Abstract
Corneal injury induces an inflammatory reaction and damages the sensory nerves that exert trophic influences in the corneal epithelium. Alterations in normal healing disrupt the integrity and function of the tissue with undesirable consequences, ranging from dry eye and loss of transparency to ulceration and perforation. Lipids play important roles in this complex process. Whereas lipid mediators such as platelet activating factor (PAF) and cyclooxygenease-2 metabolites contribute to tissue damage and neovascularization, other mediators, such as the lipoxygenase (LOX) derivatives from arachidonic acid, 12- and 15-hydroxy/hydroperoxyeicosatetraenoic acids, and lipoxin A4, act as second messengers for epidermal growth factor to promote proliferation and repair. Stimulation of the cornea with pigment epithelial derived factor in the presence of docosahexaenoic acid gives rise to the synthesis of neuroprotectin D1, a derivative of LOX activity, and increases regeneration of corneal nerves. More knowledge about the role that lipids play in corneal wound healing can provide insight into the development of new therapeutic approaches for treating corneal injuries. PAF antagonists, lipoxins, and neuroprotectins can be effective therapeutic tools for maintaining the integrity of the cornea.
Keywords: cornea, docosahexaenoic acid, neuroprotectin D1, platelet activating factor, epidermal growth factor, pigment epithelial derived factor
The cornea, known as the “window of the eye,” has two major functions: to protect the intraocular structures and to refract light. In fact, the cornea accounts for two-thirds of the refractive power of the eye. The most important characteristic of this tissue is its transparency, and the layers of the cornea function in a synchronized way to ensure this transparency.
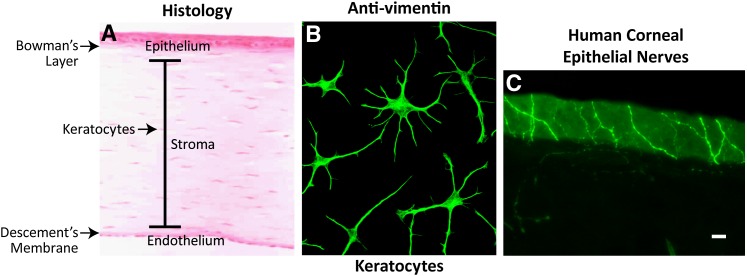
Corneal tissue has three distinct layers, the epithelial, stroma, and endothelial layers, and two acellular regions, the Bowman's layer, which separates the epithelium from the stroma, and the Descemet's membrane, which is between the stroma and the endothelium (Fig. 1A). All three layers have a uniform and consistent arrangement throughout the tissue in order to precisely bend and transmit light, first to the lens and then to the retina.
Fig. 1.
A: Corneal structure showing the different layers. B: Culture keratocytes taken from rabbit corneas and stained with vimentin. The cell projections make contact with each other and form a mesh between the collagen lamella. C: Human corneal nerves stained with β-tubulin penetrating the epithelium with nerve endings at the surface (Courtesy of Drs. Jiucheng He and Haydee E. P. Bazan).
The corneal epithelium is the most outer layer, consisting of five to seven layers of stratified nonkeratinized epithelia. The basal cells have a prominent nucleus, are mitotically active, and adhere to the basement membrane through an adhesion complex that anchors the epithelium to the Bowman's layer. Turnover of epithelial cells occurs every five to seven days by displacement of existing cells, which begin their movement toward the surface to then form two to three layers of wing-shaped cells; the cells then begin terminal differentiation and desquamation. The outer-most layer of the epithelium is in intimate contact with the tear film that keeps the surface moist and free of damage that can result from drying (dry eye). This is important for the formation of high quality images in the retina.
The stroma constitutes 90% of the corneal thickness. It is a highly organized connective tissue composed mainly of collagen type 1 fibrils stacked in orderly sheets, which forms approximately 200 lamellae, and of proteoglycans that maintain the regular spacing between fibrils. The cells of the stroma, known as keratocytes, are flat cells situated between the collagen lamella, and they extend projections toward other keratocytes to form a mesh (Fig. 1A, B). These cells are almost quiescent, but collagen and other extracellular matrix components (ECM) are slowly secreted to maintain the lamella. This regular organization of the stroma is necessary for preserving tissue transparency and maintaining intraocular pressure leading to the alignment of the optic pathway.
The most inner layer of the cornea is the endothelium. It forms a uniform monolayer of hexagonal cells that secrete the Descemet's membrane, which is composed predominantly of collagen IV, glycoproteins, and fibrin. The main function of the endothelium is to regulate water content in the stroma. This is done through metabolic pumps controlled by a Na+/K+-ATPase that actively transports Na+ from the stroma to the anterior chamber and forms a diffusing gradient for water, maintaining a constant stromal hydration. Dysfunction of the endothelium results in stroma edema and loss of transparency.
An important characteristic of the cornea is its heavy innervations; the cornea contains 300–400 times the number of nerve endings per mm2 than the skin. Most are sensory nerves derived from the ciliary nerves of the trigeminal ganglion ophthalmic branch. The nerves penetrate the cornea through the peripheral stroma in a radial pattern, shed their myelin sheet, and then advance to the epithelium to become nerve endings on the epithelial surface (Fig. 1C).
Between the cornea and the sclera is the limbus, a zone of approximately 1 mm that surrounds the cornea. In the limbus reside stem cells for the epithelium as well as vascular elements, which provide nutrients to the avascular cornea.
The cornea is exposed to the external environment and susceptible to injury and infection. Aging, tear deficiency, and injury caused by microorganisms, chemicals, and mechanical damage, including surgery (e.g., laser surgery), can damage the cornea. The outcome depends on the degree of injury. If only the epithelium is damaged, it will regenerate quickly, first by migration of the adjacent cells to cover the defect followed by a proliferative phase to obtain the normal epithelial thickness. At the same time, the keratocytes beneath the injured epithelium undergo apoptosis, and then the surrounding keratocytes are activated. The signaling between epithelial and stromal cells is mediated through cytokines, neuropeptides, growth factors, lipid mediators, and chemokines (1–4). Epithelial cell renewal is essential to preserve the corneal outer layer, which is crucial for preventing infiltration by pathogenic agents. Receptors activated by cytokines and growth factors result in the activation of various cell signaling pathways through the lipid mediators. Activation of these cell signaling pathways are responsible for the processes involved in corneal wound healing (i.e., proliferation, differentiation, migration, and apoptosis). The various cell signaling mechanisms interact with one another through crosstalk to modulate the signaling strength induced by receptor activation (5, 6).
If the stroma has been compromised, however, there will be a stronger activation of keratocytes and transformation of these cells into fibroblasts and myofibroblasts. As a consequence, scar tissue formation and loss of transparency results. Myofibroblasts are characterized by expression of α-smooth muscle actin (α-SMA), the synthesis of ECM components, and the acquisition of contractile properties (7).
Despite the degree of injury, there will be an initial inflammatory response that, in the avascular cornea, is usually characterized by stromal cell infiltration of polymorphonuclear neutrophils (PMNs) arriving from the limbal vessels and by generation of several pro- and anti-inflammatory lipid mediators, which play important roles in the integrity and transparency of the cornea. If inflammation is not resolved, then corneal fibrosis, pigmentation, and neovascularization take place, resulting in corneal scarring and disruption of the blood ocular barrier, which may lead to chronic immune-mediated uveitis (8).
LIPID COMPOSITION
Earlier studies on calf corneas identified phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin as the main phospholipids (9) with the highest content of PC/mg of proteins in the epithelium. Studies on the human cornea report that 34% of the total lipids correspond to neutral lipids (cholesterol, cholesterol esters, and triglycerides), and that there is also an abundance of gangliosides (10%) and sphingolipids (24%) (10). A subsequent study on the phospholipid composition shows that PC, PE, and sphingolipids are also the main components in human corneas (11). The fatty acids predominantly present within the human cornea are oleic acid, palmitic acid, and stearic acid (12). The major acyl group that is esterified to phospholipids in the corneal layers of oxen and rabbits is oleic acid (13, 14). In rabbit corneal epithelia, oleic acid comprises 57% of the total fatty acids of phospholipids, followed by palmitic acid at 18.7%. The percentage of esterified arachidonic acid (AA) in phospholipids is higher in the stroma and endothelium than in the epithelium (14). The higher proportion of esterified AA in the endothelium (11%) may play a role in regulating membrane permeability properties. The tear film contains a lipid layer that functions as a smooth surface for the cornea and retards water evaporation from the eye. This layer consists of a mixture of nonpolar lipids (wax esters, cholesterol, and cholesterol esters) that make up about 60–70% of the layer; the rest is composed of phospholipids, glycolipids, and a small amount of free fatty acids and mono- and di-glycerides (15, 16).
LIPIDS IN CORNEAL PATHOLOGIES
Several pathologies are attributed to lipid depositions. Corneal arcus results from lipid infiltration into the peripheral cornea of both eyes in humans. The lipids are carried by the blood and infiltrate the cornea through the limbal vessels. This pathology worsens with age (arcus senilis) (17, 18). A link has been found between corneal arcus and hyperlipoproteinemia, which is characterized by increased cholesterol as well as LDL and VLDL. Lipids present in the human cornea with arcus are more saturated than those of plasma lipoproteins (12). Cholesterol-rich lipid particles deficient in apolipoprotein (apo)-B accumulates in the cornea. Because of their large size, which precludes their diffusion through the matrix of the cornea, these lipid particles are instead derived from LDL, resulting in loss of apoB and subsequent fusion of apoB-deficient particles (17). Studies in dogs show that phospholipid-rich lipids accumulate in the Descemet's membrane, where cholesterol in both the free and esterified forms accumulates in the stroma (19, 20). Another pathology attributed to lipid deposition is lipid keratopathy. This condition, which is most common in women, can occur in one or both corneas and is often associated with vascularization and hypercholesterolemia. Accumulation of cholesterol esters occurs as droplets deposited in the stroma by lipid-overloaded fibroblasts (20). In dogs with lipid keratopathy, cholesterol levels in the cornea increase 5-fold (21). In general, corneal neovascularization provides an easy route for lipid access to the cornea; conditions such as herpetic keratitis and chemical injuries also can produce severe lipid keratopathy (22). Another disease, granular corneal dystrophy, an autosomal-dominant heritable condition characterized by granular deposits in the cornea, shows an increase in phospholipids but no changes in cholesterol (11).
Lipids also have been involved in the pathology of dry eye. Dry eye, or keratoconjuctivitis sicca, is a disorder that affects the tear film and causes damage to the ocular surface. The three major components of the tear film are the previously-mentioned tear lipids, water, and mucins. Mucins are high-molecular-weight hydrophobic glycoproteins that, by reducing tear surface tension, facilitate the spread of the tear film on the ocular surface. Conjunctiva globet cells are the main source of secreted mucins, followed by the corneal epithelium (23).
