Abstract
Ectopic expression of caveolin-1 in HEK293 cells enhances FA sequestration in membranes as measured by a pH-sensitive fluorescent dye (1). We hypothesized that sequestration of FA is due to the enrichment of caveolin in the cytosolic leaflet and its ability to facilitate the formation of lipid rafts to buffer high FA levels. Here we show that ectopic expression of caveolin-3 also results in enhanced FA sequestration. To further discriminate the effect that caveolins have on transmembrane FA movement and distribution, we labeled the outer membrane leaflet with fluorescein-phosphatidylethanolamine (FPE), whose emission is quenched by the presence of FA anions. Real-time measurements made with FPE and control experiments with positively charged fatty amines support our hypothesis that caveolins promote localization of FA anions through interactions with basic amino acid residues (lysines and arginines) present at the C termini of caveolins-1 and -3.
Keywords: caveolae, caveolin-1, caveolin-3, cholesterol, lipid raft, transport, fatty acid
Caveolins are small (151-178 amino acids) integral membrane proteins consisting of caveolin-1 and 2, which are abundantly coexpressed in endothelial and adipose cells, and caveolin-3, which is expressed in both skeletal and cardiac muscle. These proteins are required for the formation of caveolae, small (60–100 nm) invaginations of the plasma membrane that are present in particular abundance in the above-mentioned cell types (2). The C termini of caveolin-1 and 3 are postulated to be α helical in structure (3), and they are rich in positively charged residues. This domain of the caveolins lies along the cytosolic surface of the plasma membrane, where it is anchored by palmitoylated cysteine residues (4, 5). Until recently, it was thought that caveolin expression alone was sufficient to form caveolae, but an additional protein called cavin, or polymerase transcription releasing factor (PTRF), has also been shown to be necessary for caveolae formation (6–8). Mice with a genetic deletion of cavin/PTRF have no caveolae in any tissue examined (7).
Caveolins and caveolae have been postulated to be involved in numerous biochemical processes and physiological functions (9), one of which is to modulate lipid movement and storage (as reviewed in Refs. 10 and 11). Mice lacking caveolae in adipocytes due to deletion of caveolin-1 (12) or cavin/PTRF (7) have a reduced fat mass and are hyperlipidemic; i.e., their adipocytes are unable to store fat normally. Although their biochemical roles in droplet function remain unclear, wild-type and mutant caveolins are either associated with or targeted to lipid droplets in a variety of cell types (13–17). It has been suggested that caveolae may be sites for the initiation of triglyceride synthesis in adipocytes (18). Caveolin-1 has been shown to contain FA binding sites (19), but as this protein family is imbedded within the caveolae membranes, FA binding has not been well characterized, as is the case for all membrane bound proteins which bind FA when compared with soluble FA binding proteins (20). Our studies aimed at characterizing FA binding in vivo have shown that caveolin-1 expression modulates FA movement and transmembrane distribution in a model system of HEK293 cells overexpressing this protein (1).
Caveolins/caveolae could play a protective role in cells, particularly adipocytes, by buffering the cell against transient and very high levels of FA or even chronic, high circulating levels of FA that are associated with insulin resistance and diabetes (21). The cytotoxic effects of FA have been termed lipotoxicity and are evident in insulin-producing cells of the pancreas and in liver and muscle (22, 23). The molecular mechanisms of these lipotoxic actions remain unclear. They could represent localized disruptions of membranes (detergent effects) or metabolic effects. They may also be tissue-dependent (24, 25), but at the cellular level, concentrations of FA in the high physiological range cause cell death in pancreatic β cells (26), cardiomyocytes (27), and hepatocytes (28) as well as in commonly used cultured cells, such as Chinese hamster ovary (CHO) fibroblasts (29).
We have previously described the selection of HEK293 cell lines expressing caveolin-1. These cells exhibit slow metabolism of FA into esterified products and are therefore ideal for observing both rapid and slow phases of FA flux into cells. We showed that levels of caveolin-1 above a certain threshold result in an approximately 2-fold increase in FA partitioning to the inner leaflet of the plasma membrane (1). We hypothesized that caveolin expression could lead to the formation of lipid raft domains that are able to sequester higher levels of FA compared with cells lacking these domains. Structural features of caveolin-1, specifically the C terminus which contains numerous arginines and lysines, may serve as a “reservoir” to sequester and stabilize high levels of FA anions. Here we investigate whether caveolin-3 and its similar structural features functions in the same way. To add precision to our fluorescence measurements, we employ a new fluorescent phospholipid to demonstrate that the uptake modulated by caveolins involves movement of FA from the outer leaflet of the plasma membrane as it is sequestered more abundantly into the inner leaflet. As a control experiment, we showed that caveolins do not cause redistribution of positively charged fatty amines in the leaflets of the plasma membrane. Finally, we show that expression of caveolin-1 and 3 enhance TG storage and protect HEK293 cells from the cytotoxic effects of high FA concentrations.
EXPERIMENTAL PROCEDURES
Materials
FuGENE 6 Transfection Reagent was purchased from Roche Diagnostics (Indianapolis, IN). Sphingomyelinase (SMase), methyl-β-cyclodextrin (MβCD), and oleic acid were purchased from Sigma (St. Louis, MO). Dulbecco's modified Eagle's medium (DMEM) was purchased from Mediatech Inc. (Herndon, VA). Fetal bovine serum and calf serum, and the fluorescence probes 2′7′-bis-(2-carboxyethyl)-5-(and6)-carboxyfluorescein (BCECF) acetoxymethylester, and N-(fluoresceinthio-5-carbamoyl)1,2-dihexadecanoyl-sn-glycerol-3-phosphoethanolamine (FPE) triethyammonium salt were obtained from Invitrogen/Molecular Probes (Carlsbad, CA). A stock solution of FPE (1.24 mg/ml; 1.8 mM) was prepared in a mixture of chloroform:methanol as described previously (30). Stocks of oleate and oleoylamine (10 mM) (from Nu Chek Prep, Elysian, NM) were prepared by dissolving them in DMSO. Alternatively, oleic acid (OA) was complexed to fatty acid-free BSA from Sigma (St. Louis, MO) in serum-free DMEM at a molar ratio of 4:1 OA:BSA to an effective concentration of 1.6 mM. The complex was mixed for 3 h until completely cleared of turbidity, then filtered for use as is or diluted in DMEM to 0.4 and 0.8 mM.
