Abstract
The CETP inhibitor, torcetrapib, was prematurely terminated from phase 3 clinical trials due to an increase in cardiovascular and noncardiovascular mortality. Because nearly half of the latter deaths involved patients with infection, we have tested torcetrapib and other CETPIs to see if they interfere with lipopolysaccharide binding protein (LBP) or bactericidal/permeability increasing protein (BPI). No effect of these potent CETPIs on LPS binding to either protein was detected. Purified CETP itself bound weakly to LPS with a Kd ≥ 25 uM compared with 0.8 and 0.5 nM for LBP and BPI, respectively, and this binding was not blocked by torcetrapib. In whole blood, LPS induced tumor necrosis factor-α normally in the presence of torcetrapib. Furthermore, LPS had no effect on CETP activity. We conclude that the sepsis-related mortality of the ILLUMINATE trial was unlikely due to a direct effect of torcetrapib on LBP or BPI function, nor to inhibition of an interaction of CETP with LPS. Instead, we speculate that the negative outcome seen for patients with infections might be related to the changes in plasma lipoprotein composition and metabolism, or alternatively to the known off-target effects of torcetrapib, such as aldosterone elevation, which may have aggravated the effects of sepsis.
Keywords: atherosclerosis, high density lipoprotein, lipopolysaccharide
In order to reduce the incidence of cardiovascular disease beyond that achieved through the use of statins and other lipid lowering treatments, efforts have been ongoing to identify agents for raising the levels of HDL. To date, the most potent means for raising plasma HDL has been through the use of cholesteryl ester transfer protein (CETP) inhibitors. Both torcetrapib (1, 2) and anacetrapib (3) have demonstrated 2-fold elevations in human trials. However, the development of these new lipid-modulating drugs suffered a major setback in December 2006 with the termination of the phase 3 trials of torcetrapib due to an excess in overall mortality and adverse cardiovascular events (4, 5). The largest of these trials, the Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events (ILLUMINATE), involved 15,067 patients at high cardiovascular risk. At study termination, 93 deaths had occurred in the torcetrapib/atorvastatin group compared with 59 in atorvastatin group. Of the 34 excess deaths in the torcetrapib group, 14 were cardiovascular-related whereas 20 were noncardiovascular-related. Although the occurence of new infections during the trial was similar for the torcetrapib versus the atorvastatin group (182 versus 177), there were nine deaths from infection in the former group versus zero for the latter.
That nearly half of the noncardiovascular excess in mortality for the ILLUMINATE trial was associated with infection raises the question as to whether torcetrapib, apart from its intended effect on CETP, might have interfered with the function of two other proteins in the same family of lipid binding proteins, both of which play important roles in antibacterial defense (6). The first of these, lipopolysaccharide binding protein (LBP), is an acute phase protein produced mainly by the liver. It binds lipopolyscaccharide (LPS) released from the outer coat of Gram-negative bacteria and catalyses its transfer to CD14 receptors, thereby initiating the activation of macrophages, neutrophils, and other cells required for the immune response. LBP in plasma is mostly lipoprotein-bound, both to triglyceride-rich lipoproteins and to HDL, and by binding LPS it also serves to direct excess LPS to the liver for excretion in the bile. In contrast to LBP, bactericidal/permeability increasing protein (BPI) is directly bactericidal. BPI binding to LPS damages the bacterial coat causing immediate growth arrest and eventual death. The BPI-LPS complex does not, however, interact productively with CD14 receptors. Therefore, by binding LPS, BPI counters CD14 mediated signaling via LBP and serves to prevent an overly intense inflammatory response (endotoxemia) (6, 7).
The crystal structure of BPI (8) has shown it to be an elongated boomerang-shaped protein consisting of topologically similar N-terminal and C-terminal domains joined by a central β-sheet. Apolar pockets situated at the concave surface of each domain were found to have a bound molecule of phosphatidylcholine, the acyl chains of which were buried within the protein with the polar head groups located at the pocket entrances exposed to solvent. The crystal structure for LBP has not been reported but homology modeling indicates it shares structural features with BPI including the elongated shape, pseudosymmetry of N- and C-terminal domains, and presence of two apolar lipid binding pockets (9). Multiple regions of BPI and LBP appear to be involved in binding LPS. The apolar pockets are expected to incorporate two or more acyl chains of the lipid A moiety whereas the highly cationic N-termini electrostatically interact with the phosphorylated sugar groups. The recently resolved crystal structure for CETP shows similarities to BPI and LBP in overall architecture (10). However, CETP and to a lesser extent, phospholipid transfer protein (PLTP), lack the concentration of conserved positively charged residues that seem important for the high affinity binding of LPS. Although PLTP has been reported to be able to bind and neutralize LPS (11), little evidence exists that CETP might do the same. The purpose of this study was to determine whether or not torcetrapib or other CETP inhibitors interfere with the normal binding of LPS by BPI and LBP. Additionally, the affinity of CETP itself for LPS was compared with that of BPI and LBP and the effects of torcetrapib tested.
MATERIALS AND METHODS
Materials
Purified recombinant human LBP was purchased from R and D Systems. Invitrogen plasmid pcDNA3.1 and psec(IgK)v5/His, as well as Optimem media and 293 Fectin, were utilized for BPI expression. Freestyle 293 media was from Gibco. Hi-Trap SP, Lentil Lectin Sepharose 4B and Ni-NTA columns were obtained from GE Healthcare. Human LBP and BPI ELISA kits were purchasd from Hycult Biotechnolgy. Biotin LPS (Escherichia coli O111:B4 coupled through oxidized carbohydrate to hydrazide LC biotin) used for SPR experiments was from InvivoGen. Nonlabeled E. coli and P. aeruginosa LPS as well as routine chemicals were from Sigma.