Patients with dry eye experience a reduction in mucin on the ocular surface (24). In animal models of dry eye, nanomolar concentrations of topically-applied 15-hydroxy/hydroperoxyeicosatetraenoic acid [15(S)-HETE], a lipoxygenease metabolite derived from AA, protected the cornea from desiccating-induced damage (25) and also increased mucin secretion (26, 27). The molecular mechanisms of 15(S)-HETE action are not understood. In human corneal epithelial cells, 15(S)-HETE induces faster translocation of protein kinase C-α (PKCα) to the plasma membrane when stimulated by growth factors such as epidermal growth factor (EGF) or hepatocyte growth factor (HGF) (28). This translocation requires mitogen-activated protein kinase (MAPK)-ERK1/2 and cytosolic phospholipase A2 (cPLA2) activation. The effect of 15(S)-HETE is specific; for example, note that neither the hydroxyperoxide 15(S)-HpTE nor 5(S)-HETE has an effect on PKCα translocation (28). It has been shown that EGF activation of PKCα is necessary for globlet cell proliferation (29), and therefore increases mucin secretion.
How 15(S)-HETE activates translocation of PKCα has not been clarified. One possibility is that 15(S)-HETE is esterified to phospholipids and induces plasma membrane changes that could facilitate translocation of the kinase. In fact, in rabbit corneal epithelial cells, 12(S)-HETE is rapidly esterified to membrane lipids, mainly phosphatidylinositol (PI) (30). Hydrolysis of PI generates diacylglycerol-containing 12(S)- or 15(S)-HETE that could then activate PKCα.
AA DERIVATIVES INCREASE AFTER CORNEAL INJURY: ITS BIOLOGICAL ACTIONS
Injury to the cornea activates the release of AA as well as its conversion to prostaglandins (PGs), tromboxanes, and lipoxygenase (LOX) derivatives (31–33). Earlier studies have shown eicosanoid production in microsomal fractions of rabbit corneas (34, 35). Two h after in vivo cryogenic lesions that damaged all layers of the rabbit corneas, incubation of individual layers with labeled AA showed a 100% increase in cyclooxygenase (COX) products in the stroma and epithelium. Increased production in the endothelium occurs 5 days after injury (32). The major products are PGF2α, PGE2, and 6-keto PGF1α (the stable metabolite of PGI). Subsequent work confirmed that injury of rabbit corneal endothelial cells increases PGE2 synthesis (36). Pseudomonas aeruginosa infection in mice also induces increased PGE2 synthesis, with a peak at 5 days after infection (37). The main LOX product in the rabbit cornea is 12(S)-HETE (38), which increases more than 600% in the stroma, more than 700% in the epithelium, and almost three times in the endothelium after 2 h of a cryogenic injury (32). In another experiment in which labeled AA was introduced into the rabbit's anterior chamber, four h after the injection most of the AA was incorporated by membrane lipids (31). Two h after applying cryogenic lesions to labeled corneas, there was an increase in COX and LOX products in the anterior chamber concomitant with a decrease of these products in the stroma, suggesting a rapid release of the compounds through the injured endothelium.
These early experiments demonstrate a very active PLA2 activity that releases AA. Recent work has shown that human corneal epithelia express cytosolic PLA2 (cPLA2) α and γ and several secretory PLA2s (sPLA): GIII, GX, and GXIIIA (39). sPLA2s also have been found in tears and are involved in antibacterial protection after corneal injury (40, 41). cPLA2α selectively releases AA and is a good candidate for activation after corneal injury. cPLA2 activation occurs in the epithelium after stimulation by platelet-activated factor (PAF) (42, 43), a bioactive lipid that increases after corneal injury (44).
Our earlier studies also imply the presence of an active COX that releases eicosanoids after injury. The subsequent discovery of COX-2 as an inducible enzyme that is activated by a wide variety of inflammatory stimuli suggests that the increase in prostaglandins after corneal injury is derived from the activation of this particular isoform. In fact, COX-1 is expressed throughout the cornea, whereas COX-2 is predominantly expressed close to the wound following injury by epithelial debridement (45). Several other studies have also confirmed these early findings, and COX-2 expression has been observed in the corneal layers of dogs with keratitis, a common disease in these animals (46), and with herpetic keratitis (47). Experimental photorefractive keratectomy surgery (PRK) in rabbits shows COX-2 expression in the epithelium (48). In addition, topical administration of a specific COX-2 inhibitor reduces stromal swelling after epithelial debridement (49), demonstrating the involvement of COX-2 in the inflammatory reaction after epithelial injury, the initial step in preparation for PRK. Another common refractive surgery, laser in situ keratomileusis (LASIK), which damages the stroma, induces expression of COX-2 in the central epithelium and in the keratocytes adjacent to the wound (48).
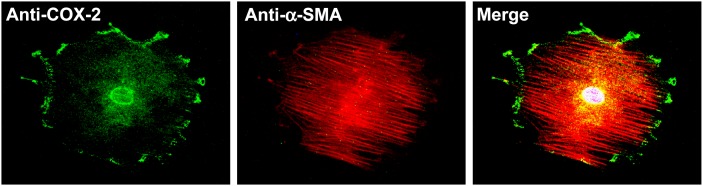
COX-2 expression is also stimulated by another important lipid mediator, PAF (50), which increases in the cornea after injury (44). It has been reported that COX-2 localizes in the endoplasmic reticulum (ER) and the nuclear envelope (51). In isolated rabbit myofibroblasts, we found expression of COX-2 in the nuclei, surrounding the nuclei, possibly in the ER and the plasma membrane (Fig. 2). Because of the expression of COX-2 in the ER and nuclear envelope, these areas are considered to be sites for prostaglandin synthesis because cPLA2 and PGE synthetase are also localized in these regions of stimulated cells (51). However, the presence of COX-2 in the plasma membrane is uncommon. It had been reported that COX-2 and caveolin-1 colocalize in human skin fibroblasts, suggesting that this compartmentalization may play a role in prostaglandin synthesis coupled to sPLA2 (52). There is also colocalization of COX-2 and its downstream enzymes in subcellular organelles. In a rabbit model of alkali burn that had damaged the three layers of the cornea, it has been reported that microsomal prostaglandin E synthetase- 1 colocalizes with COX-2 in α-SMA positive cells, suggesting that myofibroblasts synthesize PGE2 (53).
Fig. 2.
Expression of COX-2 in myofibroblasts in both plasma membrane and nuclear envelope. There is also a positive staining in the area surrounding the nuclei, possibly the ER. Rabbit corneal stromal myofibroblasts were double stained with goat anti-COX-2 polyclonal antibody and mouse anti-α-SMA monoclonal antibody. As a negative control, the primary antibody anti-COX-2 was replaced by normal goat serum (Courtesy of Drs. Jiucheng He and Haydee E. P. Bazan).
The effects of prostaglandins on the cornea are diverse and depend on a particular eicosanoid; thus, secretion of PGD2 can cause epithelial defects and chemotaxis of eosinophils from the conjunctiva (54). PGE2 inhibits endothelial cell proliferation (55) and is important for the maintenance of the polygonal shape of endothelial cells in culture (56). PGF2α does not have this effect; instead, this eicosanoid increases bovine epithelial cell proliferation (57). Topical application of PGE2 agonists to rabbit eyes produces aqueous flare, a sign of inflammation of the anterior chamber (58). Subconjuctival injections of PGE2 resulted in corneal edema, whereas PGD2 or PGF2α had no adverse effects (59). Release of PGE2 after injury contributes to corneal nerve stimulation (60), and COX-2 inhibitors have analgesic effects after corneal surgery (61).
Prostaglandins also exert therapeutic effects. PGF2α-isopropyl ester (latanoprost), a derivative of PGF2α, is commonly used as a potent ocular hypotensive drug in the management of glaucoma (62); however, glaucomatous patients often need to use these types of drugs for extensive periods, and some adverse effects have been reported. In a rabbit model of acute herpetic keratitis, latanoprost increases the severity and recurrence of this infection (63). Several studies have reported reduction of central corneal thickness after short (8 weeks) or prolonged (24 months) treatment with prostaglandin analogs (64). The mechanisms have not been elucidated, but they could be related to activation of metalloproteinases (MMPs), enzymes responsible for degradation of extracellular matrix compounds in the basal membrane. Prostaglandins are activators of MMPs in the cornea and conjunctiva (65, 66).
Our studies have also demonstrated a very active 12-LOX in rabbit corneas that synthesize 12(S)-HETE after injury (31–33, 38). The expression of this enzyme is species-specific; in rabbit and bovine corneal epithelia, the main product is 12(S)-HETE, which is derived from the activity of 12-LOX (38, 67). Human and monkey corneas mainly express 15(S)-HETE, a product of 15-LOX (67), and mouse corneas express a dual 12/15-LOX. Three types of 12-LOXs have been characterized: platelet-type, leukocyte-type, and epidermal 12-LOX. Platelet-type 12-LOX synthesizes 12-hydroperoxyeicosatetraenoic acid [12(S)-HPETE] and 12(S)-HETE from AA, whereas leukocyte-type 12-LOX synthesizes 12(S)-HETE and 15(S)-HETE (68). Each 12-LOX isoenzyme has been detected in various cell types, including smooth muscle cells, keratinocytes, endothelial cells, and tumor cells. In addition, elevated 12-LOX activity has been implicated in hypertension, inflammation, thrombosis, and the development of skin tumors (69). Along with 12/15-LOX, mice corneal epithelia express platelet-type 12-LOX (ALOX 12) and epidermal-type 12-LOX (Aloxe) (70–72). In human corneal epithelia, two 15-LOXs with different subcellular locations have been described: 15-LOX-1 in the cytoplasm and 15-LOX-2 in the cytoplasm and nucleus (73). Although 15-LOX-1 converts AA into 15(S)- and 12(S)-HETE at a rate of 4:1, 15-LOX-2 converts AA exclusively to 15(S)-HETE.
The activity of the 12- and 15-LOX products in the cornea is beginning to be elucidated. The primary oxidation products of these enzymes are reduced into secondary oxidized lipids like HETEs, leukotrienes, lipoxins, and hepoxilins, which act as lipid mediators. In addition to their function as secretogogues for the previously-mentioned mucins, 12(S)- and 15(S)-HETEs in the cornea are intermediates in a proliferative pathway involving EGF (74), which stimulates the synthesis of 12(S)- or 15(S)-HETE, depending on the species. Therefore, EGF released after corneal injury (75–77) increases epithelial proliferation through induction of 12/15-LOX. More recently, lipoxin A4 (LxA4), another product of 12/15-LOX, has been shown to be generated in mouse corneas and stimulate epithelial wound healing (78). In rabbit corneas, LxA4 synthesis is stimulated by EGF (79). The protective effects displayed by 12/15-LOX products explain why, in earlier studies, injection of 12(S)- or 15(S)-HETE into the anterior chamber of different animal models did not produce adverse effects (for review, see reference 80) and why LOX inhibitors delayed epithelial migration and wound closure in rat corneas (81). Another compound with anti-inflammatory properties is aspirin-triggered LxA4, a 15-epimeric LxA4 (epi-LxA4) that is synthesized by COX-2 when the enzyme is acetylated by aspirin (82). LxA4 and epi-LxA4 act through a G-protein coupled receptor (ALX/FPR2, called ALX here). The receptor is expressed in the mouse corneal epithelium (78). Interestingly, we recently found that there is a strong expression of ALX in human corneal endothelial cells and that addition of low epi-LxA4 concentrations stimulate cell proliferation (83). As mentioned, human endothelial cells have a very low mitotic rate, and with aging cell loss occurs. Stress caused by contact lens wear and refractive and intraocular surgery can also alter endothelial density; therefore, epi-LxA4 could be important for protecting the integrity of the endothelium and could be useful in eye banking procedures for storing corneas for transplants after previous incubation with epi-LxA4 (80).