Cell lines
HEK293 cells transfected with or without caveolin-1 were established and grown as described (1, 31). Transient transfection of caveolin-3 was done using FuGENE 6 in accordance with the manufacturer's instructions. The caveolin-3 gene was cloned into the PcB7 vector and transfected into HEK293 parental cells. Total cell extracts were prepared from 60 mm plates of HEK293 cells after transient transfection with 1–3 µg of DNA for 24 h. Cells were washed with cold PBS and scraped into 100 µl of lysis buffer (50 mM Tris pH 7.4, 100 mM NaCl, 1% sodium deoxycholate, 4% Nonidet, 0.4% SDS) containing a protease inhibitor mixture from American Bioanalytical (Natick, MA). Soluble protein concentration was determined from the supernatant after microcentrifugation using the BCA protein assay from Pierce (Rockford, IL). Following SDS-PAGE, Western blotting was performed to confirm protein expression. Caveolin-3 expression levels from each transfection were confirmed by Western blotting and found to be comparable. A crude rat skeletal muscle membrane preparation (32) was also analyzed in this way as a control for the level of expression. Primary antibody against caveolin-3 was obtained from BD Transduction Laboratories (Lexington, KY) and was subsequently detected using a secondary antibody conjugated to horseradish peroxidase from Sigma and a chemiluminescence substrate from Perkin Elmer Life Sciences (Boston, MA).
Sphingomyelinase and MβCD treatment of cells
Sphingomyelinase from Bacillus cereus was added at a final concentration of 100 mU/ml for 3 h at 37°C to digest plasma membrane sphingomyelin (SM). To extract plasma membrane cholesterol, cells were incubated with DMEM supplemented with or without 10 mM MβCD for 20 min at 37°C. In each case, the cells were then washed following pretreatment and prepared for fluorescence and cholesterol measurements as previously described (1).
Quantification of sphingomyelin
Lipids from HEK293 cells were extracted by the Folsch method (33). Briefly, the lipid phase of cellular extracts was dried and spotted onto Uniplate Silica Gel G TLC plates from Analtech Inc. (Newark, DE), and the lipids were separated using a chloroform:methanol:water solvent system (65:25:4). Bands were quantified by densitometry using the TotalLab program (nonlinear dynamics).
Labeling cells with FPE and BCECF for fluorescence measurements of FA binding and movement across the membrane
HEK293 cells were grown in DMEM supplemented with 5% fetal bovine serum and 5% calf serum and loaded with the pH probe BCECF as described previously (1). To label the outer leaflet of the plasma membrane, the desired amount of FPE stock was dried under nitrogen, resuspended in DMSO, and added to a separate batch of cells at a final concentration of 9 µM. Cells were incubated for 1 h in the dark, washed in 20 mM MOPS-KRB buffer (pH 7.4), and resuspended in buffer after treatment with 0.1% Trypsin-EDTA for 10 s.
Fluorescence measurements
Fluorescence measurements were made using a Spex® Fluoromax-2 from Jobin Yvon (Edison, NJ). The fluorescence of BCECF was measured using a ratiometric signal of excitation at 439 nm and 505 nm (R = ex505/ex439) with an emission of 535 nm. The emission of FPE was measured at 520 nm after exciting the probe at 490 nm. Experiments monitoring the binding and transmembrane movement of FA into cultured HEK293 cells were carried out at 37°C as described previously (1). Because FPE and BCECF are not compatible for use in the same cells (overlapping excitation wavelengths), separate cell batches were used to measure each probe. Briefly, the fluorescence signals of each probe (ratiometric excitation or single λ emission) were measured as unbound oleate (20 μM final concentration) was added through the injection port above a rapidly stirred cuvette to ensure instantaneous mixing of FA with cells. Vehicle alone, DMSO, or KOH added at 1:500 dilution to the external buffer did not affect intracellular pH or FPE fluorescence. When monitoring the fluorescence of a single probe, changes in fluorescence can be resolved to within 2 s.
For single-cell imaging experiments, the desired amount of FPE stock was dried and resuspended in DMSO for addition to cells that were attached to the bottom of cover slips and grown as described above (4.2 μM final FPE concentration). In the case of BCECF, cells were incubated in culture medium containing 2 mM BCECF-AM. After an incubation time of 30–40 min in the dark with gentle agitation, the culture medium was replaced by MOPS buffer (pH 7.4), and cells were incubated for 40 min at 37°C prior to microscopic imaging. For imaging changes in FPE and BCECF fluorescence in single cells, a concentration of 20 μM OA was added to 2 ml of MOPS buffer in a collagen-coated glass bottom culture dish from MatTek Corporation (Ashland, MA) containing HEK293 cells. Cells were allowed to incubate with FA for several minutes before images were obtained with a two-photon laser scanning confocal microscope (excitation wavelength = 780 nm). Unlike the online fluorescence measurements described above, kinetic measurements with this approach are limited by the inability to mix the FA rapidly with the cells. Therefore, detection of FA binding and transmembrane movement by each probe is limited by the time required for the FA to diffuse through the buffer and reach the cell surface.
Oil Red O staining
Oil Red O staining was performed essentially as described (34). Cells supplemented with FA for the indicated time were washed with PBS and fixed with 3.7% formaldehyde (Sigma) in PBS for 15 min. Cells were stained for 1.5 h in freshly diluted Oil Red O solution (six parts 0.5% Oil Red O stock solution in isopropanol and four parts water). The stain was removed, and cells were washed with water.
Determination of triglyceride content
TG content was determined in cell lysates using a colorimetric assay (Triglycerides reagent) as previously described according to the manufacturer's instructions (Point Scientific Inc., Canton, MI). Cell lysates were prepared using 1% NP-40 in PBS, and results were normalized to 100 µg of total cellular protein.