Purified CETP and BPI
Human CETP was purified from medium conditioned by CETP expressing Chinese hamster ovary (CHO) cells as described previously (12). For BPI, for which a commercial source was not available, the BPI coding region (amino acid residues 32-487 without the native signal sequence) was PCR amplified from human bone marrow cDNA and cloned into either pSec(IgK) V5/His for tagged BPI or pcDNA3.1 for nontagged BPI.
For transient BPI expression, HEK-293F cells were grown in suspension in one liter of Freestyle 293 media in Fernbach flasks (Corning) incubated at 37°C, in 8% CO2 at 90 rpm on an Innova shaker to a cell density of ∼1.1 × 106 cells/ml. To two separate 35 ml aliquots of Optimem media were added 1.3ml of 293 Fectin and 1 mg plasmid DNA, respectively. After 5 min of static incubation at room temperature, the plasmid DNA and 293 Fectin solutions were combined, incubated for an additional 25 min, then the entire mixture was added to the one liter suspension of HEK-293F cells and placed on an incubated shaker (Forma Scientific). At 48 h, sodium butyrate was added at 2 mM final concentration. The conditioned media was harvested at 120 h (70–80% cell viability) by refrigerated centrifugation, 0.2 um filtered, and stored at 4°C until purification.
For purification of nontagged BPI, one liter of BPI conditioned media was loaded onto a 5 ml Hi-Trap SP column which has been equilibrated with PBS. After washing the column with PBS protein was eluted with 15 bed volumes of a salt gradient (PBS to 0.8 M NaCl/PBS). The BPI-containing fractions were pooled based on SDS-PAGE analysis and dialyzed into 20 M Tris, pH7.4, 0.3 M NaCl (loading buffer) at 4°C overnight. The BPI pool was then injected onto a 5 ml Lentil Lectin Sepharose 4B column, the column washed with loading buffer and protein then eluted with 15 bed volumes of a 0 to 0.4 M methy a-D-manno-pyranoside gradient in the loading buffer. BPI-containing fractions were again pooled based on SDS-PAGE and dialyzed against PBS buffer.
For purification of the His-tagged BPI, one liter of conditioned media was injected onto a 5ml Hi-Trap Ni-NTA column, which had been equilibrated with PBS buffer. After loading, the column was washed with 20 mM imidazole in PBS buffer. Bound protein was then eluted with 15 bed volumes of a 20–300 mM imidazole gradient in PBS at pH 7.5. His-BPI fractions were pooled, dialyzed into PBS buffer, and loaded onto a 5 ml HiTrap SP sepharose column equilibrated with PBS. After washing with PBS, the column was eluted with 15 bed volumes of a salt gradient (PBS to 0.8M NaCl/PBS buffer). His-BPI fractions were pooled according to SDS-PAGE analysis.
LBP, BPI and CETP binding to LPS: SPR methods
The effects of compounds on either BPI or LBP binding to immobilized LPS were examined in a Biacore 3000 surface plasmon resonance (SPR) instrument at 25°C in 10 mM Hepes pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.05% Tween 20, 1 mg/ml BSA, 1% DMSO. Biotinylated LPS was immobilized on a neutravidin (Pierce) surface that was prepared by standard amine coupling procedures on a CM5 biosensor chip. Binding surfaces were regenerated between protein injections with a 6 s injection of 0.5% SDS followed by a 6 s injection of 0.12M KSCN, 0.46M MgCl2, 0.23M urea, 0.46M GuHCl, 0.08% CHAPS, 0.08% zwitterent 3-12, 0.08% tween 80, 0.08% tween 20, 0.08% triton X-100. Binding data were Y-aligned, X-aligned, and double referenced against an unmodified neutravidin surface and buffer injections using Scrubber 2 software (Biologics Inc.). Kinetic analyses were performed using Biaeval software (GE Healthcare). Preincubation with soluble LPS completely inhibited binding of either BPI or LBP to the immobilized LPS. Control experiments with untreated proteins showed that binding rates were protein concentration dependent and reproducible. The binding rate of 45 nM protein could be clearly distinguished from that of 50nM protein, suggesting that a 10% inhibition in binding could readily be detected. To determine the effects of compounds on BPI or LBP binding to LPS, proteins at 50nM were preincubated with compounds at 0.33, 1.0, or 3.0 uM. Compound-treated samples were injected over the immobilized LPS and their rates of binding were compared with those of untreated controls. Binding was measured as the total apparent on-constant obtained by fitting the data to a kinetic model for 1:1 Langmuir binding. Compounds alone showed no detectible binding to the LPS surface
LPS binding to LBP and BPI: ELISA methods
In contrast to the SPR assay, for ELISA assay the additions were in reverse order. LBP and BPI were immobilized first followed by addition of biotinylated LPS. For assay of LPS binding to LBP a commercial kit (Hycult Biotechnology) was used. LBP (0.5 nM) preincubated for 15 min at 37°C with or without test compound was added to wells precoated with anti-LBP antibody at 100 ul/well. After 1 h at room temperature, the contents were removed, the wells washed four times, and 100 ul biotinylated LPS was added for the binding step (also 1 h, RmT). The LPS was then removed, washed four times and streptavidin-peroxidase added for detection followed by development with tetramethylbenzine (TMB). For some assays, a modified protocol was utilized in which LBP was first preincubated before compound addition with a mix of human LDL and HDL, isolated by sequential ultracentrifugation, at a concentration of 12 and 4 mg/dl cholesterol, respectively. An ELISA for determining LPS binding to BPI combined anti-BPI antibody coated plates provided in an ELISA kit for measuring BPI mass with the biotinylated LPS from the LBP-LPS ELISA kit (both from Hycult Biotechnolgy) utilizing the same protocol, except in this case BPI was used at 2 nM for the preincubation and immobilization steps. For both ELISAs, each compound concentration, as well as minus compound controls, were tested in triplicate wells. Absorbance values for inhibitor conditions were compared with controls after first correcting for nonspecific background determined from minus LBP and minus BPI control wells. The specificity of both ELISAs was demonstrated by the ability of both nonbiotinylated E. coli O111:B4 and P. aeruginosa serotype 10 LPS to dose-dependently abolish the detection of the biotinylated LPS. For testing the ability of purified CETP to compete with the immobilized LBP and BPI, 50 ul CETP at 2× the final designated concentration was added to the immobilized LBP or BPI, mixed well, then 50 ul biotinylated LPS, also at 2× final concentration, was added and mixed. The remainder of the protocol was identical to that described above.