Another activity for metabolizing AA in the corneal epithelium is through the cytochrome P450 monooxygenase (84). Two main compounds are formed: 12-R-hydroxieicosatetraenoic acid [12(R)-HETE], and its degradative product 12-R-hydroxyeicosatrienoic acid [12(R)-HETrE], which is formed by oxidation of the hydroxyl group followed by two keto reductase steps. Unlike LOX products, these derivatives have inflammatory properties. 12(R)- HETrE is a potent inflammatory and angiogenic mediator in hypoxia caused by contact lens wear and chemical- induced injuries in the cornea (85, 86). This mediator also increases in human tears during inflammation (87). The other previously-mentioned compound, 12(R)-HETE, produces swelling when applied to the endothelial side of the cornea by inhibiting Na+/K+-ATPase (88, 89). Contact lenses also induce 8-(R)-hydroxyl-hexadecatrienoic acid [8(R)-HETrE] production in corneal epithelia. These products can diffuse across the stroma and inhibit the endothelial Na+/K+-ATPase, which causes loss of transparency (90).
PAF IS A CENTRAL PLAYER IN THE INFLAMMATORY RESPONSE OF THE CORNEA
A wide variety of responses induced by PAF in corneal cells point toward a central role for this inflammatory mediator. In rabbits, PAF synthesis increases soon after alkali injury occurs, with an almost linear increase that takes place in the first 4 h and continue to increase up to 24 h after injury (44). PAF is a potent chemotactic agent of neutrophils (91) that, in this type of injury, arrives to the cornea around 3 h after injury (92). As such, accumulation of PAF at longer times is due to contributions from corneal tissue as well as inflammatory cell infiltration. Synthesis of PAF is through the remodeling pathway. This pathway was demonstrated when corneas labeled with 3H-acetate and stimulated with a Ca2+ ionosphere synthesized 3H-PAF (44), suggesting the involvement of an acetyl-transferase and a Ca-sensitive PLA2. An active microsomal acetyl transferase activity has been shown in corneal epithelia (93). In addition, 4% of the choline-derived phospholipids of the cornea are alkyl-acyl derivatives, precursors of PAF in the remodeling pathway (94).
PAF actions are mediated by activation of a seven transmembrane receptor (PAF-R) coupled to G proteins (95). The receptor is expressed by corneal epithelial and endothelial cells, stroma keratocytes and myofibroblasts, but not by fibroblasts. This lack of PAF-R expression in fibroblasts could be a mechanism for avoiding excessive degradation of ECM components, one of the actions of PAF as described below. After in vivo and in vitro injury, corneal epithelial cells upregulate PAF-R gene expression, and PAF treatment increases PAF-R mRNA levels 3-fold (96). The induction of PAF-R by its agonist is blocked by PAF-R antagonists. Stimulation of epithelial cells with growth factors that increase during injury, such as HGF, keratinocyte growth factor (KGF) and transforming growth factor (TGF)-β1, β2, and β3, upregulates PAF-R gene expression (96). Therefore, increases in PAF synthesis and upregulation of the receptor in the corneal epithelium constitute a feedback mechanism that maintains a prolonged PAF response after injury. In fact, in rabbit and human corneas where the epithelia have been removed and incubated in an organ culture model in the presence of PAF, significant inhibition of epithelial wound healing occurred (97). The inhibition persisted even when growth factors such as EGF, HGF, or KGF are added to the organ culture.
As mentioned, when inflammation is controlled, corneal epithelial defects heal quickly due to active migration and proliferation of the cells; this helps prevent excessive apoptosis of the keratocytes beneath the uncovered wound that induces a strong response of the stroma cells outside of the wound area and from the corneal limbus (98, 99). This rapid response of the epithelium is important because persistent epithelial defects and recurrent erosions could lead to ulceration and, in extreme cases, perforation. Corneal repair requires remodeling of ECM components, such as collagens, fibronectin, and laminin, by the action of MMPs and urokinase plasminogen activator (uPA). Also, re-epithelization after injury involves attachment of these cells to the provisional basement membrane formed by the aforementioned components of the ECM before migration. In corneas in organ culture where PAF inhibits epithelial wound healing, the proliferation rate of epithelial cells is unaffected; instead, there is a decreased attachment of these cells to fibronectin, laminin, and collagens I and IV (97).
One of the most important actions of PAF is the activation of MMP-1, MMP-9, and uPA (100–103). Activation of these proteolytic enzymes is regulated by tissue inhibitors of MMPs (TIMPs) (104) and plasminogen activator inhibitors (PAIs) (105), which are coexpressed with the enzymes to maintain a balance that promotes normal wound healing. PAF also stimulates the expression of TIMP-1, TIMP-2, and PAI-1 through receptor-mediated mechanisms. However, PAF induction of MMP-9 far exceeds the induction of the inhibitors (106); therefore, excessive degradation of the basement membrane by MMP-9 impairs migration and epithelial wound healing and could lead to corneal ulceration (107). As mentioned, PAF attracts PMNs and other leukocytes to the site of injury, contributing to further increase of various proteases and other degradative enzymes of ECM components. In fact, in an in vivo model of rabbit corneas damaged by alkali burn, there was an increase in PMN infiltration and a persistent epithelial defect that at 4 weeks after injury resulted in 90% perforation of the corneas (108). Treatment with a potent PAF-R antagonist, a 2, 4, 6 trimethyl-1, 4 dihidro pyridine-3, 5 dicarboxylic acid 5-ethyl ester (LAU-0901) (109), reduced PMN infiltration and prevented corneal perforation, demonstrating the powerful action of PAF in the destruction of this tissue when sustained inflammation occurs.
The inflammatory response is also enhanced by PAF action in stimulating AA release (42), induction of COX-2 (50), and increased synthesis of the prostaglandins PGF2α, PGE2, and PGD2. Furthermore, COX-2 induction and prostaglandin synthesis mediate the stimulation of uPA, MMP-1, and MMP-9 activity by PAF in rabbit corneas (65). Interestingly, the release of AA after cPLA2 stimulation induced by PAF does not increase the synthesis of 12(S)-HETE in rabbit corneal epithelia (42, 43). This indicates that 12-LOX activation does not require PAF stimulation and strengthens the findings that LOX derivatives act as protective agents that are not in the cascade of inflammatory lipids contributing to tissue damage. Release of AA by PAF occurs 5 min after stimulation, continues to increase for up to 1 h, and subsequently plateaus for at least 6 h (42). During the first 30 min, most of the AA release is derived from choline, ethanolamine, and inositol phosphoglycerides, suggesting a PLA2 and PLC activation by PAF. The most probable phospholipase activated by PAF is cPLA2 because: a) there is selective release of AA and linoleic acid is not affected by PAF (42), b) PAF activates MAPK-ERK1/2 (110), c) phosphorylation of cPLA2 by ERK1/2 increases the activity of the enzyme (111), d) PAF triggers the influx of Ca2+ into corneal epithelial cells (50) that could also be involved in cPLA2 activation, and e) the corneal epithelium expresses cPLA2α (39).
Another action of PAF is on selective apoptosis of corneal cells. After mechanical removal of the epithelium, as mentioned, the underlying keratocytes enter into apoptosis (99). Incubation of wounded corneas in the presence of PAF significantly increases keratocyte apoptosis (97). In a rabbit model of diffuse lamellar keratitis (DLK), an inflammatory condition produced when cells infiltrate the corneal flap after LASIK, treatment with LAU-0901 decreases cell infiltration and inhibition of keratocyte apoptosis (112). In the rabbit alkali-burn model, there are strong terminal deoxynucleotidyl transferase-mediated-UTP-nick end labeling (TUNEL)-positive keratocytes, which limit the amount of cells that can be converted into fibroblasts and myofibroblasts for repopulating the stroma and promoting wound healing (98). In this model, there is a significant decrease in apoptosis in corneas treated with LAU-0901. These data indicate that PAF plays a central role in stroma keratocyte apoptosis after injury and inhibits the corneal repair process.
Epithelial cells and myofibroblasts, however, are resistant to apoptosis stimulated by PAF, and therefore a preinducer is required. This concept of “priming” has been shown in other cells, such as in PMNs pretreated with TNFα in which PAF significantly increases free radical production, and in the recruitment of eosinophils by PAF in skin pretreated with lipopolysaccharides (LPS) (113, 114). Cultures of rabbit and human corneal epithelial cells exposed to UV radiation prior to treatment with PAF induced a 20% increase in cell apoptosis with an increase in DNA laddering as early as 2 h after PAF treatment, suggesting that the inflammatory mediator accelerates the apoptotic process (115). Activation of cPLA2, release of cytochrome c from mitochondria, and caspase-3 activation are involved in the signaling pathway. Preincubation with PAF antagonists before irradiation and treatment with PAF decreases the ratio of apoptotic cells.