Cytotoxicity assay
HEK293 cells (∼2.5 × 104) were seeded in 96 well plates and grown overnight to confluence. FA complexed to BSA at different concentrations were then added, and cells were incubated for 48 h. Cell viability was then assessed using a CellTiter 96 AQ One Solution Cell Proliferation Assay according to the manufacturer's instructions (Promega, Madison, WI).
RESULTS
Caveolin-3 modulates transmembrane FA movement
We proposed a mechanistic model whereby caveolin-1 at the inner leaflet of the bilayer enhances the amount of FA anions at this locus by virtue of its multiple positive charges, particularly in the C terminus region, which is expected to lie close to the inner leaflet of the plasma membrane (1). Like caveolin-1, caveolin-3 contains multiple lysine and arginine residues in the same region of its structure and, therefore, would be expected to enhance partitioning of FA into the cytosolic leaflet.
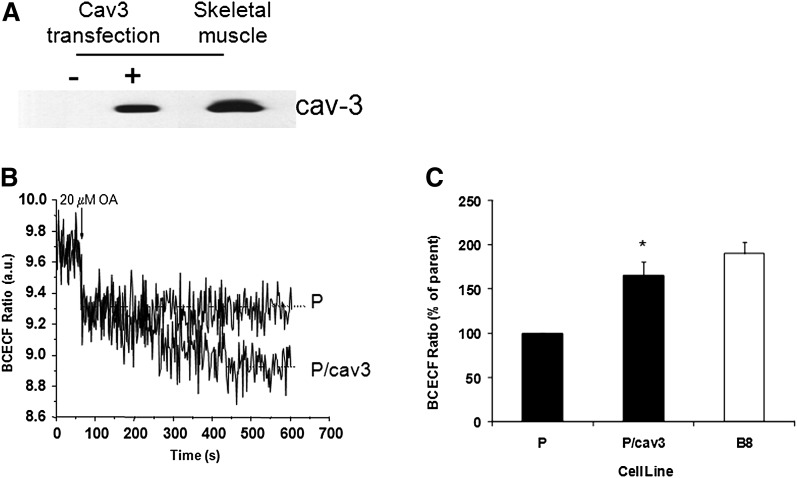
To test this hypothesis, parental HEK293 cells were transiently transfected with caveolin-3 cDNA. After 24 h, cells were harvested for Western blotting and for real-time fluorescence assays of FA uptake by measurement of intracellular pH using BCECF as we have done previously (1). As shown in Fig. 1A, transfected caveolin-3 was expressed in HEK293 cells (P-cav3) at a level that is slightly lower than that found naturally in rat skeletal muscle. FA uptake was then assessed in these and parental cells by monitoring BCECF fluorescence (Fig. 1B). Addition of 20 µM oleic acid (arrow) resulted in a very rapid (t1/2 ≤ 2 s) decrease in fluorescence, which was equal in magnitude in both cell lines. However, an additional slow decrease in pH (minutes) was seen only in cells expressing high levels of caveolin-3 so that the total fluorescence drop of BCECF, which is linearly related to pH (35), was twice that of the parental cells. This altered and unique response is the same as that previously observed in cells expressing high levels of caveolin-1 (1). Fig. 1C shows the total change in BCECF fluorescence from parental and B8 cells in four independent experiments with different cell preparations.
Fig. 1.
Caveolin-3 modulates transmembrane FA movement. A: Parental cells were transiently transfected with caveolin-3 (or not) as outlined in “Experimental Procedures.” Whole-cell extracts were prepared and subjected to SDS-PAGE as was a rat muscle cell membrane preparation. B: The proteins were transferred and subjected to Western blotting, and detection was carried out with HRP-conjugated secondary antibody and chemiluminescence. A representative trace for BCECF fluorescence is shown in both parental (P) and high caveolin-1 expressing (B8) cells. C: The data are presented as the average of triplicates from four independent experiments comparing fluorescence values after 600 s for caveolin-3 transfected (P/cav3) and untransfected cells (P) as well as B8 cells (* = P ≤ 0.003, P/Cav3 versus P). BCECF, 2′7′-bis-(2-carboxyethyl)-5-(and6)-carboxyfluorescein.
The expression of caveolin in HEK293 cells significantly increases the levels of membrane cholesterol (1). Since cholesterol and sphingolipids levels are coordinately regulated (36), the observed effects of the caveolins on transmembrane FA movement could also be due to the elevated levels of these lipid species. Cholesterol can be rapidly extracted from cells by methyl-β-cyclodextrin (MβCD) (37), and SM can be digested with sphingomyelinase. Accordingly, we applied these reagents to parental and high caveolin-1 expressing cells and then monitored the binding of FA to the membrane surface and change in intracellular pH (as in Fig. 1B). Quantitative responses of BCECF in each cell type are given in Table 1. Within 5 min, mβCD removed 56% of the cholesterol in B8 cells but had no significant effect on the rate or magnitude of BCECF fluorescence. There was no effect of mβCD on any parameter in the parental cells. The same result was found after 20 min of MβCD exposure (data not shown). Sphingomyelinase reduced SM levels in B8 cells by 46 ± 9% and did not affect caveolin-1 expression. Additionally, caveolin-1 remained localized only at the cell surface in appreciable abundance, as was the case following MβCD treatment (data not shown). However, net FA movement from the extracellular medium across the plasma membrane was modestly but significantly (∼25%, P ≤ 0.05) decreased as assessed by BCECF fluorescence. These data indicate that SM present in the outer leaflet may play a partial role in modulating the acute uptake of FA into cells, possibly by modulating raft formation, and that the significant increase in FA uptake into B8 cells is largely SM-independent.
TABLE 1.