CETP activity assay
The effects of LPS on cholesteryl ester transfer activity in whole human plasma was determined using 3H- and 14C-cholesteryl oleate labeled LDL and HDL, respectively, as donor lipoproteins as previously described (1) except is this case the 3H- and 14C-assays were conducted separately. Three strains of bacterial LPS, E. coli 0111:B4, E. coli 026:B6, and Pseudomonas aeruginosa serotype 10 were tested in triplicate assays at 5, 50, 500 and 5000 ng/ml.
Whole blood LPS activation assay
For testing the effects of torcetrapib on the ability of LPS to activate blood monocytes/macrophages, an ex vivo whole blood assay was performed. All steps were conducted under sterile conditions. Fresh blood from human donors fasted overnight was collected into heparin-containing tubes. Within 30 min of collection, blood was mixed with an equal volume of RPMI 1640 media + glutamine at 37°C equilibrated to 5% CO2. Torcetrapib in DMSO was added to 1.0 uM, with DMSO only serving at the minus inhibitor control. The samples were mixed and 180 ul aliquots added to a 96-well plate containing 20 ul LPS (E. coli 0111:B4) at 10× the indicated final concentrations. All conditions were run in triplicate wells. The plate was placed in a 37°C incubator at 5% CO2 for 4 h. After the incubation the plate was centrifuged for 5 min at 2000 rpm (450 g) to pellet blood cells. Tumor necrosis factor (TNF)-α levels were measured using the single analyte ELISA kit from SABiosciences per the instructions using 50 ul of 1 in 5 diluted plasma.
RESULTS
Effects of CETPIs on LPS binding by LBP and BPI
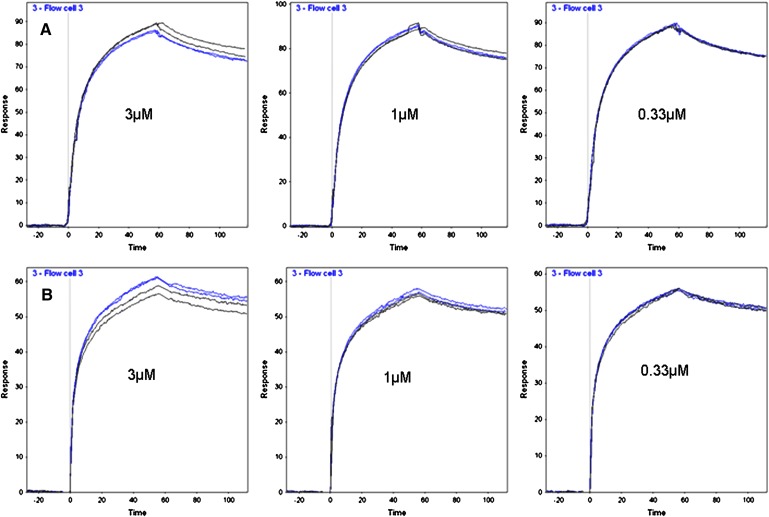
For this study, CETP inhibitors (CETPIs) representing two series were tested, including torcetrapib and its close analog CP-532623 (1, 13), and the Merck inhibitor anacetrapib (3). Orlistat, a nonselective lipase inhibitor, was included as a control nonCETPI compound with similar high lipophilicity (ElogD = 8.95) to that of the CETPIs (ElogD = 7.4–10.1). As determined by Biacore, the dose-dependent binding of LBP and BPI to immobilized LPS displayed high affinity with calculated Kds of 0.8 and 0.5 nM, respectively (supplementary Fig. I). For analysis of the potential effects of CETPIs, the inhibitors were preincubated at three concentrations with a fixed concentration, 50 nM, of the test proteins. Two injections were performed for each compound concentration, accompanied by two control injections, minus compound, for comparison. Figure 1 shows representative sensorgrams for one experiment each, examining the effects of torcetrapib on the binding of LBP (Fig. 1A) and of BPI (Fig. 1B) to LPS. The mean results for three experiments each for Torcetrapib and anacetrapib showed no significant effect on either LBP or BPI binding to LPS at any concentration (supplementary Fig. II, for all treatments P > 0.05 by Student t-test).