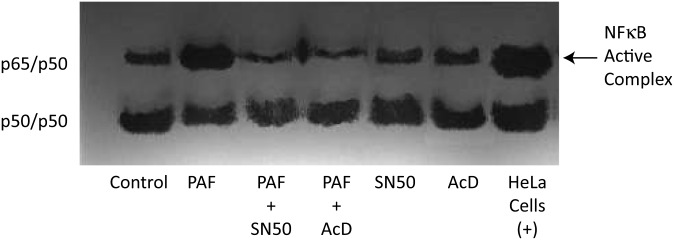
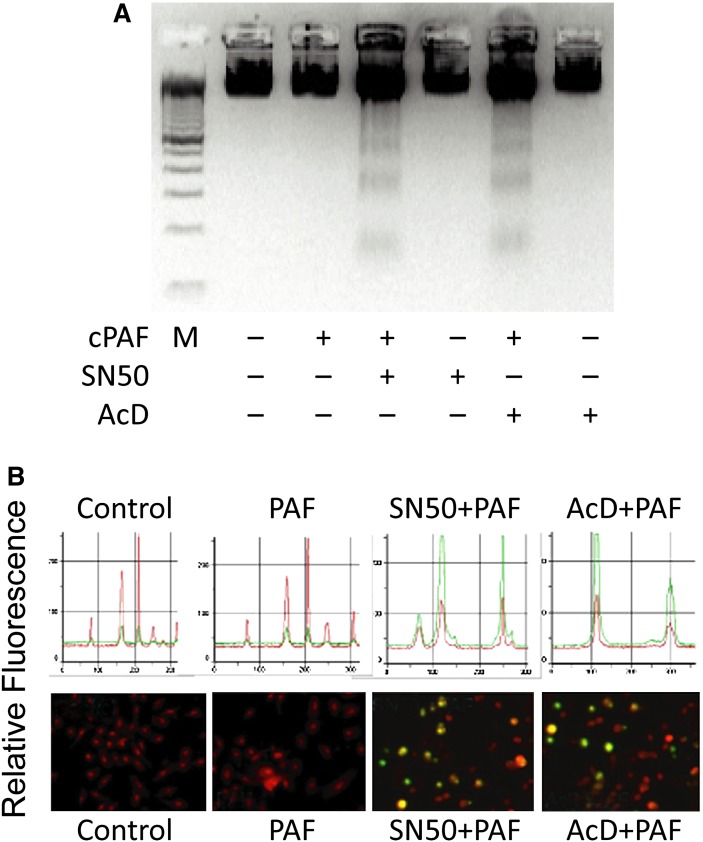
The resistance to apoptosis by PAF involves activation of the transcription factor NF-κB (116). In gel shift assays, increased NF-κB-DNA binding by PAF was inhibited by SN50 and actinomycin D (AcD) (Fig. 3) and primary cultures of human corneal epithelial cells treated with PAF in the presence of NF-κB inhibitor SN50 or with the inhibitor of transcription AcD increased apoptosis (Fig. 4A, B). This suggests that translocation of the transcription factor to the nuclei induced by PAF prevents epithelial cell apoptosis and also demonstrates a regulatory action of NF-κB in PAF-induced apoptosis. Recent experiments have shown that activation of NF-κB is required for gene induction of MMP-9 by PAF in corneal epithelial cells (117).
Fig. 3.
PAF increases activation of NF-κB in human corneal epithelial cells. NF-κB-DNA binding was found in nontreated cells. Increase in the active complex NF-κB-DNA binding was found after 3 h of incubation with PAF. SN50 and AcD block the activation of NF-κB by PAF. HeLA cells were used as a positive control (116).
Fig. 4.
PAF increases apoptosis of human corneal epithelial cells after blocking activation of NF-κB. Cells were treated with the NF-κB inhibitor SN-50 (100µg/ml) or the inhibitor of transcription, actinomycin D (AcD, 5 µg/ml) with or without 500 nM PAF. A: DNA fragmentation was evident 24 h after treatment. No fragmentation was noted in cultures exposed to PAF alone, AcD alone, or SN50 alone. M is a 100bp DNA marker. B: TUNEL staining. The lower part of the figure shows representative fields in each condition. TUNEL positive cells were identified in SN50- and AcD-treated cells stimulated with PAF. Total cells were stained with propidium iodide. The histogram in the upper part of the figure shows the profile of green (apoptotic cells) and red (non- apoptotic cells) nuclear material carried out in similar areas of the field (116).
Treatment of alkali-burned rabbit corneas with LAU-0901 increases the number of myofibroblasts in the limbal area (108). As mentioned, these cells are important for tissue repair; however, myofibroblasts in culture, despite their expression of PAF-R, are also resistant to apoptosis, and after 24 h of treating isolated myofibroblasts with PAF, only 12% of the cells tested TUNEL positive (118). A recent study using TNFα knockout mice in which corneas were alkali burnt also shows an increase in myofibroblasts, compared with wild-type mice (119). TNFα modulates human corneal fibroblast apoptosis through inhibition of NF-κB (120); in addition, myofibroblasts express TNFα-R1, a major signaling receptor for TNFα. We found that in isolated rabbit myofibroblasts, 28% of the cells were apoptotic after 24 h of TNFα treatment. Combining PAF and TNFα produces 77% of apoptotic cells (118). The synergistic increase in apoptosis is through PAF-R stimulation, as apoptosis is inhibited by LAU-0901. The results suggest either: a) the existence of two independent pathways controlling apoptosis, or b) the involvement of TNFα in increasing PAF synthesis, which amplifies the apoptotic response to TNFα. With respect to the second possibility, there are reports that TNFα in other cells stimulates PAF synthesis (121–123); however, we are more inclined to favor the first possibility because our experiments with LAU-0901 showed no effect on apoptosis induced by TNFα (118).
PAF is also an important player in corneal neovascularization, or angiogenesis, which is a condition that causes loss of corneal transparency. Herpes simplex, bacterial keratitis, chemical burns, trauma, and hypoxia due to prolonged contact lens wear are some of the most frequent causes of neovascularization. Experiments involving implantation of slow-release pellets containing PAF in mouse corneas produced a strong angiogenic response (124). There was no PAF-induced neovascularization in PAF-R knockout mice or in animals treated with PAF antagonists. More recently, we found that PAF upregulates the expression of the pro-angiogenic vascular endothelial growth factor (VEGF) and downregulates the expression of the anti-angiogenic trombospodin (TSP) in isolated myofibroblasts (125). Cocultures of myofibroblasts with human umbilical vein endothelial cells (HUVEC) in a collagen gel induce vessel formation that increases in the presence of PAF and is blocked by LAU-0901. These results demonstrate an important role for myofibroblasts in the development of corneal neovascularization and point to the action of PAF in augmenting the angiogenic response. In addition, during corneal neovascularization, degradation of ECM components occurs, which allows endothelial cells to migrate through the basement membrane. PAF is an inducer of MMPs in corneal myofibroblasts and stimulates membrane type 1-MMP (MT1-MMP) and MMP-9 (126), both of which facilitate the migration of vascular endothelial cells.
ω-3 FATTY ACIDS AND THEIR DERIVATIVES ARE IMPORTANT IN THE REPARATIVE PROCESS OF THE CORNEA
Recently, the importance of the ω-3 fatty acids and their derivatives has been revealed. In clinical studies, dietary supplementation of ω-3 fatty acids has shown beneficial effects in decreasing the symptoms of dry eye and improving meibomian gland dysfunction (127, 128). Elevated gene expression of interleukin (IL)-1, IL-6, IL-8, and TNFα in the conjunctival epithelium and a higher tear concentration of IL-1 has been reported in patients with dry eye (129, 130). α-Linolenic acid treatment in a mouse model of dry eye results in decreased corneal and conjunctival expression of IL-1 and TNFα. These cytokines are released early in response to epithelial cell damage. α-Linolenic acid also decreases epithelial damage and reduces leukocyte infiltration (128). These beneficial effects could be due to the action of α-linolenic acid and to the elongation and desaturation products, eicosapentaenoic acid (20:5, EPA) and docosahexaenoic acid (22:6, DHA), or to the recently-discovered derivatives of EPA and DHA, resolvins and neuroprotectins, respectively (82, 131, 132).
Resolvins are derived from EPA, which is converted first into 18-R-hydroxyeicosapentaenoic acid by acetylated COX-2 followed by the action of a 5-LOX and an epoxy intermediate, and then converted into the bioactive 5,12,18-R-trihydroxy-eicosapentaenoic acid called resolvin E1 (RvE1) (for a recent review, see reference 82). RvE1 has been shown to block PMN infiltration and, in various animal models of inflammation, reduce leukocyte-mediated tissue injury. There is an inflammatory component in the pathology of dry eye, and because resolvins are important for counteracting inflammation, we tested the action of RvE1 methyl ester derivatives in a mouse model of dry eye. After 2 and 4 days of topical application of the compounds, there was an increase in tear production as well as epithelial cell density, suggesting that resolvins could play a role in maintaining the integrity of epithelia damaged by dry eye (133). A very recent study has shown that RvE1 also reduces corneal neovascularization by reducing the number of infiltrated neutrophils and macrophages as well as the gene expression of inflammatory cytokines (134).
Corneal surgery, diabetes, chemical burns, and extensive contact lens wear are some of the main causes of damage to corneal sensory nerves. The lacrimal gland responds to neural control and loss of efferent sensory input leads to diminished lacrimal secretion, reduced nutritional support, and production of a dry ocular surface; all of these conditions could result in different degrees of damage, such as haziness leading to reduced transparency and, in the most severe cases, corneal melting and complete opacity (for review, see reference 135). One of the most frequent causes of nerve damage is refractive surgery, a very common procedure for correcting vision. The most commonly used techniques are PRK and LASIK, both of which damage the corneal nerves. Clinical studies using confocal microscopy to measure corneal nerve density following these procedures have reported that it took 2 years for patients to recover 80% of the original nerve density after PRK, whereas after LASIK (in which there is nerve damage at the stromal level) it took almost 5 years to obtain an 80% recovery (136). As a consequence, about 48% of patients reported ocular dryness, and 20% of these patients suffered recurrent epithelial erosions (137).
Nerve growth factor (NGF) stimulates growth, differentiation, and survival of sensory neurons and accelerates wound healing (138). In the cornea, neurotrophins as well as the corresponding Trk-receptors are expressed in epithelial cells and keratocytes, thereby allowing epithelial cells to release NGF, which promotes neurite extension and survival (139). DHA is the precursor of several derivatives. The most studied today is neuroprotectin D1 (NPD1, 10R, 17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z- hexaenoic acid), which has protective bioactivity in oxidative stress challenged retinal pigment epithelial cells (140). NPD1 also inhibits PMN infiltration and pro-inflammatory gene expression in brains damaged by ischemia-reperfusion (132). Synthesis of NPD1 occurs after free DHA is converted by a 15-LOX-1 and further epoxydation (131, 141). In the cornea, DHA is a minor component of membrane phospholipids (14), but synthesis of NPD1 has been reported in mice fed with ω-3 enriched diets (78). In retinal pigment epithelial cells, NGF stimulates NPD1 synthesis (142). Because topical application of NGF has a beneficial effect in early recovery of corneal sensitivity after LASIK (143), a study was done in rabbits in which PRK was performed and corneas treated with DHA, NGF, and DHA plus NGF delivered by collagen shields (144). A significant increase in nerve density was found in animals treated with DHA plus NGF, compared with the other groups and to nontreated animals. Most importantly, there was a significant increase in epithelial cell proliferation after NGF plus DHA, demonstrating a healthy epithelium. The mechanisms of action have not been elucidated, but one possibility is that derivatives of DHA, such as NPD1, are involved.
