Abstract
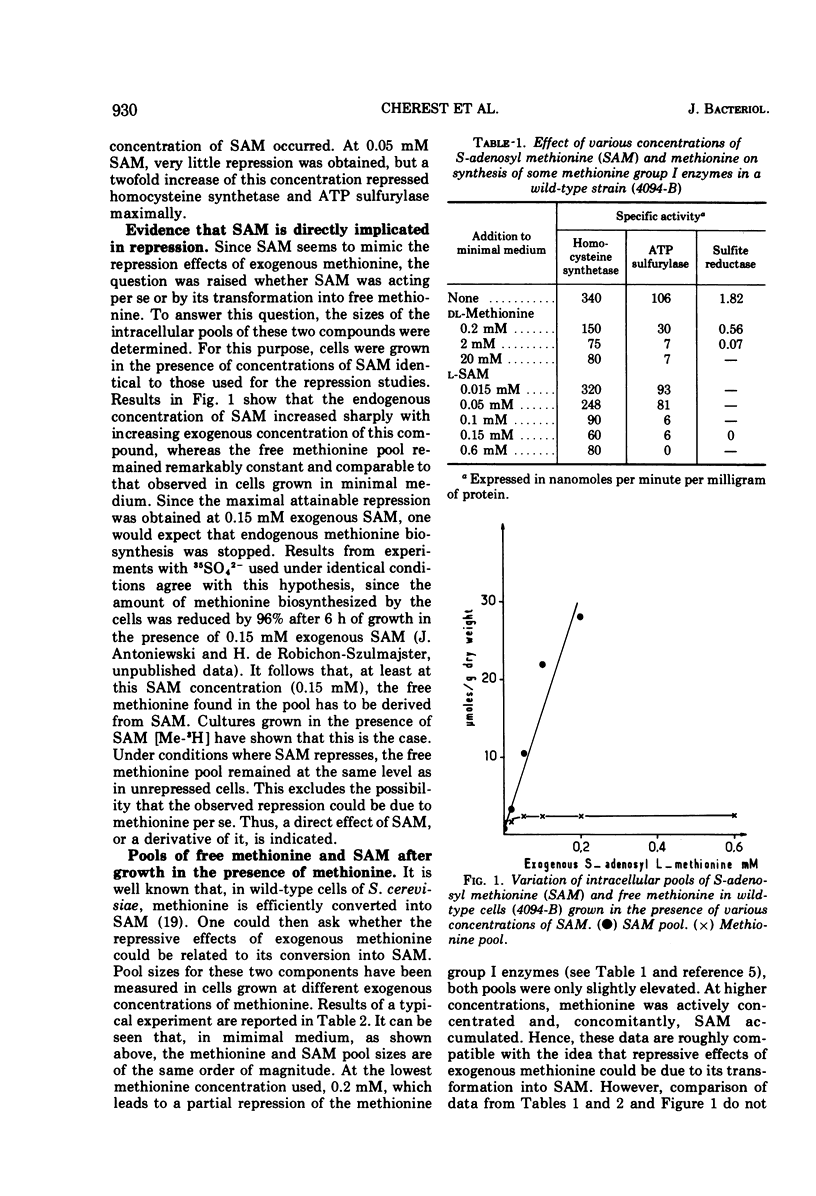
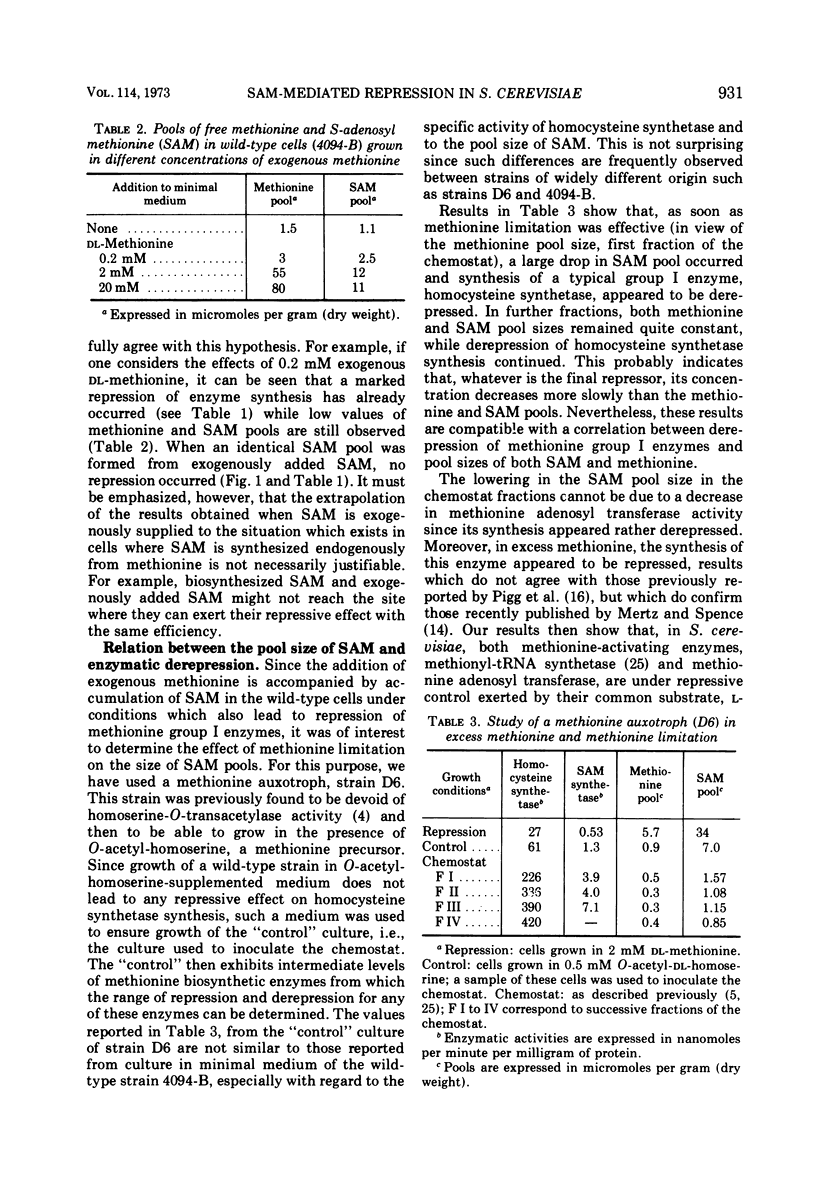
S-adenosylmethionine (SAM) has been shown to provoke repression of some methionine-specific enzymes in wild-type cells, namely, adenosine triphosphate sulfurylase, sulfite reductase, and homocysteine synthetase. Repressive effects observed in SAM-supplemented cultures should be due to SAM per se, since the intracellular pool of SAM increases while the intracellular pool of methionine remains low and constant. Derepression brought about by methionine limitation is accompanied by a severe decrease in SAM as well as methionine pool sizes, although methionine adenosyl transferase is slightly derepressed. Different hypotheses have been considered to account for the previously reported implication of methionyl transfer ribonucleic acid and the presently reported SAM effects in this regulatory process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibold E. R., Williams L. S. Regulation of synthesis of methionyl-, prolyl-, and threonyl-transfer ribonucleic acid synthetases of Escherichia coli. J Bacteriol. 1972 Mar;109(3):1020–1026. doi: 10.1128/jb.109.3.1020-1026.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Cherest H., Eichler F., Robichon-Szulmajster H. Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jan;97(1):328–336. doi: 10.1128/jb.97.1.328-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Robichon-Szulmajster H. Methionine-mediated repression in Saccharomyces cerevisiae: a pleiotropic regulatory system involving methionyl transfer ribonucleic acid and the product of gene eth2. J Bacteriol. 1971 Jun;106(3):758–772. doi: 10.1128/jb.106.3.758-772.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S. Some roles of polyamines in microbial physiology. Adv Enzyme Regul. 1972;10:207–223. doi: 10.1016/0065-2571(72)90015-5. [DOI] [PubMed] [Google Scholar]
- DEVITO P. C., DREYFUSS J. METABOLIC REGULATION OF ADENOSINE TRIPHOSPHATE SULFURYLASE IN YEAST. J Bacteriol. 