Fig. 1.
Effects of torcetrapib on LBP and BPI binding to immobilized LPS. 50 nM protein preincubated with torcetrapib (blue lines) or without (black lines) was injected for 60 s. Each treatment was injected in duplicate. A: Representative sensorgrams from one experiment with LBP. B: Sensorgrams for one experiment with BPI.
The same inhibitors, as well as CP-532623, were also tested in ELISA assays in which the compounds were preincubated at 0.3, 1.0, and 3.0 uM with LBP or BPI before the proteins were immobilized on antibody coated wells, followed by the addition of biotinylated LPS and subsequent detection using streptavidin-HRP. As was the case for the SPR assays, mean results for a series of such ELISAs found none of the inhibitors had a significant effect (P > 0.05 for all concentrations) compared with control on LPS binding to either LBP or BPI (supplementary Fig. III). LBP in plasma is largely associated with lipoproteins (6). Furthermore, for CETPIs such as torcetrapib at concentrations greater than that of plasma CETP, the excess inhibitor is expected to distribute into lipoproteins as well (12). Therefore, the LBP-LPS ELISA was repeated for torcetrapib, CP-532623, and Orlistat using a protocol in which LBP was first preincubated for 15 min at 37°C with a mix of human LDL and HDL, followed by addition of the test compounds and then continuation of the assay as before. The three inhibitors were tested together in four separate ELISAs. Under these more physiological conditions, the ability of LBP to bind LPS remained unaffected in the presence of the CETPIs (relative to control all P > 0.05, data not shown).
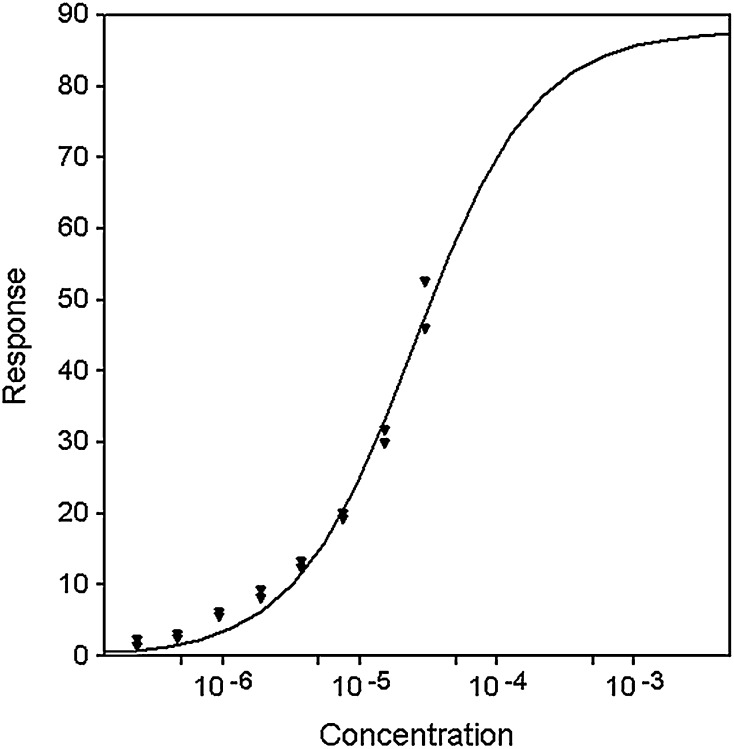
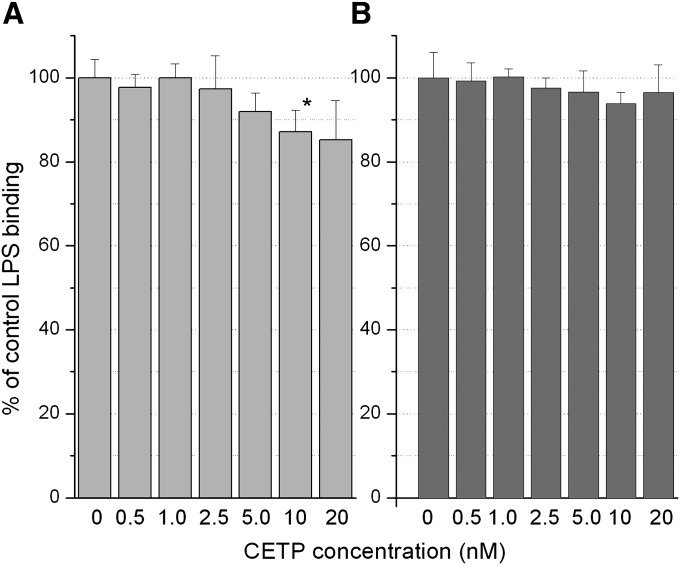
Given the lack of effect of the CETPIs on LBP and BPI, a question was to what degree does CETP itself bind LPS and how does this compare with that of the former proteins. Equilibrium binding of CETP to immobilized LPS was measured by SPR at concentrations ranging from 0.1uM to 30 uM. Because the highest concentration of CETP that could be tested, 30 uM, failed to saturate the surface, it is unclear whether or not binding is 1:1 or possibly multiple sites (non-specific). If a 1:1 Langmuir equilibrium binding model is assumed then the data can be fit to yield a KD of 25 ± 3 uM (Fig. 2). If data are fitted to multiple sites models, then values obtained are always greater than 25uM. Therefore, it is reasonable to conclude that the KD for CETP binding LPS ≥ 25uM. This is >25,000-fold higher than that of LBP and BPI. In three separate experiments, two injections each, in which CETP was preincubated with torcetrapib (3 uM CETP: 9 uM torcetrapib), no difference in binding to LPS relative to control was found (data not shown). Additionally, purified CETP was tested for its ability to compete with the immobilized LBP and BPI for binding of LPS in the ELISA assays (Fig. 3). An apparent trend toward a reduction in LPS binding to LBP was observed (Fig. 3A), which, however, reached significance only at the 10 nM concentration of CETP (P = 0.045). Assuming all LBP was captured in the immobilization step, these effects at 10–20 nM CETP occurred at 20–40:1 ratios of CETP to LBP (20–40:0.5 nM). For the BPI-LPS ELISA (Fig. 3B), no significant effect was seen in the presence of CETP, which at the highest 20 nM dose in this ELISA is equivalent to a 10:1 ratio of CETP to BPI (20:2 nM).