In retinal pigment epithelial cells, several nurotrophins increase NPD1 synthesis, but PEDF stimulates NPD1 synthesis about 10 times greater than NGF (142). PEDF is expressed in corneal epithelia, so we decided to evaluate whether treatment with PEDF plus DHA after corneal surgery increases NPD1 synthesis concomitant with increased nerve regeneration. In this case, rabbits underwent lamellar keratectomy, which is used frequently in corneal transplants and dissects all the corneal nerves converging radially in the stroma. Animals were treated with PEDF, DHA, or PEDF plus DHA with a collagen shield as before. One week after treatment, increased NPD1 synthesis was observed in corneas treated with DHA, suggesting that PEDF increases after corneal injury and could be used by DHA to synthesize NPD1. A greater increase in NPD1 was seen in corneas treated with DHA in the presence of PEDF (145). Sprouting of stromal nerves was observed in the stroma of animals treated with PEDF plus DHA as soon as 1 week after treatment; by 2 weeks there were nerves appearing in the epithelium. Wholemounts of corneal nerves stained with β-tubulin demonstrated a significant increase in nerve density at 8 weeks in animals treated with DHA plus PEDF. The other treatments did not show differences with respect to nontreated animals. The effects of DHA in synergizing with NGF and PEDF to increase nerve regeneration suggest that some of the DHA derivatives, such as NPD1, play a role in regenerating corneal innervation and could provide new therapies for protecting and repairing the ocular surface.
CONCLUSIONS
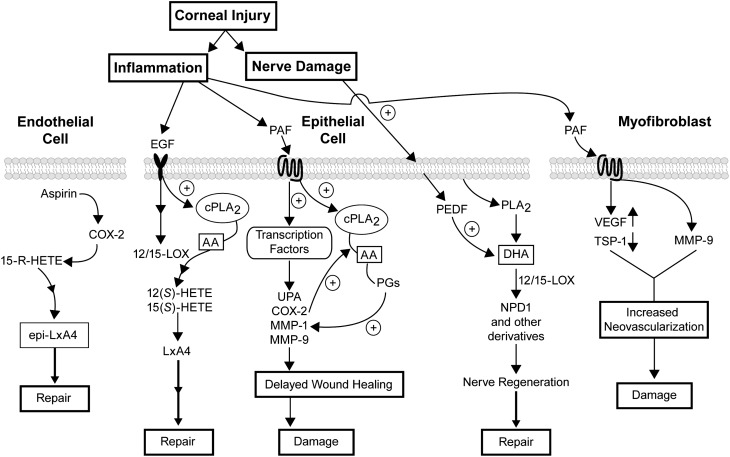
The first responses to corneal insult are inflammation and nerve damage (Fig. 5). Corneal cells respond by releasing growth factors and cytokines that activate receptors and a network of signal transduction pathways. Although changes in lipids after corneal injuries and pathologies have been known for some time, the importance of bioactive lipids in the inflammatory response and in the corneal repair process is just being elucidated. Figure 5 focuses on the action of AA metabolites, PAF and DHA, and on the most recent data obtained from studies conducted in our laboratory. An important conclusion is that there is a balance between pro- and anti-inflammatory lipids and that an uneven response will determine if damage or repair occurs.
Fig. 5.
Schematic diagram of the lipid action in corneal damage and repair. Injury to the cornea produces inflammation and damage to the sensory nerves. During sustained inflammation, PAF activation in the epithelium stimulates expression of COX-2, MMPs, and uPA and delays wound healing. PAF by itself does not have an effect on apoptosis in epithelial cells; it instead activates NF-κB, which is required for the gene expression of MMP-9 (117). It also increases VEGF and MMP-9 and decreases TSP-1 expression in myofibroblasts promoting vessel formation. In epithelial cells, however, increased synthesis of LxA4 after EGF stimulation of 12- or 15-LOX and transcellular reaction with products of the 5-LOX of inflammatory cells (e.g., LTA4) counter-arrest PAF damage, thereby promoting epithelial wound healing. In endothelial cells, epi-LxA4 stimulates proliferation and repair. Note that our experiments suggest two different pools of AA are activated after injury; PAF did not stimulate synthesis of LOX metabolites. Our results also show that PEDF in conjunction with DHA stimulates the synthesis of NPD1 and augments nerve regeneration. NPD1 and/or other 12/15-LOX metabolites of DHA could have a neuroprotective effect that may decrease the incidence of neurotrophic keratitis.
The cells of the cornea respond to injury by releasing AA and PAF from membrane lipids and converting AA to eicosanoids. If inflammation persists, cells that infiltrate the cornea amplify the response, increasing the activation of MMPs and COX-2 derivatives. This resulted in delayed wound healing and induction of epithelial damage and ulceration, thereby increasing the expression of angiogenic factors in stromal myofibroblasts and promoting neovascularization.
As a consequence of injury, growth factors are also released that activate 12/15-LOX, promoting LxA4 synthesis from AA and NPD1 synthesis from DHA, thus potentiating repair and nerve regeneration. In endothelial cells, epi-LxA4 promotes proliferation and could be useful for the preservation of the endothelium during prolonged periods of corneal storage for transplants.
The discovery of new lipid mediators and their actions in counteracting damage to corneal tissue has opened new avenues for future development of more effective drugs for treating injuries and pathologies that affect the cornea.
Footnotes
Abbreviations:
- 12- and 15-HETE/HPETE
- 12- and 15-hydroxy/hydroperoxyeicosatetraenoic acids
- α-SMA
- α-smooth muscle actin
- AA
- arachidonic acid
- AcD
- actinomycin D
- ALX
- lipoxin receptor
- apo
- apolipoprotein
- cPLA2
- cytosolic phospholipase A2
- COX-2
- cyclooxygenease-2
- DHA
- docosahexaenoic acid
- ECM
- extracellular matrix
- EGF
- epidermal growth factor
- EPA
- eicosapentaenoic acid
- ER
- endoplasmic reticulum
- HGF
- hepatocyte growth factor
- HUVEC
- human umbilical vein endothelial cells
- KGF
- keratinocyte growth factor
- LASIK
- laser in situ keratomileusis
- LOX
- lipoxygenase
- LxA4
- lipoxin A4
- MAPK
- mitogen-activated protein kinase
- MMP
- metaloproteinase
- NGF
- nerve growth factor
- NPD1
- neuroprotectin D1
- PEDF
- pigment epithelial derived factor
- PAF
- platelet activating factor
- PAF-R
- platelet activating factor receptor
- PAI
- plasminogen activator inhibitor
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- prostaglandin
- PKC
- protein kinase C
- PMN
- polymorphonuclear neutrophil
- PRK
- photorefractive keratectomy
- RvE1
- resolvin E1
- TGF
- transforming growth factor
- TIMP
- tissue inhibitor of metalloproteinases
- TNFα
- tumor necrosis factor-α
- TSP
- trombospodin
- uPA
- urokinase plasminogen activator
- VEGF
- vascular endothelial growth factor
The authors’ work described here was supported by the National Institutes of Health, National Eye Institute grants R01 EY004928 and R01 EY019465, and by a Translational Research Initiative grant from the Louisiana State University Health Sciences Center, New Orleans. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Wilson S. E., Liu J. J., Mohan R. R. 1999. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 18: 293–309. [DOI] [PubMed] [Google Scholar]
- 2.Imanishi J., Kamiyama K., Iguchi I., Kita M., Sotozono C., Kinoshita S. 2000. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 19: 113–129. [DOI] [PubMed] [Google Scholar]
- 3.Gabison E. E., Huet E., Baudouin C., Menashi S. 2009. Direct epithelial-stromal interaction in corneal wound healing: role of EMMPRIN/CD147 in MMPs induction and beyond. Prog. Retin. Eye Res. 28: 19–33. [DOI] [PubMed] [Google Scholar]
- 4.Klenkler B., Sheardown H. 2004. Growth factors in the anterior segment: role in tissue maintenance, wound healing and ocular pathology. Exp. Eye Res. 79: 677–688. [DOI] [PubMed] [Google Scholar]
- 5.Sharma G. D., He J., Bazan H. E. 2003. p38 and Erk1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of crosstalk activation between MAP kinase cascades. J. Biol. Chem. 278: 21989–21997. [DOI] [PubMed] [Google Scholar]
- 6.Kakazu A., Sharma G. D., Bazan H. E. 2008. Association of protein tyrosine phosphatases (PTPs)-1B with c-Met receptor and modulation of corneal epithelial wound healing. Invest. Ophthalmol. Vis. Sci. 48: 2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jester J. V., Petroll W. M., Cavanagh H. D. 1999. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog. Retin. Eye Res. 18: 311–356. [DOI] [PubMed] [Google Scholar]
- 8.Robbins S. L., Cotran R. M., Kumar V., Collins T., Schmitt B. 1999. Acute and chronic inflammation. Robbin's Pathological Basis of Diseases. 6th ed WB Saunders, Philadelphia, PA; 50–88. [Google Scholar]
- 9.Broekhuyse R. M. 1968. Phospolipds in the tissues of the eye. Isolation characterization and quantitative analysis by two-dimensional thin layer chromatography of diacyl and vinyl-ether phospolipids. Biochim. Biophys. Acta. 152: 307–315. [DOI] [PubMed] [Google Scholar]
- 10.Feldman G. L. 1967. Human ocular lipids: their analysis and distribution. Surv. Ophthalmol. 12: 207–243. [PubMed] [Google Scholar]
- 11.Rodrigues M. M., Streeten B. W., Krachmer J. H., Laibson P. R., Salem N., Jr., Passonneau J., Chock S. 1983. Microfibrillar protein and phospholipid in granular corneal dystrophy. Arch. Ophthalmol. 101: 802–810. [DOI] [PubMed] [Google Scholar]
- 12.Tschetter R. T. 1966. Lipid analysis of the human cornea with and without arcus senilis. Arch. Ophthalmol. 76: 403–405. [DOI] [PubMed] [Google Scholar]
- 13.Bartley W., Heyningen V. R., Notton B. M., Renshaw W. A. 1962. Fatty acid composition of lipids present in different parts of the ox eye. Biochem. J. 85: 332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazan H. E., Bazan N. G. 1984. Composition of phospholipids and free fatty acids and incorporation of labeled arachidonic acid in rabbit cornea. Comparison of epithelium, stroma and endothelium. Curr. Eye Res. 3: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 15.Andrews J. S. 1970. Human tear film lipids. I. Composition of the principal non-polar component. Exp. Eye Res. 10: 223–227. [DOI] [PubMed] [Google Scholar]
- 16.Tiffany J. M. 1987. The lipid secretion of the meibomian glands. Adv. Lipid Res. 22: 1–62. [DOI] [PubMed] [Google Scholar]