1964 Nov;88:1341–1348. doi: 10.1128/jb.88.5.1341-1348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Su C. H., Holloway C. T. S-Adenosylmethionine synthetase deficient mutants of Escherichia coli K-12 with impaired control of methionine biosynthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1120–1126. doi: 10.1016/0006-291x(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Holloway C. T., Greene R. C., Su C. H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970 Nov;104(2):734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Spence K. D. Methionine adenosyltransferase and ethionine resistance in Saccharomyces cerevisiae. J Bacteriol. 1972 Sep;111(3):778–783. doi: 10.1128/jb.111.3.778-783.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. T., Spence K. D. Transport of S-adenosylmethionine in Saccharomyces cerevisiae. J Bacteriol. 1972 Feb;109(2):499–504. doi: 10.1128/jb.109.2.499-504.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIGG C. J., SORSOLI W. A., PARKS L. W. INDUCTION OF THE METHIONINE-ACTIVATING ENZYME IN SACCHAROMYCES CEREVISIAE. J Bacteriol. 1964 Apr;87:920–923. doi: 10.1128/jb.87.4.920-923.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbury R. J., Lawrence D. A., Smith D. A. Regulation of the methionine-specific aspartokinase and homoserine dehydrogenase of Salmonella typhimurium. J Gen Microbiol. 1968 Dec;54(3):337–342. doi: 10.1099/00221287-54-3-337. [DOI] [PubMed] [Google Scholar]
- SCHLENK F., DEPALMA R. E. The formation of S-adenosylmethionine in yeast. J Biol Chem. 1957 Dec;229(2):1037–1050. [PubMed] [Google Scholar]
- SCHLENK F., DEPALMA R. E. The preparation of S-adenosylmethionine. J Biol Chem. 1957 Dec;229(2):1051–1057. [PubMed] [Google Scholar]
- SIEGEL L. M. A DIRECT MICRODETERMINATION FOR SULFIDE. Anal Biochem. 1965 Apr;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Savin M. A., Flavin M., Slaughter C. Regulation of homocysteine biosynthesis in Salmonella typhimurium. J Bacteriol. 1972 Aug;111(2):547–556. doi: 10.1128/jb.111.2.547-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Smith D. A. S-amino acid metabolism and its regulation in Escherichia coli and Salmonella typhimurium. Adv Genet. 1971;16:141–165. doi: 10.1016/s0065-2660(08)60357-0. [DOI] [PubMed] [Google Scholar]
- Surdin-Kerjan Y., Cherest H., Robichon-Szulmajster H. Relationship between methionyl transfer ribonucleic acid cellular content and synthesis of methionine enzymes in Saccharomyces cerevisiae. J Bacteriol. 1973 Mar;113(3):1156–1160. doi: 10.1128/jb.113.3.1156-1160.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]