Fig. 2.
SPR equilibrium binding of CETP to LPS and the effect of torcetrapib. Equilibrium binding of CETP to immobilized LPS was measured at concentrations ranging from 0.11 to 30 uM. Binding data (triangles) were fit to a 1:1 Langmuir model to obtain a fit (solid line) that suggests a KD of 25+/−3 uM.
Fig. 3.
Effects of CETP on LPS binding to LBP and BPI. Purified CETP was added to wells containing immobilized LBP or BPI followed by addition of biotinylated LPS to achieve the final CETP concentrations indicated. Results for LPS binding to LBP (A) and BPI (B) represent the mean for four experiments each. * For effects on LPS-LBP binding at 10 nM CETP relative to control P = 0.045.
There have been two reports of inhibition of CETP activity by LPS for in vitro assays (14, 15) with a third finding no effect (16). These studies utilized isolated lipoprotein and/or CETP at low levels relative to that of human plasma but LPS concentrations extremely high compared with those in human subjects, even in patients with sepsis or septic shock (17). We therefore tested three strains of bacterial LPS in whole human plasma CE-transfer assays at final concentrations ranging from 5–5000 ng/ml. Determined using both labeled LDL and HDL as donor lipoproteins, no difference was seen between LPS treated and nontreated plasma. (all P > 0.05, data not shown).
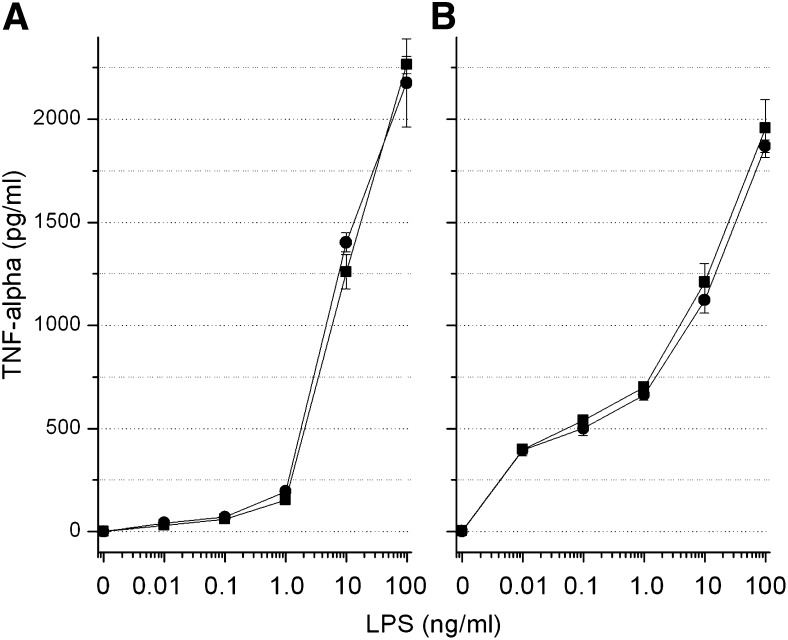
LBP serves both to bind LPS through its N-terminal domain and to transfer LPS to CD14 receptors for the process of immune cell activation to occur (6). The latter process is mediated through the C-terminal domain of the protein. For a final test of whether torcetrapib interferes with either of these functions of LBP, a whole blood LPS activation assay was performed with the endpoint being induction of TNF-α. Figure 4 shows the results of two separate experiments using different pools of blood from human donors. For both experiments, an approximate 1000- to2000-fold induction of TNF-α was produced at the 10 and 100 ng/ml LPS concentrations with no difference observed for blood containing 1.0 uM torcetrapib, a level of the drug causing ≥ 95% inhibition of CETP activity.
Fig. 4.
Whole blood LPS activation assay with TNF-α induction as endpoint. Two separate pools of whole blood from two donors each, A and B, were tested for the effects of torcetrapib (1.0 uM final concentration) on TNF-α induction by LPS as described in Materials and Methods. Results are expressed as mean plasma TNF-α concentration ± SD for torcetrapib treated (circles) versus DMSO control (squares) blood.