Effect of MβCD and SMase on HEK293 cell FA movement
| Cholesterol (μg/mg) |
BCECF fluorescence (a.u.) |
|||
|---|---|---|---|---|
| Parental | B8 | Parental | B8 | |
| Vehicle | 24.6 ± 1.2 | 42.6 ± 6.7 | 0.15 ± 0.01 | 0.42 ± 0.03 |
| MβCD | 20.9 ± 2.4 | 18.8 ± 0.6a | 0.14 ± 0.02 | 0.37 ± 0.04 |
| Sphingomyelin (ng/mg) | BCECF fluorescence (a.u.) | |||
| Parental | B8 | Parental | B8 | |
| Vehicle | 25.9 ± 1.9 | 40.2 ± 1.2 | 0.23 ± 0.04 | 0.43 ± 0.05 |
| SMase | 17.1 ± 1.7 | 21.8 ± 3.9 | 0.25 ± 0.04 | 0.32 ± 0.04b |
Total cholesterol, total sphingomyelin, and BCECF fluorescence were measured in parental and high caveolin-1 expressing cells (B8) treated with or without incubation with 10 mM MβCD for 5 min at 37°C. BCECF data are the mean ± SD of 3–4 independent experiments. Statistical significance for cholesterol levels in B8 cells after 5 min incubation with MβCD as compared with untreated cells was determined by Student's t-test. Cholesterol was not reduced in parental cells. There was no significant difference in FA partitioning due to this treatment in either cell preparation as determined by BCECF fluorescence. Similarly, total sphingomyelin and BCECF fluorescence were measured in parental and high caveolin-1 expressing cells (B8) treated for 3 h with 100 mU/ml sphingomyelinase (SMase), which significantly decreased SM levels (P ≤ 0.01) in both parental and B8 cells. However, a SMase-dependent decrease in the BCECF signal, compared with the vehicle, was observed only in the B8 cells and was significantly different from the fluorescence values in the parental cell line.
Abbreviations: BCECF, 2′7′-bis-(2-carboxyethyl)-5-(and6)-carboxyfluorescein; FPE, fluorescein-phosphatidylethanolamine; MβCD, methyl-β-cyclodextrin; SM, sphingomyelin; SMase, sphingomyelinase.
P ≤ 0.06.
P < 0.02.
FPE reports the binding (and loss) of FA anions in the extracellular leaflet of the plasma membrane
Our measurements of FA movement into and across the plasma membrane in the experiments described above detect the arrival of FA at the inner leaflet. Some (∼50%) of the protonated FA lose a proton, which is detected by the pH-sensitive probe BCECF (1, 38, 39). To discriminate the steps of FA binding to the extracellular leaflet of the membrane from its translocation across the lipid bilayer, we performed new measurements with the fluorescent probe FPE. This phospholipid molecule is labeled with a fluorescein group that lies at the polar interface with the aqueous phase and is very sensitive to the membrane surface potential (40, 41). Thus, changes in its fluorescence are directly proportional to the number of FA anions found in the lipid bilayer. Furthermore, when added to the extracellular leaflet, FPE remains in this leaflet because of its inability to translocate to the inner leaflet (42).
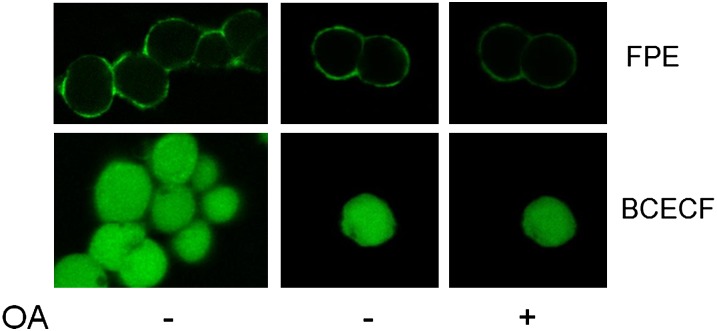
We first examined single cells by confocal microscopy to verify that loading FPE into HEK293 cells resulted in labeling of the plasma membrane and to verify that it is not internalized into the cells (Fig. 2). Representative fields of HEK293 cells labeled with FPE confirmed that the fluorescence is restricted to the cell rim (Fig. 2A), whereas cells with entrapped BCECF exhibited the expected uniform distribution of the probe in the cytosol (Fig. 2B). Furthermore, addition of OA to the external medium resulted in a decrease in fluorescence in the individual cells (Fig. 2C) as well as in populations monitored by online fluorescence measurements (Fig. 3). In these experiments, the addition of exogenous OA also caused a reduction in BCECF fluorescence, which coincides with a reduction in intracellular pH as in Fig. 1.
Fig. 2.
Fluorescence imaging of HEK293 cells demonstrates localization of probes, binding, and transmembrane movement of oleic acid. HEK293 cells were labeled with either FPE (top panel) or loaded with BCECF (bottom panel) prior to the imaging with a two-photon confocal microscope (see “Methods”). The probes were excited at 780 nm and images were captured under a 40× objective (oil immersion) at a pixel resolution of 512 × 512 using an image acquisition time of ∼10 s. Image of multiple cells in a field were obtained before the addition of oleate (left panels) and confirmed the correct localization of each probe. Single cells (center panels) were chosen to monitor the response of each fluorophore to oleate. Addition of 20 μM oleate (right panels) results in a decrease of fluorescence intensity due to fatty acid binding (FPE) and transmembrane diffusion (BCECF). In these two independent experiments (FPE and BCECF), the images were taken 4 min after the addition of oleate. All images were pseudo-colored using the Image J software. BCECF, 2′7′-bis-(2-carboxyethyl)-5-(and6)-carboxyfluorescein; FPE, fluorescein-phosphatidylethanolamine; OA, oleic acid.
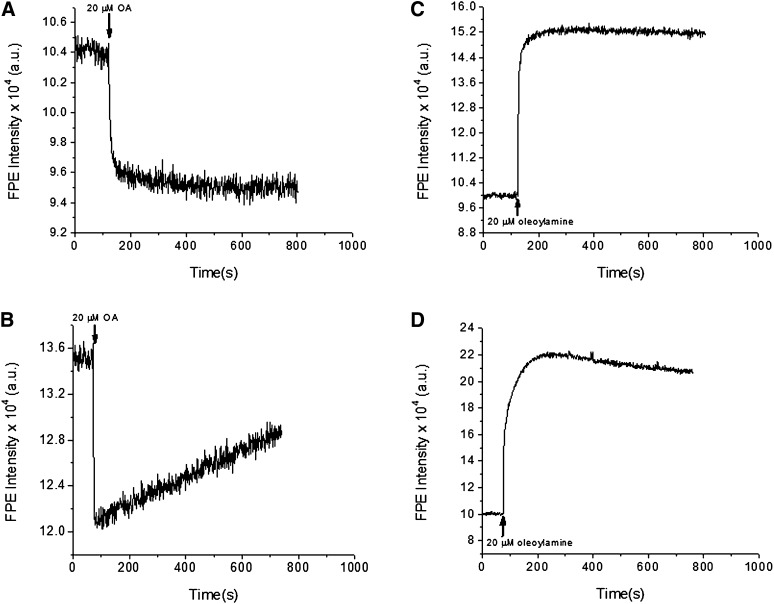
Fig. 3.
