cholesterol staining. Clin. Chem. 41: 1761–1767. [PubMed] [Google Scholar]
- 13.Sato I., Taniguchi T., Ishikawa Y., Kusuki M., Hayashi F., Mukai M., Kawano S., Kondo S., Yamashita S., Kumagai S. 2006. The lipoprotein fraction between VLDL and LDL detected by biphasic agarose gel electrophoresis reflects serum remnant lipoprotein and Lp(a) concentrations. J. Atheroscler. Thromb. 13: 55–61. [DOI] [PubMed] [Google Scholar]
- 14.Usui S., Kakuuchi H., Okamoto M., Mizukami Y., Okazaki M. 2002. Differential reactivity of two homogeneous LDL-cholesterol methods to LDL and VLDL subfractions, as demonstrated by ultracentrifugation and HPLC. Clin. Chem. 48: 1946–1954. [PubMed] [Google Scholar]
- 15.Gaubatz J. W., Chari M. V., Nava M. L., Guyton J. R., Morrisett J. D. 1987. Isolation and characterization of the two major apoproteins in human lipoprotein(a). J. Lipid Res. 28: 69–79. [PubMed] [Google Scholar]
- 16.Kostner G. M., Ibovnik A., Holzer H., Grillhofer H. 1999. Preparation of stable fresh frozen primary lipoprotein(a) (Lp(a)) standard. J. Lipid Res. 40: 2255–2263. [PubMed] [Google Scholar]
- 17.Hirowatari Y., Yoshida H., Kurosawa H., Doumitu K., Tada N. 2003. Measurement of cholesterol of major serum lipoprotein classes by anion-exchange HPLC with perchlorate ion-containing eluent. J. Lipid Res. 44: 1404–1412. [DOI] [PubMed] [Google Scholar]
- 18.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumaker V. N., Puppione D. L. 1986. Sequential flotation ultracentrifugation. Methods Enzymol. 128: 155–170. [DOI] [PubMed] [Google Scholar]
- 20.Berneis K. K., Krauss R. M. 2002. Metabolic origns and clinical significance of LDL heterogeneity. J. Lipid Res. 43: 1363–1379. [DOI] [PubMed] [Google Scholar]
- 21.Ensign W., Hill N., Heward C. B. 2006. Disparate LDL phenotypic classification among 4 different methods assessing LDL particle characteristics. Clin. Chem. 52: 1722–1727. [DOI] [PubMed] [Google Scholar]
- 22.Wood R. J., Volek J. S., Liu Y., Shachter N. S., Contois J. H., Fernabdez M. L. 2006. Carbohydrate restriction alters lipoprotein metabolism by modifying VLDL, LDL, and HDL subfraction distribution and size in overweight men. J. Nutr. 136: 384–389. [DOI] [PubMed] [Google Scholar]
- 23.Nestel P., Billington T., Tada N., Nugent P., Fidge N. 1983. Heterogenety of very-low-density lipoprotein metabolism in hyperlipidemic subjects. Metabolism. 32: 810–817. [DOI] [PubMed] [Google Scholar]
- 24.Winocour P. H., Durrington P. N., Bhatnager D., Ishola M., Mackness M., Arrol S. 1991. Influence of early diabetic nephropathy of very low density lipoprotein (VLDL), intermediate density lipoprotein (IDL), and low density lipoprotein (LDL) composition. Atherosclerosis. 89: 49–57. [DOI] [PubMed] [Google Scholar]
- 25.Dachet C., Cavallero E., Martin C., Girardot G., Jacotot B. 1995. Effect of gemfibrozil on the concentration and composition of very low density and low density lipoprotein subfractions in hypertriglyceridemic patients. Atherosclerosis. 113: 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Meyer B. J., Caslake M. J., McConnell M. M., Packard C. J. 2000. Two subpopulations of intermediate density lipoprotein and their relationship to plasma triglyceride and cholesterol levels. Atherosclerosis. 153: 355–362. [DOI] [PubMed] [Google Scholar]
- 27.Gotto A. M., Jr., Pownall H. J., Havel R. J. 1986. Introduction to the plasma lipoproteins. Methods Enzymol. 128: 3–41. [DOI] [PubMed] [Google Scholar]