DISCUSSION
The purpose of this investigation was two-fold. The first objective was to assess whether the mortality due to sepsis observed on treatment in the ILLUMINATE trial (5) might be related to a direct interference by torcetrapib with two homologous proteins known to play important roles in immunosurveillance and the response to infection (6). The second goal was to examine whether CETP itself binds LPS with relative high affinity and therefore might be able to neutralize LPS and so play a supporting role to those of LBP and BPI. If so, does torcetrapib binding to CETP prevent this? In the first case, anacetrapib, representing a second inhibitor series, was tested as well. Two sensitive methods, SPR (Biacore) and ELISA, for measuring the binding of LBS by LBP and BPI detected no significant effect of any of the inhibitors. For LBP, this included conditions where the protein was first preincubated with human LDL and HDL, followed by addition of the inhibitors, thus more resembling a physiological state where both the protein and these lipophilic compounds are associated with lipoprotein. The lack of effect of these inhibitors is not unexpected. Although the crystal structures of CETP (10) and BPI (8) demonstrate overall similarities in 3-dimensional structure for the two proteins, the lipid binding sites are quite different. Whereas CETP possesses a hydrophobic tunnel large enough to accommodate two neutral lipids and two phospholipids, BPI has two small pockets, each for accepting the acyl chains of a phospholipid. LBP is predicted to be much more similar to BPI than to CETP, both in sequence homology (LBP:BPI 44% identity, LBP:CETP 25% identity) and ability to bind lipid. The binding site for torcetrapib has recently been defined to the N-terminal side of the CETP tunnel (13), which is about twice the size of a BPI pocket and shares no apparent similarity in lipid binding modes.
As expected, the affinity of LBP and BPI for LPS was high with SPR determined Kd values of 0.8 and 0.5 nM. In contrast, purified CETP bound weakly to LPS with a Kd ≥ 25 uM. Addition of CETP to the LBP ELISA slightly reduced LPS binding but only at relatively high ratios of CETP:LBP. For BPI-LPS binding, no significant effect was seen. In humans, typical plasma CETP levels range from 20 to 60 nM (1, 12) whereas plasma LBP levels might average 80 nM (6, 17). However, in patients with severe sepsis or septic shock, LBP levels would be expected to be induced to much higher levels ranging from 80 nM to >3 uM (17). Likewise, most BPI is stored within the primary granules of neutrophils and is expected to be used in large part for intracellular bactericidal activity, following phagocytosis, with the remainder released into the surrounding fluid to contribute to extracellular killing of bacteria and neutralization of endotoxin (18). In both cases, the local concentration of BPI is expected to exceed that of CETP. Therefore, the possibility that CETP might play a significant role involving LPS binding relative to that of LBP and BPI appears remote.
The binding of LPS by LBP occurs through its N-terminal domain, whereas the transfer of LPS to membrane and soluble CD14 receptors rely on its C-terminal region. Both processes are required for LBP to fulfill its role in both cell activation and endotoxin neutralization. The ex vivo LPS activation assay described in this study found that 1.0 uM torcetrapib, a concentration that inhibits plasma CETP activity by ≥ 95%, had no effect on monocyte/macrophage activation as indicated by induction of TNF-α. This demonstrates that torcetrapib does not interfere with either function of LBP. It also argues against a role for CETP in these processes that would have been blocked by torcetrapib in the ILLUMINATE trial, worsening the severity of sepsis. Although there have been two reports of CETP activity being inhibited by LPS (14, 15) these studies employed assays in which lipoprotein and/or CETP concentrations were low relative to that of whole human plasma but LPS concentrations (1–200 ug/ml) were extremely high compared with that typical for human subjects with sepsis or septic shock (≤ 5 ng/ml) (17). In this study, three strains of bacterial LPS were tested for their effect on CE-transfer in whole human plasma assays at concentrations up to 5 ug/ml, a level 500-fold greater than that required for the approximately 1250-fold induction of TNF-α observed in the ex vivo blood activation assays. No effect of LPS on CETP activity was seen. This suggests that the inhibition of CETP reported in the previous studies may have arisen from the low concentrations of lipoprotein and transfer protein used relative to LPS, leading to nonspecific LPS-substrate interactions that might have excluded or sequestered CETP, conditions that do not resemble the far more lipoprotein- and protein-rich environment of plasma. Recently, it was reported that expression of human CETP in mice conferred protection against LPS induced mortality (19). However, information important for interpreting these results including plasma LPS, CETP and lipoprotein concentrations were not determined. LPS injection in human CETP transgenic mice of the type used in this study, for instance, has been shown to decrease hepatic mRNA levels as well as plasma CETP mass. A drop in plasma CETP concentration would be expected to raise HDL levels and lower nonHDL lipoprotein, which could in turn alter the response to LPS, yet the data allowing a comparison of plasma lipoprotein for wild-type versus CETP transgenics is absent. The 40% decrease in apparent CETP activity is comparable to magnitude of CETP mass reduction reported in the previous study using comparable doses of LPS (15). The authors suggested a direct interaction between CETP and LPS in this study but did not provide any binding data, referring instead to previous reports of inhibition by LPS (14, 15).
In the current study, a lack of effect of potent CETPIs on LBP and BPI function is demonstrated. Additionally, strong evidence is provided to suggest that a direct physiologically relevant interaction between CETP and LPS is unlikely. Finally, LPS activation in whole blood proceeded normally in the presence of a high concentration of torcetrapib. Together, these results suggest that if the increased mortality related to sepsis in the ILLUMINATE trial was mechanistically related to CETP inhibition, it is likely a result of the changes in lipoprotein composition, levels, and metabolism, rather than direct effects of the inhibitor on immune response proteins. These effects of torcetrapib and other CETPIs include shifts in cholesterol and triglyceride content, slowed clearance of HDL, and accelerated clearance of triglyceride-rich lipoproteins and LDL from the plasma resulting in elevations of the former and decreases in the latter (1, 3, 20–23). ApoA-I and especially apoE levels in the HDL fraction increase. Both apolipoproteins, either alone or associated with lipoprotein, are able to bind and neutralize LPS and through uptake mediated by hepatic apoB/E and SR-B1 receptors, promote its clearance and excretion (24–29). In this process, both triglyceride-rich lipoproteins and HDL are reported to play important roles. Aside from the liver, the steroidogenic tissues such as the adrenals also display high expression of SR-B1 and depend heavily on HDL cholesterol uptake for sterol production (25, 26). This raises the question in the context of CETP inhibition as to whether or not the balance between hepatic and extrahepatic uptake of LPS is negatively affected.