The binding and transmembrane movement of fatty acids, but not fatty amines, is modulated by caveolins. The binding of oleic acids (left panels) and fatty amines (right panels) to the cell membrane was assessed by measuring the changes of FPE intensity upon addition of lipid to either parent (top panels) or B8 cells (bottom panels). For all cell types, addition of oleic acid or oleoylamine (20 μM) to a rapidly stirred suspension of cells results in an initial rapid fluorescence change representing the binding of each lipid to the plasma membrane surface. The addition of oleic acid causes a FPE fluorescence decrease while the addition of 20 μM oleoylamine results in a FPE fluorescence increase in both cell types. For both lipids, no secondary slow phase of fluorescence change was observed upon addition to parental cells. Only in the case of oleic acid addition to B8 was a significant fluorescence recovery observed, indicating the sequestering of oleate, but not fatty amines, at the inner leaflet by caveolin. FPE, fluorescein-phosphatidylethanolamine.
Addition of oleic acid to a cuvette containing FPE-loaded parental cells (P) and cells expressing high caveolin-1 (B8) revealed identical rapid decreases (t1/2 ≤ 2 s) in FPE fluorescence in both cell lines (Fig. 3, left panels). After this initial fast phase, a second slower kinetic phase was evident only in the B8 (high-caveolin) cells. Within minutes, the FPE fluorescence increased again by ∼50%, whereas the parental line showed minimal recovery of fluorescence (<5%) during the time of the measurement. This change (i.e., fluorescence recovery) reflects a decrease in concentration of negative charges at the outer leaflet, or more simply, a loss of FA from the outer membrane leaflet with time. The slower time course was nearly identical to that observed for the second slower phase of cytoplasmic acidification detected by BCECF observed here (see Fig. 1) and previously (1).
To test our hypothesis that the positive charges of caveolins mediate these effects on FA uptake by binding the FA anion, we performed parallel experiments with fatty amines, which have a net positive charge in the membrane interface (43). Thus, binding of the amine is expected to cause an increase in FPE fluorescence. These results were indeed observed for C18:1 (oleoyl) amine, as illustrated for the FPE (Fig. 3B, D). In the parent cells, both the FA and the fatty amine showed a fast initial change and no subsequent changes in fluorescence over a period of ten minutes. In the B8 cells, the FA resulted in a fast decrease followed by a slower increase, signifying a slow decrease in FA anions in the extracellular leaflet. Alternatively, caveolin-1 expressing cells showed only a small recovery (fluorescence decrease) of the signal upon addition of the fatty amine, an effect which was not at all comparable to the effect of a much stronger attraction of caveolin for the FA anion than for the positively charged amine.
Caveolin expression enhances FA accumulation and protects against FA-induced lipotoxicity
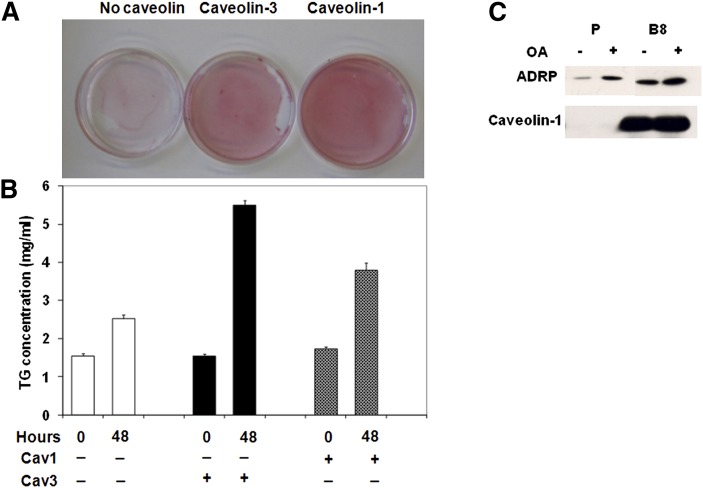
Mice lacking caveolae as a consequence of caveolin-1 (12) or cavin/PTRF (7) gene deletion have smaller fat depots and are hyperlipidemic. These changes suggest that adipocytes from these animals have either compromised triglyceride storage or enhanced constitutive lipolysis or both. Our HEK293 cell model is not suitable for studies of lipolysis because these cells do not express necessary members of the perilipin family (44). However, they are useful for determining the effects of caveolin expression on lipid accumulation. As shown by Oil Red O staining of lipids (Fig. 4A), expression of caveolin-1 and caveolin-3 results in substantially more lipid accumulation than in the parental cells, and quantitative analysis reveals an approximate doubling of triglyceride accumulation (Fig. 4B). Interestingly, we isolated the lipid droplets from the caveolin-1 expressing cells and found a substantial association of this protein in the droplet along with the usual lipid droplet protein ADRP (adipose differentiation-related protein) (Fig. 4C), consistent with data from other cell types (16, 17).
Fig. 4.
Caveolin expression enhances cellular FA accumulation. A: After 48 h with 80 μM oleic acid, cells were fixed and stained with Oil Red O as described in “Experimental Procedures.” B: Triglyceride accumulation was determined and normalized to total cellular protein. The results are from 8 cell preparations with P ≤ 0.001 for high caveolin-1 expressing cells (B8) and caveolin-3 transfected (P/ Cav3) versus untransfected (P) cells at 48 h. C: Lipid droplets were isolated as described by Liu et al. (17). After SDS-PAGE and transfer, they were immunoblotted for the indicated proteins as in Fig 1. ADRP, adipose differentiation-related protein; OA, oleic acid; TG, triglyceride.