- 17.Cogan D. G., Kuwabara T. 1959. Arcus senilis. Its pathology and histochemistry. Arch. Ophthalmol. 61: 553–560. [PubMed] [Google Scholar]
- 18.Broekhuyse R. M. 1976. Lipids in tissues of the eye. XIV. Corneoscleral lipids during ageing and in arcus senilis. Doc. Ophthalmol. 7: 313–321. [Google Scholar]
- 19.Crispin S. 2002. Ocular lipid deposition and hyperlipoproteinaemia. Prog. Retin. Eye Res. 21: 169–224. [DOI] [PubMed] [Google Scholar]
- 20.Gaynor P. M., Zhang W. Y., Salehizadeh B., Pettiford B., Kruth H. S. 1996. Cholesterol accumulation in human cornea: evidence that extracellular cholesteryl ester-rich lipid particles deposit independently of foam cells. J. Lipid Res. 37: 1849–1861. [PubMed] [Google Scholar]
- 21.Crispin S. M. 1984. Lipid keratopathy in the dog. Ph.D. Thesis, University of Edinburgh. [Google Scholar]
- 22.Forsius H. 1961. Lipid keratopathy: a clinical and serum lipid chemical study of sixteen cases. Acta Ophthalmol. (Copenh.). 39: 272–283. [PubMed] [Google Scholar]
- 23.Gipson I., Inatomi T. 1997. Mucin genes expressed by the ocular surface epithelium. Prog. Retin. Eye Res. 16: 81–88. [Google Scholar]
- 24.Danjo Y., Watanabe H., Tisdale A. S., George M., Tsumura T., Abelson M. B., Gipson I. K. 1998. Alteration of mucin in human conjunctival epithelia in dry eye. Invest. Ophthalmol. Vis. Sci. 39: 2602–2609. [PubMed] [Google Scholar]
- 25.Gamache D. A., Wei Z. Y., Weimer L. K., Miller S. T., Spellman J. M., Yanni J. M. 2002. Corneal protection by the ocular mucin secretagogue 15(S)-HETE in a rabbit model of desiccation-induced corneal defect. J. Ocul. Pharmacol. Ther. 18: 349–361. [DOI] [PubMed] [Google Scholar]
- 26.Jackson R. S., Van Dyken S. J., McCartney M. D., Ubels J. L. 2001. The eicosanoid, 15-(S)-HETE, stimulates secretion of mucin-like glycoprotein by the corneal epithelium. Cornea. 20: 516–521. [DOI] [PubMed] [Google Scholar]
- 27.Jumblatt J. E., Cunningham L. T., Yang L., Jumblatt M. M. 2002. Characterization of human ocular mucin secretion mediated by 15(S)-HETE. Cornea. 21: 818–824. [DOI] [PubMed] [Google Scholar]
- 28.Sharma G. D., Ottino P., Bazan N. G., Bazan H. E. 2005. Epidermal and hepatocyte growth factors, but not keratinocyte growth factor, modulate protein kinase Cα translocation to the plasma membrane through 15(S)-hydroxyeicosatetraenoic acid synthesis. J. Biol. Chem. 280: 7917–7924. [DOI] [PubMed] [Google Scholar]
- 29.Shatos M. A., Hodges R. R., Oshi Y., Bair J. A., Zoukhri D., Kublin C., Lashkari K., Dartt D. A. 2009. Role of cPKCα and nPKCϵ in EGF-stimulated goblet cell proliferation. Invest. Ophthalmol. Vis. Sci. 50: 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst J. S., Bazan H. E., Balazy M. 1994. The incorporation and release of 12(S)-hydroxyeicosatetraenoic acid and its major metabolite, 8(S)-hydroxyhexadecatrienoic acid, from rabbit corneal lipids. Exp. Eye Res. 59: 97–105. [DOI] [PubMed] [Google Scholar]
- 31.Bazan H. E. 1987. Corneal injury alters eicosanoid formation in the rabbit anterior segment in vivo. Invest. Ophthalmol. Vis. Sci. 28: 314–319. [PubMed] [Google Scholar]
- 32.Bazan H. E., Birkle D. L., Beuerman R., Bazan N. G. 1985. Cryogenic lesion alters the metabolism of arachidonic acid in rabbit cornea layers. Invest. Ophthalmol. Vis. Sci. 26: 474–480. [PubMed] [Google Scholar]
- 33.Bazan H. E., Birkle D. L., Beuerman R. W., Bazan N. G. 1985. Inflammation-induced stimulation of the synthesis of prostaglandins and lipoxygenase-reaction products in rabbit cornea. Curr. Eye Res. 4: 175–179. [DOI] [PubMed] [Google Scholar]
- 34.Kass M. A., Holmberg N. J. 1979. Prostaglandin and thromboxane synthesis by microsomes of rabbit ocular tissues. Invest. Ophthalmol. Vis. Sci. 18: 166–171. [PubMed] [Google Scholar]
- 35.Bhattacherjee P., Kulkarni P. S., Eakins K. E. 1979. Metabolism of arachidonic acid in rabbit ocular tissues. Invest. Ophthalmol. Vis. Sci. 18: 172–178. [PubMed] [Google Scholar]
- 36.Jumblatt M. M., Willer S. S. 1996. Corneal endothelial repair, regulation of prostaglandin E2 synthesis. Invest. Ophthalmol. Vis. Sci. 37: 1294–1301. [PubMed] [Google Scholar]
- 37.Kernacki K. A., Berk R. S. 1995. Characterization of arachidonic acid metabolism and the polymorphonuclear leukocyte response in mice infected intracorneally with pseudomonas aeruginosa. Invest. Ophthalmol. Vis. Sci. 36: 16–23. [PubMed] [Google Scholar]
- 38.Hurst J. S., Balazy M., Bazan H. E., Bazan N. G. 1991. The epithelium, endothelium, and stroma of the rabbit cornea generate (12S)-hydroxyl eicosatetraenoic acid as the main lipoxygenase metabolite in response to injury. J. Biol. Chem. 266: 6726–6730. [PubMed] [Google Scholar]
- 39.Landreville S., Coulombe S., Carrier P., Gelb M. H., Guérin S. L., Salesse C. 2004. Expression of phospholipases A2 and C in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 45: 3997–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hume E. B., Cole N., Parmar A., Tan M. E., Aliwarga Y., Schubert T., Holden B. A., Willcox M. D. 2004. Secretory phospholipase A2 deposition on contact lenses and its effect on bacterial adhesion. Invest. Ophthalmol. Vis. Sci. 45: 3161–3164. [DOI] [PubMed] [Google Scholar]
- 41.Qu X. D., Lehrer R. I. 1998. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram- positive bacteria in human tears. Infect. Immun. 66: 2791–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurst J. S., Bazan H. E. 1995. Platelet-activating factor preferentially stimulates the phospholipase A2/cyclooxygenase cascade in the rabbit cornea. Curr. Eye Res. 14: 769–775. [DOI] [PubMed] [Google Scholar]
- 43.Hurst J. S., Bazan H. E. 1995. Activation of the phospholipase/cyclooxygenase cascade in the rabbit cornea by platelet-activating factor is challenged by PAF receptor antagonists. Ocul. Pharmacol. Ther. 11: 329–337. [DOI] [PubMed] [Google Scholar]
- 44.Bazan H. E., Reddy S. T., Lin N. 1991. Platelet-activating factor (PAF) accumulation correlates with injury in the cornea. Exp. Eye Res. 52: 481–491. [DOI] [PubMed] [Google Scholar]
- 45.Amico C., Yakimov M., Catania M. V., Giuffrida R., Pistone M., Enea V. 2004. Differential expression of cyclooxygenase-1 and cyclooxygenase-2 in the cornea during wound healing tissue. Cell. 36: 1–12. [DOI] [PubMed] [Google Scholar]
- 46.Sellers R. S., Silverman L., Khan K. N. M. 2004. Cyclooxygenase-2 expression in the cornea of dogs with keratitis. Vet. Pathol. 41: 116–121. [DOI] [PubMed] [Google Scholar]
- 47.Daheshia M., Kanangat S., Rouse B. T. 1998. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp. Eye Res. 67: 619–624. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto T., Saika S., Okada Y., Kawashima Y., Sumioka T., Fujita N., Suzuki Y., Yamanaka A., Ohnishi Y. 2004. Expression of cyclooxygenase-2 in corneal cells after photorefractive keratectomy and laser in situ keratomileusis in rabbit. J. Cataract Refract. Surg. 30: 2612–2617. [DOI] [PubMed] [Google Scholar]
- 49.Karon M. D., Klycle S. D. 2003. Effect of inhibition of inflammatory mediators on trauma-induced stromal edema. Invest. Ophthalmol. Vis. Sci. 44: 2507–2511. [DOI] [PubMed] [Google Scholar]
- 50.Bazan H. E., Tao Y., DeCoster M. A., Bazan N. G. 1997. Platelet activating factor induces cyclooxygenase-2 gene expression in corneal epithelium. Requirement of calcium in the signal transduction pathway. Invest. Ophthalmol. Vis. Sci. 38: 2492–2501. [PubMed] [Google Scholar]
- 51.Spencer A. G., Woods J. W., Arakawa T., Singer I., Smith W. L. 1998. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J. Biol. Chem. 273: 9886–9893. [DOI] [PubMed] [Google Scholar]
- 52.Liou J. Y., Deng W. G., Gilroy D. W., Shyue S. K., Wu K. K. 2001. Colocalization and interaction of cyclooxygenase-2 with caveolin-1 in human fibroblasts. J. Biol. Chem. 276: 34975–34982. [DOI] [PubMed] [Google Scholar]
- 53.Kawamura A., Tatsuguchi A., Ishizaki M., Takahashi H., Fukuda Y. 2008. Expression of microsomal prostaglandin e synthase-1 in fibroblasts of rabbit alkali-burned corneas. Cornea. 27: 1156–1163. [DOI] [PubMed] [Google Scholar]
- 54.Fujishima H., Fukagawa K., Okada N., Takano Y., Tsubota K., Hirai H., Nagata K., Matsumoto K., Saito H. 2005. Prostaglandin D2 induces chemotaxis in eosinophils via its receptor CRTH2 and eosinophils may cause severe ocular inflammation in patients with allergic conjunctivitis. Cornea. 24: S66–S70. [DOI] [PubMed] [Google Scholar]
- 55.Chen K. H., Hsu W. M., Chiang C. C., Li Y. S. 2003. Transforming growth factor-beta2 inhibition of corneal endothelial proliferation mediated by prostaglandin. Curr. Eye Res. 26: 363–370. [DOI] [PubMed] [Google Scholar]
- 56.Neufeld A. H., Jumblatt M. M., Matkin E. D., Raymond G. M. 1986. Maintenance of corneal endothelial cell shape by prostaglandin E2: effects of EGF and indomethacin. Invest. Ophthalmol. Vis. Sci. 27: 1437–1442. [PubMed] [Google Scholar]
- 57.Conconi M. T., Spinazzi R., Tommasini M., Limoli A., Parnigotto P. P. 2001. Prostaglandin F2 alpha can modulate the growth and the differentiation of bovine corneal epithelial cells cultured in vitro. Ann. Anat. 183: 567–573. [DOI] [PubMed] [Google Scholar]
- 58.Kitagawa K., Hayasaka S., Watanabe K., Nagaki Y. 2001. Aqueous flare elevation induced by transcorneal application of highly selective agonists for prostaglandin E2 receptor subtypes in pigmented rabbits: effect of tetramethylpyrazine. Prostaglandins Other Lipid Mediat. 65: 189–198. [DOI] [PubMed] [Google Scholar]