Alternatively, the ILLUMINATE trial (5) found aldosterone levels to be increased with torcetrapib treatment, an effect of this inhibitor confirmed in recent studies (30, 31), but which occurs independently of CETP inhibition and is not shared by all CETP inhibitors. Could the increase in aldosterone have resulted in the increased oxidative stress and endothelial dysfunction associated with elevations of this hormone (32, 33) and thus converged in the setting of sepsis to worsen the outcome? In this regard, it is important to note that of the nine deaths from infection in the torcetrapib group, seven were in patients with diabetes, a condition that in itself is strongly associated with increased oxidative stress and cardiovascular disease (34, 35). Whether the failure of torcetrapib was due to the molecule or the mechanism is an open question and will remain so until CETPIs free of these known off-target effects progress through clinical outcome studies.
Supplementary Material
Acknowledgments
The authors thank Drs Mark Bamberger and Charles Shear for their review of this manuscript.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- BPI
- bactericidal/permeability increasing protein
- CETP
- cholesteryl ester transfer protein
- CETPI
- CETP inhibitor
- LBP
- lipopolysaccharide binding protein
- LPS
- lipopolysaccharide
- SPR
- surface plasmon resonance
- SR-BI
- scavenger receptor BI
- TNF
- tumor necrosis factor
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Clark R. W., Sutfin T. A., Ruggeri R. B., Willauer A. T., Sugarman E. D., Magnus-Aryitev G., Cosgrove P. G., Sand T. M., Wester R. T., Williams J. A., et al. 2004. Raising high density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler. Thromb. Vasc. Biol. 24: 490–497. [DOI] [PubMed] [Google Scholar]
- 2.Brousseau M. E., Schaefer E. J., Wolfe M. L., Bloeden L., Digenio A. G., Clark R. W., Mancuso J., Rader D. J. 2004. Effects of a potent inhibitor of cholesteryl ester transfer protein on plasma lipoproteins in patients with low HDL cholesterol. N. Engl. J. Med. 350: 1505–1515. [DOI] [PubMed] [Google Scholar]
- 3.Krishna R., Anderson M. S., Bergman A. J., Jin B., Fallon M., Cote J., Rosko K., Chavez-Eng C., Lutz R., Bloomfield D. M., et al. 2007. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomized placebo-controlled phase 1 studies. Lancet. 370: 1907–1914. [DOI] [PubMed] [Google Scholar]
- 4.Tall A. R., Yvan-Charvet L., Wang N. 2007. The failure of torcetrapib. Was it the molecule or the mechanism? Arterioscler. Thromb. Vasc. Biol. 27: 257–260. [DOI] [PubMed] [Google Scholar]
- 5.Barter P. J., Caufield M., Eriksson M., Grundy S. M., Kastelein J. J. P., Komajda M., Lopez-Sendon J., Mosca L., Tardif J-C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 6.Van Amersfoort E. S., Van Berkel T. J. C., Kuiper J. 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 16: 379–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dentener M. A., Von Asmuth E. J. U., Francot G. J. M., Marra M. N., Buurman W. A. 1993. Antagonistic effects of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein on lipopolysaccharide-induced cytokine release by mononuclear phagocytes. J. Immunol. 151: 4258–4265. [PubMed] [Google Scholar]
- 8.Beamer L. J., Carroll S. F., Eisenberg D. 1997. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science. 276: 1861–1864. [DOI] [PubMed] [Google Scholar]
- 9.Beamer L. J., Carroll S. F., Eisenberg D. 1998. The BPI/LBP family of proteins: a structural analysis of conserved regions. Protein Sci. 7: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu X., Mistry A., Ammirati M. J., Chrunyk B. A., Clark R. W., Cong Y., Culp J. S., Danley D. E., Freeman T. B., Geoghegan K. F., et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14: 106–113. [DOI] [PubMed] [Google Scholar]
- 11.Hailman E., Albers J. J., Wolfbauer G., Tu A., Wright S. D. 1996. Neutralization and transfer of lipopolysaccharide by phospholipid transfer protein. J. Biol. Chem. 271: 12172–12178. [DOI] [PubMed] [Google Scholar]
- 12.Clark R. W., Ruggeri R. B., Cunningham D., Bamberger M. J. 2006. Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J. Lipid Res. 47: 537–552. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D., Lin W., Hoth L. R., Danley D. E., Ruggeri R. B., Geoghegan K. F., Chrunyk B. A., Boyd J. G. 2008. Biophysical and biochemical approach to locating an inhibitor binding site on cholesteryl ester transfer protein. Bioconjug. Chem. 19: 1604–1613. [DOI] [PubMed] [Google Scholar]
- 14.Connolly D. T., Krul E. S., Heuvelman D., Glenn K. C. 1996. Inhibition of cholesteryl ester transfer protein by apolipoproteins, lipopolysaccharides, and cholesterol sulfate. Biochim. Biophys. Acta. 1304: 145–160. [DOI] [PubMed] [Google Scholar]