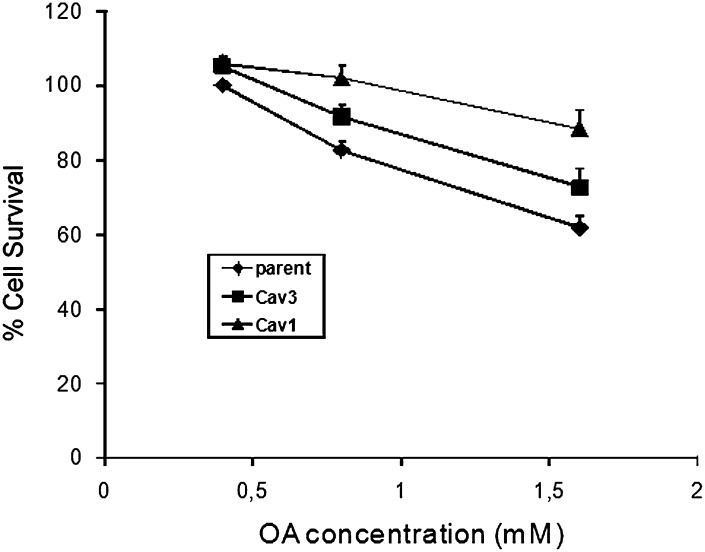
From these novel results, we postulate that caveolin expression may protect cells from lipotoxicity by enabling them to tolerate high levels of FA within the plasma membrane as well as by enhancing storage. Accordingly, we incubated the indicated cell lines with 0.4, 0.8, and 1.6 mM of albumin-bound OA for 48 h, after which cell viability was assessed (Fig. 5). At all three concentrations of FA, cells expressing caveolin 1 were resistant to OA-induced cell death as compared with the parental line. The decrease in survival of parental cells was already significant (P < 0.05) at 0.4 mM albumin-bound OA compared with cells expressing high levels of caveolin-1, and the protective effect of caveolin-1 was more pronounced at 0.8 and 1.6 mM albumin-bound FA. Similarly, caveolin-3 cells were significantly resistant to the cytotoxic effects of 0.8 and 1.6 mM albumin-bound oleate.
Fig. 5.
Caveolins protect cells from FA-induced lipotoxicity. Cells were supplemented with the indicated concentration of oleate bound to BSA in a 4:1 ratio for 48 h. Control wells were incubated in BSA alone. At the end of the incubation time, media was aspirated. Cell survival was assessed as described in “Experimental Procedures.” The data are expressed as % cell survival normalized to unsupplemented cells. The data are the mean ± SD from 3–4 independent experiments, each done in triplicate. P/Cav3 (≠) versus Parent, P ≤ 0.016, 0.8 mM FA, 0.16, P ≤ 0.002. Cav1/B8 (*) versus Parent, P ≤ 0.015 and 0.004, respectively. OA, oleic acid.
DISCUSSION
Dysregulation of adipocyte lipid metabolism leading to chronically elevated levels of free FA is a major contributor to the development of insulin resistance and type 2 diabetes (21). Fundamental biophysical and metabolic processes that can affect circulating FA concentrations include its cellular uptake and transport across the plasma membrane, its incorporation into triglyceride, and its release there from by hormonally regulated lipolysis. Here we address the former two processes in a model cell system (1) without some of the complications inherent in a more complex cell, such as the adipocyte. For example, in addition to high levels of caveolin-1, fat cells highly express CD36 (45) and fatty acid transport proteins (FATP) 1 and 4 (46), proteins whose expression substantially affects adipocyte FA uptake and metabolism. Thus, the effect of caveolins on FA uptake and storage in HEK293 cells can be studied under more defined conditions and in the absence of proteins known to modulate these processes in a variety of different ways.
The very high level of caveolae in adipocytes (11) is suggestive of a role for caveolins/caveolae in FA movement across the plasma membrane. It is further supported by the lipodystrophic lean phenotype of mice lacking caveolin in their adipocytes (7, 12). We previously obtained direct data for FA transport in the plasma membrane with a real-time fluorescence assay that supports a role of caveolin-1 in modulating transmembrane FA movement; we postulated that the C terminus region of this protein provides a juxtamembrane sink of positive charge that can sequester FA and “buffer” the membrane from high concentrations of FA (1). We showed in this study that ectopic caveolin expression increases membrane cholesterol levels, which in turn are known to increase membrane sphingomyelin (36), raising the possibility that these lipids could also be involved in modulating FA movement. However, we ruled out any effect of cholesterol on FA movement and showed that sphingomyelin had a small effect (Table 1). Thus we obtained further support for our original model by three independent means.
First, we showed that caveolin-3 behaved identically to caveolin-1 with regard to FA flux (Fig. 1), as would be predicted from our model. There is a slow recovery of fluorescence change reflecting proton release to the cytosol as FA anions accumulate and are stabilized by caveolin in the inner membrane leaflet.
Second, we used a new assay for assessing transmembrane FA movement using FPE (Figs. 2 and 3) to show that movement from the outer to the inner leaflet is the step affected by caveolin expression. Single-cell imaging (Fig. 2) is an important complement to our fluorescence measurements in cell suspensions because it (i) reveals the behavior of individual cells as opposed to the average of many cells and (ii) shows that the fluorescent probes are properly localized, which can only be assumed in measurements obtained from cell suspensions. Thus our results (Fig. 2) demonstrated both the localization of FPE in the plasma membrane and the expected decrease in fluorescence after addition of OA. The cell suspension data (Fig. 3) show that, following the initial rapid binding of FA at the outer leaflet (and simultaneous diffusion across the bilayer), the slow change in FPE fluorescence reflects a slow decrease in the number of FA anions in the outer leaflet. Thus the second slow phase of transmembrane movement inferred from the internal pH [Fig. 1 and (1)] is due to the slow movement of FA from the outer leaflet to the inner leaflet.
Third, we used a fatty amine, which would be positively charged, and showed that caveolin expression had little or no effect on transmembrane FA movement (Fig. 3) as would be predicted by our model.