- 59.Ueda M., Sugatani J., Unezaki S., Ito Y., Miki H., Uyama M., Ito S. 1997. Involvement of prostaglandin E2 in rabbit corneal injury by anterior segment ischemia. Prostaglandins Leukot. Essent. Fatty Acids. 57: 285–291. [DOI] [PubMed] [Google Scholar]
- 60.Belmonte C., Acosta M. C., Gallar J. 2004. Neuronal basis of sensation in intact and injured corneas. Exp. Eye Res. 78: 513–525. [DOI] [PubMed] [Google Scholar]
- 61.Chen X., Gallar J., Belmonte C. 1977. Reduction by anti-inflammatory drugs of the response of corneal sensory nerve fibers to chemical irritation. Invest. Ophthalmol. Vis. Sci. 38: 1944–1953. [PubMed] [Google Scholar]
- 62.Bito L. Z. 2001. A new approach to the medical management of glaucoma, from the bench to the clinic, and beyond: the Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 42: 1126–1133. [PubMed] [Google Scholar]
- 63.Kaufman H. E., Varnell E. D., Thompson H. W. 1999. Latanoprost increases the severity and recurrence of herpetic keratitis in the rabbit. Am. J. Ophthalmol. 127: 531–536. [DOI] [PubMed] [Google Scholar]
- 64.Hatanaka M., Vessani R. M., Elias I.R., Morita C., Susanna R., Jr 2009. The effect of prostaglandin analogs and prostamide on central corneal thickness. J. Ocul. Pharmacol. Ther. 25: 51–53. [DOI] [PubMed] [Google Scholar]
- 65.Ottino P., Bazan H. E. 2001. Corneal stimulation of MMP-1, -9 and uPA by platelet-activating factor is mediated by cyclooxygenase-2 metabolites. Curr. Eye Res. 23: 77–85. [DOI] [PubMed] [Google Scholar]
- 66.Mietz H., Schrehardt U. S., Strassfeld C., Krieglstein G. K. 2001. Effect of latanoprost and timolol on the histopathology of the rabbit conjunctiva. Invest. Ophthalmol. Vis. Sci. 42: 679–687. [PubMed] [Google Scholar]
- 67.Liminga M., Hörnsten L., Sprecher H., Oliw E. H. 1994. Arachidonate 15-lipoxygenase in human corneal epithelium and 12- and 15-lipoxygenases in bovine corneal epithelium: comparison with other bovine 12-lipoxygenases. Biochim. Biophys. Acta. 1210: 288–296. [DOI] [PubMed] [Google Scholar]
- 68.Brash A. R. 1999. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274: 23679–23682. [DOI] [PubMed] [Google Scholar]
- 69.Nie D., Tang K., Diglio C., Honn K. V. 2000. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 95: 2304–2311. [PubMed] [Google Scholar]
- 70.Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 71.Chen X. S., Kurre U., Jenkins N. A., Copeland N. G., Funk C. D. 1994. cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J. Biol. Chem. 269: 13979–13987. [PubMed] [Google Scholar]
- 72.McDonnell M., Davis W., Li H., Funk C. D. 2001. Characterization of the murine epidermal 12/15-lipoxygenase. Prostagl Oth Lipid Mediat. 63: 93–107. [DOI] [PubMed] [Google Scholar]
- 73.Chang M. S., Schneider C., Roberts R. L., Shappell S. B., Haselton F. R., Boeglin W. E., Brash A. R. 2005. Detection and subcellular localization of two 15S-lipoxygenases in human cornea. Invest. Ophthalmol. Vis. Sci. 46: 850–856. [DOI] [PubMed] [Google Scholar]
- 74.Ottino P., Taheri F., Bazan H. E. 2003. Growth factor-induced proliferation in corneal epithelial cells is mediated by 12(S)-HETE. Exp. Eye Res. 76: 613–622. [DOI] [PubMed] [Google Scholar]
- 75.Wilson S. E., Chen L., Mohan R. R., Liang Q., Liu J. 1999. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp. Eye Res. 68: 377–397. [DOI] [PubMed] [Google Scholar]
- 76.Schultz G., Khaw P.T., Oxford K., MaCauley S., Setten G. V., Chegini N. 1994. Growth factors and ocular wound healing. Eye. 8: 184–187. [DOI] [PubMed] [Google Scholar]
- 77.Wilson S. E., He Y. G., Weng J., Zieske J. D., Jester J. V., Schultz G. S. 1994. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp. Eye Res. 59: 665–678. [DOI] [PubMed] [Google Scholar]
- 78.Gronert K., Maheshwari N., Khan N., Hassan I. R., Dunn M., Schwartzman M. L. 2005. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 280: 15267–15278. [DOI] [PubMed] [Google Scholar]
- 79.Kenchegowda S., Jackson F. R., Johnson J. A., Bazan N. G., Bazan H. E. 2009. EGF promotes corneal epithelial wound healing through the induction of 12/15 lipoxygenase and synthesis of lipoxin A4. ARVO. 2595: D-1062. [Google Scholar]
- 80.Bazan H. E. 2005. Cellular and molecular events in corneal wound healing: significance of lipid signalling. Exp. Eye Res. 80: 453–463. [DOI] [PubMed] [Google Scholar]
- 81.Gupta A. G., Hirakata A., Proia A. D. 1993. Effect of inhibitors of arachidonic acid metabolism on corneal reepithelialization in the rat. Exp. Eye Res. 56: 701–708. [DOI] [PubMed] [Google Scholar]
- 82.Serhan C. N., Yacoubian S., Yang R. 2008. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol Mech Dis. 3: 279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He J., Bazan N. G., Bazan H. E. 2008. Aspirin-triggered lipoxin A4(Epi LXA4) promotes corneal endothelial proliferation and wound healing. ARVO. 3945: D-693. [Google Scholar]
- 84.Schwartzman M. L., Abraham N. G., Masferrer J., Dunnand M. W., McGiff J. C. 1985. Cytochrome P450 dependent metabolism of arachidonic acid in bovine corneal epithelium. Biochem. Biophys. Res. Commun. 15: 343–351. [DOI] [PubMed] [Google Scholar]
- 85.Conners M. S., Stoltz R. A., Webb S. C., Rosenberg J., Dunn M. W., Abraham N. G., Schwartzman M. L. 1995. A closed eye-contact lens model of corneal inflammation. I. Induction of cytochrome P450 arachidonic acid metabolism. Invest. Ophthalmol. Vis. Sci. 36: 828–840. [PubMed] [Google Scholar]
- 86.Conners M. S., Urbano F., Vafeas C., Stoltz R. A., Dunn M. W., Schwartzman M. L. 1997. Alkali burn-induced synthesis of inflammatory eicosanoids in rabbit corneal epithelium. Invest. Ophthalmol. Vis. Sci. 38: 1963–1971. [PubMed] [Google Scholar]
- 87.Mieyal P. A., Dunn M. W., Schwartzman M. L. 2001. Detection of endogenous 12-hydroxyeicosatrienoic acid in human tear film. Invest. Ophthalmol. Vis. Sci. 42: 328–332. [PubMed] [Google Scholar]
- 88.Edelhauser H. F., Geroski D. H., Woods W. D., Holley G. P., Schwartzman M. L. 1993. Swelling in the isolated perfused cornea induced by 12(R)hydroxyl eicosatetraenoic acid. Invest. Ophthalmol. Vis. Sci. 34: 2953–2961. [PubMed] [Google Scholar]
- 89.Schwartzman M. L., Balazy M., Masferrer J., Abraham N. G., McGiff J. C., Murphy R. C. 1987. 12(R)-hydroxyicosatetraenoic acid: a cytochrome-P450-dependent arachidonate metabolite that inhibits Na+,K+-ATPase in the cornea. Proc. Natl. Acad. Sci. USA. 84: 8125–8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams K. K., Woods W. D., Edelhauser H. F. 1996. Corneal diffusion and metabolism of 12(R)-hydroxyeicosatetraenoic acid (12(R)HETE). Curr. Eye Res. 15: 852–859. [DOI] [PubMed] [Google Scholar]
- 91.Zimmerman G. A., McIntyre T. M., Prescott S. M., Stafforini D. M. 2002. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit. Care Med. 30: S294–S301. [DOI] [PubMed] [Google Scholar]
- 92.Pfister R. R. 1975. The healing of corneal epithelial abrasions in the rabbit: a scanning electron microscope study. Invest. Ophthalmol. Vis. Sci. 14: 648–661. [PubMed] [Google Scholar]
- 93.Hurst J. S., Bazan H. E. 1997. The sensitivity of bovine cornealepithelial lyso-PAF acetyltransferase to cyclooxygenase and lipoxygenase inhibitors is independent of arachidonate metabolites. J. Ocul. Pharmacol. Ther. 13: 415–426. [DOI] [PubMed] [Google Scholar]
- 94.Bazan H. E., Hurst J. S., Bazan N. G. 1994. Differences in the acyl composition of the platelet-activating factor (PAF) precursor and other choline phosphoglycerides of the rabbit retinal rod outer segments and neural retina. Curr. Eye Res. 13: 45–50. [DOI] [PubMed] [Google Scholar]
- 95.Sugimoto T., Tsuchimochi H., McGregor C. G., Mutoh H., Shimizu T., Kurachi Y. 1992. Molecular cloning and characterization of the platelet-activating factor receptor gene expressed in the human heart. Biochem. Biophys. Res. Commun. 189: 617–624. [DOI] [PubMed] [Google Scholar]
- 96.Ma X., Bazan H. E. 2000. Increased platelet-activating factor receptor gene expression by corneal epithelial wound healing. Invest. Ophthalmol. Vis. Sci. 41: 1696–1702. [PubMed] [Google Scholar]
- 97.Chandrasekher G., Ma X., Lallier T. E., Bazan H. E. 2002. Delay of corneal epithelial wound healing and induction of keratocyte apoptosis by platelet-activating factor. Invest. Ophthalmol. Vis. Sci. 43: 1422–1428. [PubMed] [Google Scholar]
- 98.Wilson S. E., Kim W. J. 1998. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest. Ophthalmol. Vis. Sci. 39: 220–226. [PubMed] [Google Scholar]
- 99.Mohan R. R., Hutcheon A. E., Choi R., Hong J., Lee J., Mohan R. R., Jr., Ambrósio R., Zieske J. D., Wilson S. E. 2003. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 76: 71–87. [DOI] [PubMed] [Google Scholar]
- 100.Bazan H. E., Tao Y., Bazan N. G. 1993. Platelet-activating factor induces collagenase expression in corneal epithelial cells. Proc. Natl. Acad. Sci. USA. 90: 8678–8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tao Y., Bazan H. E., Bazan N. G. 1995. Platelet-activating factor induces the expression of metalloproteinases-1 and -9, but not -2 or -3, in the corneal epithelium. Invest. Ophthalmol. Vis. Sci. 36: 345–354. [PubMed] [Google Scholar]
- 102.Tao Y., Bazan H. E., Bazan N. G. 1996. Platelet-activating factor enhances urokinase-type plasminogen activator gene expression in corneal epithelium. Invest. Ophthalmol. Vis. Sci. 37: 2037–2046. [PubMed] [Google Scholar]
- 103.Bazan H. E., Ottino P. 2002. The role of platelet-activating factor in the corneal response to injury. Prog. Retin. Eye Res. 21: 449–464. [DOI] [PubMed] [Google Scholar]
- 104.Moses M. A., Marikovsky M., Harper J. W., Vogt P., Eriksson E., Klagsbrun M., Langer R. 1996. Temporal study of the activity of matrix metalloproteinases and their endogenous inhibitors during wound healing. J. Cell. Biochem. 60: 379–386. [DOI] [PubMed] [Google Scholar]