- 15.Masucci-Magoulas L., Moulin P., Jiang X. C., Richardson H., Walsh A., Breslow J. L., Tall A. 1995. Decreased cholesteryl ester transfer protein (CETP) mRNA and protein and increased high density lipoprotein following lipopolysaccharide administration in human CETP transgenic mice. J. Clin. Invest. 95: 1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd D. B., Bonnette P., Thompson J. F. 2006. Protein fusions of BPI with CETP retain functions inherent to each. Biochemistry. 45: 12954–12959. [DOI] [PubMed] [Google Scholar]
- 17.Opal S. M., Scannon P. J., Vincent J., White M., Carroll S. F., Palardy J. E., Parejo N. A., Pribble J. P., Lemke J. H. 1999. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 180: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 18.Schultz H., Weiss J. 2007. The bactericidal/permeability-increasing protein (BPI) in infection and inflammatory disease. Clin. Chim. Acta. 384: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazita P. M., Barbeiro D. F., Moretti A. I. S., Quintao E. C. R., Soriano F. G. 2008. Human CETP expression enhances the mouse survival rate in an experimental inflammation model: a novel role for CETP. Shock. 30: 590–595. [DOI] [PubMed] [Google Scholar]
- 20.Brousseau M. E., Diffenderfer M. R., Millar J. S., Nortsupha C., Asztalos B. F., Welty F. K., Wolfe M. L., Rudling M., Bjorkhem I., Angelin B., et al. 2005. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein species, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler. Thromb. Vasc. Biol. 25: 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millar J. S., Brousseau M. E., Diffenderfer M. R., Barret H. R., Welty F. K., Faruqi A., Wolfe M. L., Nartsupha C., Digenio A. G., Mancuso J. P., et al. 2006. Effects of cholesteryl ester transfer protein inhibitor torcetrapib on apoprotein B100 metabolism in humans. Arterioscler. Thromb. Vasc. Biol. 26: 1350–1356. [DOI] [PubMed] [Google Scholar]
- 22.Guerin M., Le Goff W., Duchene E., Julia Z., Nguyen T., Thuren T., Shear C. L., Chapman M. J. 2008. Inhibition of CETP by torcetrapib attenuates the atherogenicity of postprandial TG-rich lipoproteins in type IIB hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 28: 148–154. [DOI] [PubMed] [Google Scholar]
- 23.Wolk R., Chen D., Clark R. W., Mancuso J., Barclay P. L. 2009. Pharmacokinetic, pharmacodynamic and safety profile of a new cholesteryl ester transfer protein inhibitor in healthy human subjects. Clin. Pharmacol. Ther. 86: 430–437. [DOI] [PubMed] [Google Scholar]
- 24.Van Oosten M., Rensen P. C. N., Van Ameersfoort E. S., Van Eck M., Van Dam A., Breve J. P., Vogel T., Panet A., Van Berkel T. J. C., Kuiper J. 2001. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. J. Biol. Chem. 276: 8820–8824. [DOI] [PubMed] [Google Scholar]
- 25.Vishnyakova T. G., Bocharov A. V., Baranova I. N., Chen Z., Remaley A. T., Csako G., Eggerman T. L., Patterson A. P. 2003. Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J. Biol. Chem. 278: 22771–22780. [DOI] [PubMed] [Google Scholar]
- 26.Cai L., Ji A., de Beer F. C., Tannock L. R., van der Westhuyzen D. R. 2008. SR-B1 protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Invest. 118: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khovidhunkit W., Kim M., Memon R. A., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45: 1169–1196. [DOI] [PubMed] [Google Scholar]
- 28.Vreugdenhil A. C., Snoek A. M., van ’t Veer C., Greve J. W., Buurman W. A. 2001. LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J. Clin. Invest. 107: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitchens R. L., Thompson P. A. 2003. Impact of sepsis-induced changes in plasma on LPS interactions with monocytes and plasma lipoproteins: roles of soluble CD14, LBP, and acute phase lipoproteins. J. Endotoxin Res. 9: 113–118. [DOI] [PubMed] [Google Scholar]
- 30.Forrest M. J., Bloomfield D., Briscoe R. J., Brown P. N., Cumiskey A-M., Ehrhart J., Hershey J. C., Keller W. J., Ma X., McPherson H. E., et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Brit. J. Pharmacol. 154: 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X., Dietz J. D., Xia C., Knight D. R., Loggin W. T., Smith A. H., Yuan H., Perry D. A., Keiser J. 2009. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 150: 2211–2219. [DOI] [PubMed] [Google Scholar]
- 32.Keidar S., Kaplan M., Pavlotzky E., Coleman R., Havek T., Hamoud S., Aviram M. 2004. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development. Circulation. 109: 2213–2220. [DOI] [PubMed] [Google Scholar]
- 33.Leopold J. A., Dam A., Maron B. A., Scribner A. W., Liao R., Handy D. E., Stanton R. C., Pitt B., Loscalzo J. 2007. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat. Med. 13: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brownlee M. 2005. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 35.Ceriello A., Assaloni R., Ros R. D., Maier A., Piconi L., Quagliaro L., Esposito K., Giugliano D. 2005. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress and inflammation in type 2 diabetic patients. Circulation. 111: 2518–2524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.