What then would be the purpose of caveolins modulating the transmembrane FA flux and increasing their levels in the cytosolic leaflet of the plasma membrane? Two possibilities that come to mind are to regulate FA storage in some way and to protect cells from the cytotoxic effects of high FA concentrations. Here we show data that supports both possibilities (Figs. 4 and 5), and indeed, these two roles are complementary. That is, it has previously been suggested that increased storage of triglycerides, in the case of pancreatic islet cells, protects them from lipotoxicity (47). Here we show that caveolin expression enhances triglyceride storage perhaps by directing caveolins (Fig. 4C) to the lipid droplet, although the mechanism of how this enhances lipid storage is not yet known. As noted previously, caveolins have been seen to associate with lipid droplets in a variety of other cell types (13–16, 48), again by an unknown mechanism. Although caveolae are not generally thought to be highly dynamic structures, we see association of caveolin with lipid droplets in 5 h, the first time that we can see appreciable lipid accumulation in the HEK293 cells (data not shown).
Finally, caveolins protect against FA-induced cell death (Fig. 5). In addition to the above-noted observation that lipid accumulation has this effect, we postulate that caveolins serve as positively charged “buffers” to accommodate high levels of membrane-associated FA anions. Indeed, all aspects of FA dynamics involve abundant proteins that bind them and modulate their concentration and metabolism. In circulation, FA are bound to albumin and in cells, to FABPs. A reason that caveolin expression is so high in adipocytes could be to protect against the very high levels of FA that can be released upon hormonally activated lipolysis, which under certain conditions, can be shown to result in adipocyte autolysis (49). We are in the process of looking at adipocytes that do or don't express caveolins to address this and other issues concerning the role of caveolins/caveolae in adipocyte FA flux and metabolism.
Footnotes
Abbreviations:
- BCECF
- 2′7′-bis-(2-carboxyethyl)-5-(and6)-carboxyfluorescein
- FPE
- fluorescein-phosphatidylethanolamine
- MβCD
- methyl-β-cyclodextrin
- OA
- oleic acid
- PTRF
- polymerase transcription releasing factor
- SM
- sphingomyelin
- SMase
- sphingomyelinase
- TG
- triglyceride
This work was supported by National Institutes of Health Grant DK-56395. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Meshulam T., Simard J. R., Wharton J., Hamilton J. A., Pilch P. F. 2006. Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry. 45: 2882–2893. (PubMed). [DOI] [PubMed] [Google Scholar]
- 2.Parton R. G., Simons K. 2007. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8: 185–194. (PubMed). [DOI] [PubMed] [Google Scholar]
- 3.Spisni E., Tomasi V., Cestaro A., Tosatto S. C. 2005. Structural insights into the function of human caveolin 1. Biochem. Biophys. Res. Commun. 338: 1383–1390. (PubMed). [DOI] [PubMed] [Google Scholar]
- 4.Dietzen D. J., Hastings W. R., Lublin D. M. 1995. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J. Biol. Chem. 270: 6838–6842. (PubMed). [DOI] [PubMed] [Google Scholar]
- 5.Monier S., Dietzen D. J., Hastings W. R., Lublin D. M., Kurzchalia T. V. 1996. Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett. 388: 143–149. (PubMed). [DOI] [PubMed] [Google Scholar]
- 6.Hill M. M., Bastiani M., Luetterforst R., Kirkham M., Kirkham A., Nixon S. J., Walser P., Abankwa D., Oorschot V. M., Martin S., et al. 2008. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 132: 113–124. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L., Brown D., McKee M., Lebrasseur N. K., Yang D., Albrecht K. H., Ravid K., Pilch P. F. 2008. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 8: 310–317. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Pilch P. F. 2008. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 283: 4314–4322. (PubMed). [DOI] [PubMed] [Google Scholar]
- 9.Cohen A. W., Hnasko R., Schubert W., Lisanti M. P. 2004. Role of caveolae and caveolins in health and disease. Physiol. Rev. 84: 1341–1379. (PubMed). [DOI] [PubMed] [Google Scholar]
- 10.Martin S., Parton R. G. 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7: 373–378. (PubMed). [DOI] [PubMed] [Google Scholar]
- 11.Pilch P. F., Souto R. P., Liu L., Jedrychowski M. P., Berg E. A., Costello C. E., Gygi S. P. 2007. Cellular spelunking: exploring adipocyte caveolae. J. Lipid Res. 48: 2103–2111. (PubMed). [DOI] [PubMed] [Google Scholar]
- 12.Razani B., Combs T. P., Wang X. B., Frank P. G., Park D. S., Russell R. G., Li M., Tang B., Jelicks L. A., Scherer P. E., et al. 2002. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 277: 8635–8647. (PubMed). [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto T., Kogo H., Ishiguro K., Tauchi K., Nomura R. 2001. Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J. Cell Biol. 152: 1079–1085. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostermeyer A. G., Paci J. M., Zeng Y., Lublin D. M., Munro S., Brown D. A. 2001. Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J. Cell Biol. 152: 1071–1078. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pol A., Luetterforst R., Lindsay M., Heino S., Ikonen E., Parton R. G. 2001. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J. Cell Biol. 152: 1057–1070. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brasaemle D. L., Dolios G., Shapiro L., Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279: 46835–46842. (PubMed). [DOI] [PubMed] [Google Scholar]
- 17.Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. 2004. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 279: 3787–3792. (PubMed). [DOI] [PubMed] [Google Scholar]
- 18.Ost A., Ortegren U., Gustavsson J., Nystrom F. H., Stralfors P. 2005. Triacylglycerol is synthesized in a specific subclass of caveolae in primary adipocytes. J. Biol. Chem. 280: 5–8. (PubMed). [DOI] [PubMed] [Google Scholar]