- 105.Keijer J., Linders M., van Zonneveld A. J., Ehrlich H. J., de Boer J. P., Pannekoek H. 1991. The interaction of plasminogen activator inhibitor 1 with plasminogen activators (tissue-type and urokinase-type) and fibrin: localization of interaction sites and physiologic relevance. Blood. 78: 401–409. [PubMed] [Google Scholar]
- 106.Ottino P., Taheri F., Bazan H. E. 2002. Platelet-activating factor induces the gene expression of TIMP-1, -2, and PAI-1: imbalance between the gene expression of MMP-9 and TIMP-1 and -2. Exp. Eye Res. 74: 393–402. [DOI] [PubMed] [Google Scholar]
- 107.Fini M. E., Cook J. R., Mohan R. 1998. Proteolytic mechanisms in corneal ulceration and repair. Arch. Dermatol. Res. 290: S12–S23. [DOI] [PubMed] [Google Scholar]
- 108.He J., Bazan N. G., Bazan H. E. 2006. Alkali-induced corneal stromal melting prevention by a novel platelet-activating factor receptor antagonist. Arch. Ophthalmol. 124: 70–78. [DOI] [PubMed] [Google Scholar]
- 109.Bazan N. G., Sunkel C., Marcheselli V. L., Alvarez-Builla J. 2003. United States patent US 6566, 359B1, 2, 4, 6-trimethyl-1, 4-dihydropyridine- 3, 5 dicarboxylic acid esters as neuroprotective drugs. Assignee: LSU Health Sciences Center. [Google Scholar]
- 110.Bazan H. E., Varner L. 1997. A mitogen-activated protein kinase (MAPkinase) cascade is stimulated by platelet activating factor (PAF) in corneal epithelium. Curr. Eye Res. 16: 372–379. [DOI] [PubMed] [Google Scholar]
- 111.Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. 1993. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 29: 269–278. [DOI] [PubMed] [Google Scholar]
- 112.Esquenazi S., He J., Bazan H. E., Bazan N. G. 2004. Prevention of experimental diffuse lamellar keratitis using a novel platelet-activating factor receptor antagonist. J. Cataract Refract. Surg. 30: 884–891. [DOI] [PubMed] [Google Scholar]
- 113.Maestre C., Zarco P., Guerrero C. G., González E., Herrero-Beaumont G., Braquet M., Egido J. 1990. Cooperation between tumor necrosis factor (TNF) and platelet-activating factor (PAF) in the inflammatory response. 1990. J. Lipid Mediat. 2(Suppl): S151–S159. [PubMed] [Google Scholar]
- 114.Macari D. M., Teixeira M. M., Ansari T., Jeffery P. K., Hellewell P. G. 1998. Priming and induction of eosinophil trafficking in guinea-pig cutaneous inflammation by tumour necrosis factor alpha. Br. J. Pharmacol. 125: 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma X., Bazan H. E. 2001. Platelet-activating factor (PAF) enhances apoptosis induced by ultraviolet radiation in corneal epithelial cells through cytochrome c-caspase activation. Curr. Eye Res. 23: 326–335. [DOI] [PubMed] [Google Scholar]
- 116.Ma X., Lukiw W. J., Bazan N. G., Bazan H. E. 2001. Platelet activating factor (PAF) activated NF-κB in human corneal epithelial cells causes inhibition of apoptosis. Suppl Invest Ophthalmol Vis Sci. 42: S892. [Google Scholar]
- 117.Taheri F., Haydee H. E. 2007. Platelet-activating factor overturns the transcriptional repressor disposition of Sp1 in the expression of MMP-9 in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 48: 1931–1941. [DOI] [PubMed] [Google Scholar]
- 118.He J., Bazan H. E. 2006. Synergistic effect of platelet-activating factor and tumor necrosis factor-alpha on corneal myofibroblast apoptosis. Invest. Ophthalmol. Vis. Sci. 47: 883–891. [DOI] [PubMed] [Google Scholar]
- 119.Saika S., Ikeda K., Yamanaka O., Flanders K. C., Okada Y., Miyamoto T., Kitano A., Ooshima A., Nakajima Y., Ohnishi Y., et al. 2006. Loss of tumor necrosis factor alpha potentiates transforming growth factor beta-mediated pathogenic tissue response during wound healing. Am. J. Pathol. 168: 1848–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mohan R. R., Mohan R. R., Kim W. J., Wilson S. E. 2000. Modulation of TNF-alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kappaB. Invest. Ophthalmol. Vis. Sci. 41: 1327–1336. [PubMed] [Google Scholar]
- 121.Gountopoulou A., Leondaritis G., Galanopoulou D., Mavri-Vavayanni M. 2008. TNF alpha is a potent inducer of platelet-activating factor synthesis in adipocytes but not in preadipocytes. Differential regulation by PI3K. Cytokine. 41: 174–181. [DOI] [PubMed] [Google Scholar]
- 122.Sun X. M., Hsueh W., Torre-Amione G. 1990. Effects of in vivo ‘priming’ on endotoxin-induced hypotension and tissue injury. The role of PAF and tumor necrosis factor. Am. J. Pathol. 136: 949–956. [PMC free article] [PubMed] [Google Scholar]
- 123.Sun X. M., Hsueh W. 1998. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J. Clin. Invest. 81: 1328–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma X., Ottino P., Bazan H. E., Bazan N. G. 2004. Platelet-activating factor (PAF) induces corneal neovascularization and upregulates vascular endothelial growth factor (VEGF) expression in endothelial cells. Invest. Ophthalmol. Vis. Sci. 45: 2915–2921. [DOI] [PubMed] [Google Scholar]
- 125.Eastlack J. P., He J., Bazan H. E. 2008. The induction of an angiogenic respose by platelet-activating factor (PAF) in corneal myofibroblasts. ARVO. 1476: D-1024. [DOI] [PubMed] [Google Scholar]
- 126.Ottino P., He J., Axelrad T. W., Bazan H. E. 2005. PAF-induced furin and MT1- MMP expression is independent of MMP-2 activation in corneal myofibroblasts. Invest. Ophthalmol. Vis. Sci. 46: 487–496. [DOI] [PubMed] [Google Scholar]
- 127.Aragona P., Bucolo C., Spinella R., Giuffrida S., Ferreri G. 2005. Systemic omega-6 essential fatty acid treatment and PGE1 tear content in Sjogren's syndrome patients. Invest. Ophthalmol. Vis. Sci. 46: 4474–4479. [DOI] [PubMed] [Google Scholar]
- 128.Rashid S., Jin Y., Ecoiffier T., Barabino S., Schaumberg D. A., Dana M. R. 2008. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch. Ophthalmol. 126: 219–225. [DOI] [PubMed] [Google Scholar]
- 129.Pflugfelder S. C., Jones D., Ji Z., Afonso A., Monroy D. 1999. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr. Eye Res. 19: 201–211. [DOI] [PubMed] [Google Scholar]
- 130.Solomon A., Dursun D., Liu Z., Xie Y., Macri A., Pflugfelder S. C. 2001. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest. Ophthalmol. Vis. Sci. 42: 2283–2292. [PubMed] [Google Scholar]
- 131.Hong S., Gronert K., Devchand P., Moussignac R. L., Serhan C. N. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J. Biol. Chem. 278: 14677–14687. [DOI] [PubMed] [Google Scholar]
- 132.Marcheselli V. L., Hong S., Lukiw W. J., Tian X. H., Gronert K., Musto A., Hardy M., Gimenez J. M., Chiang N., Serhan C. N., et al. 2003. Novel docosanoids inhibit brain ischemia-reperfusion- mediated leukocyte infiltration and proinflammatory gene expression. J. Biol. Chem. 278: 43807–43817. [DOI] [PubMed] [Google Scholar]
- 133.Li N., He J., Gjorstrup P., Bazan H. E. 2008. The resolvin E1 analogs, RX-10065 and RX-1005 improve tear production and decrease inflammation in amouse dry eye model. ARVO. 121: A-148. [Google Scholar]
- 134.Jin Y., Arita M., Zhang Q., Saban D. R., Chauhan S. K., Chiang N., Serhan C. N., Dana R. 2009. Novel anti-inflammatory and pro-resolving lipid mediators block inflammatory angiogenesis. Invest. Ophthalmol. Vis. Sci. 50: 4743–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Müller L. J., Marfurt C. F., Kruse F., Tervo T. M. 2003. Corneal nerves: structure, contents and function. Exp. Eye Res. 76: 521–542. [DOI] [PubMed] [Google Scholar]
- 136.Erie J. C., McLaren J. W., Hodge D. O., Bourne W. M. 2005. Recovery of corneal subbasal nerve density after PRK and LASIK. Am. J. Ophthalmol. 140: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 137.Hovanesian J. A., Shah S. S., Maloney R. K. 2001. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J. Cataract Refract. Surg. 27: 577–584. [DOI] [PubMed] [Google Scholar]
- 138.Verge V. M., Merlio J. P., Grondin J., Ernfors P., Persson H., Riopelle R. J., Hökfelt T., Richardson P. M. 1992. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J. Neurosci. 12: 4011–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lambiase A., Manni L., Bonini S., Rama P., Micera A., Aloe L. 2000. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest. Ophthalmol. Vis. Sci. 41: 1063–1069. [PubMed] [Google Scholar]
- 140.Mukherjee P. K., Marcheselli V. L., Serhan C. N., Bazan N. G. 2004. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 101: 8491–8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Calandria J. M., Marcheselli V. L., Mukherjee P. K., Uddin J., Winkler J. W., Petasis N. A., Bazan N. G. 2009. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 284: 17877–17882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mukherjee P. K., Marcheselli V. L., Barreiro S., Hu J., Bok D., Bazan N. G. 2007. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl. Acad. Sci. USA. 104: 13152–13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joo M. J., Yuhan K. R., Hyon J. Y., Lai H., Hose S., Sinha D., O'Brien T. P. 2004. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch. Ophthalmol. 122: 1338–1341. [DOI] [PubMed] [Google Scholar]
- 144.Esquenazi S., Bazan H. E., Bui V., He J., Kim D. B., Bazan N. G. 2005. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 46: 3121–3127. [DOI] [PubMed] [Google Scholar]
- 145.Cortina M. S., He J., Li N., Bazan N. G., Bazan H. E. 2010. PEDF plus DHA induses neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery. Invest. Ophthalmol. Vis. Sci. 51: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]