- 19.Trigatti B. L., Anderson R. G., Gerber G. E. 1999. Biochem. Biophys. Res. Commun. 255: 34–39. (PubMed). [DOI] [PubMed] [Google Scholar]
- 20.Hamilton J. A. 2004. Fatty acid interactions with proteins: what X-ray crystal and NMR solution structures tell us. Prog. Lipid Res. 43: 177–199. (PubMed). [DOI] [PubMed] [Google Scholar]
- 21.Guilherme A., Virbasius J. V., Puri V., Czech M. P. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unger R. H. 2002. Lipotoxic diseases. Annu. Rev. Med. 53: 319–336. (PubMed). [DOI] [PubMed] [Google Scholar]
- 23.Schaffer J. E. 2003. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 14: 281–287. (PubMed). [DOI] [PubMed] [Google Scholar]
- 24.Hoehn K. L., Hohnen-Behrens C., Cederberg A., Wu L. E., Turner N., Yuasa T., Ebina Y., James D. E. 2008. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7: 421–433. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., et al. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7: 45–56. (PubMed). [DOI] [PubMed] [Google Scholar]
- 26.Shimabukuro M., Zhou Y. T., Lee Y., Unger R. H. 1998. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J. Biol. Chem. 273: 3547–3550. (PubMed). [DOI] [PubMed] [Google Scholar]
- 27.Dyntar D., Eppenberger-Eberhardt M., Maedler K., Pruschy M., Eppenberger H. M., Spinas G. A., Donath M. Y. 2001. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 50: 2105–2113. (PubMed). [DOI] [PubMed] [Google Scholar]
- 28.Wei Y., Wang D., Topczewski F., Pagliassotti M. J. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291: E275–E281. (PubMed). [DOI] [PubMed] [Google Scholar]
- 29.Listenberger L. L., Ory D. S., Schaffer J. E. 2001. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276: 14890–14895. (PubMed). [DOI] [PubMed] [Google Scholar]
- 30.Simard J. R., Pillai B. K., Hamilton J. A. 2008. Fatty acid flip-flop in a model membrane is faster than desorption into the aqueous phase. Biochemistry. 47: 9081–9089. (PubMed). [DOI] [PubMed] [Google Scholar]
- 31.Wharton J., Meshulam T., Vallega G., Pilch P. 2005. Dissociation of insulin receptor expression and signaling from caveolin-1 expression. J. Biol. Chem. 280: 13483–13486. (PubMed). [DOI] [PubMed] [Google Scholar]
- 32.Chao L. C., Zhang Z., Pei L., Saito T., Tontonoz P., Pilch P. F. 2007. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol. Endocrinol. 21: 2152–2163. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. (PubMed). [PubMed] [Google Scholar]
- 34.Zuo Y., Qiang L., Farmer S. R. 2006. Activation of CCAAT/ enhancer-binding protein (C/EBP) alpha expression by C/EBP beta during adipogenesis requires a peroxisome proliferator-activated receptor-gamma-associated repression of HDAC1 at the C/ebp alpha gene promoter. J. Biol. Chem. 281: 7960–7967. (PubMed). [DOI] [PubMed] [Google Scholar]
- 35.Kamp F., Hamilton J. A. 1992. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc. Natl. Acad. Sci. USA. 89: 11367–11370. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridgway N. D. 2000. Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochim. Biophys. Acta. 1484: 129–141. (PubMed). [DOI] [PubMed] [Google Scholar]
- 37.Haynes M. P., Phillips M. C., Rothblat G. H. 2000. Efflux of cholesterol from different cellular pools. Biochemistry. 39: 4508–4517. (PubMed). [DOI] [PubMed] [Google Scholar]
- 38.Guo W., Huang N., Cai J., Xie W., Hamilton J. A. 2006. Fatty acid transport and metabolism in HepG2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G528–G534. (PubMed). [DOI] [PubMed] [Google Scholar]
- 39.Kamp F., Guo W., Souto R., Pilch P. F., Corkey B. E., Hamilton J. A. 2003. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. J. Biol. Chem. 278: 7988–7995. (PubMed). [DOI] [PubMed] [Google Scholar]
- 40.Asawakarn T., Cladera J., O'Shea P. 2001. Effects of the membrane dipole potential on the interaction of saquinavir with phospholipid membranes and plasma membrane receptors of Caco-2 cells. J. Biol. Chem. 276: 38457–38463. (PubMed). [DOI] [PubMed] [Google Scholar]
- 41.O'Shea P. 2005. Physical landscapes in biological membranes: physico-chemical terrains for spatio-temporal control of biomolecular interactions and behaviour. Philos Transact A Math Phys. Eng. Sci. 363: 575–588. [DOI] [PubMed] [Google Scholar]
- 42.Wall J., Ayoub F., O'Shea P. 1995. Interactions of macromolecules with the mammalian cell surface. J. Cell Sci. 108: 2673–2682. (PubMed). [DOI] [PubMed] [Google Scholar]
- 43.Civelek V. N., Hamilton J. A., Tornheim K., Kelly K. L., Corkey B. E. 1996. Intracellular pH in adipocytes: effects of free fatty acid diffusion across the plasma membrane, lipolytic agonists, and insulin. Proc. Natl. Acad. Sci. USA. 93: 10139–10144. (PubMed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brasaemle D. L. 2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559. (PubMed). [DOI] [PubMed] [Google Scholar]
- 45.Febbraio M., Abumrad N. A., Hajjar D. P., Sharma K., Cheng W., Pearce S. F., Silverstein R. L. 1999. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274: 19055–19062. (PubMed). [DOI] [PubMed] [Google Scholar]
- 46.Lobo S., Wiczer B. M., Smith A. J., Hall A. M., Bernlohr D. A. 2007. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J. Lipid Res. 48: 609–620. (PubMed). [DOI] [PubMed] [Google Scholar]
- 47.Cnop M., Hannaert J. C., Hoorens A., Eizirik D. L., Pipeleers D. G. 2001. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 50: 1771–1777. (PubMed). [DOI] [PubMed] [Google Scholar]
- 48.Le Lay S., Hajduch E., Lindsay M. R., Le Liepvre X., Thiele C., Ferre P., Parton R. G., Kurzchalia T., Simons K., Dugail I. 2006. Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis. Traffic. 7: 549–561. (PubMed). [DOI] [PubMed] [Google Scholar]
- 49.Stralfors P. 1990. Autolysis of isolated adipocytes by endogenously produced fatty acids. FEBS Lett. 263: 153–154. (PubMed). [DOI] [PubMed] [Google Scholar]