Abstract
Levuglandins and their stereo- and regio-isomers (termed isolevuglandins or isoketals) are γ-ketoaldehydes (IsoK) that rapidly react with lysines to form stable protein adducts. IsoK protein adduct levels increase in several pathological conditions including cardiovascular disease. IsoKs can induce ion channel dysfunction and cell death, potentially by adducting to cellular proteins. However, IsoKs also adduct to phosphatidylethanolamine (PE) in vitro, and whether PE adducts form in cells or contribute to the effects of IsoKs is unknown. When radiolabeled IsoK was added to HEK293 cells, 40% of the radiolabel extracted into the chloroform lower phase suggesting the possible formation of PE adducts. We therefore developed methods to measure IsoK-PE adducts in cells. IsoK-PE was quantified by LC/MS/MS after hydrolysis to IsoK-ethanolamine by Streptomyces chromofuscus phospholipase D. In HEK293 and human umbilical vein endothelial cells (HUVEC), IsoK dose-dependently increased PE adduct concentrations to a greater extent than protein adduct. To test the biological significance of IsoK-PE formation, we treated HUVEC with IsoK-PE. IsoK-PE dose dependently induced cytotoxicity (LC50 2.2 μM). These results indicate that cellular PE is a significant target of IsoKs, and that formation of PE adducts may mediate some of the biological effects of IsoKs relevant to disease.
Keywords: cytotoxicity, endothelial cell, inflammation, isoketal, levuglandin, oxidative stress, phosphatidylethanolamine, phospholipase D
Levuglandins and their regio- and stereo-isomers (isolevuglandins or isoketals) are highly reactive γ-ketoaldehydes (IsoK) formed by nonenzymatic rearrangement of prostaglandin H2 and their free radical generated counterparts (H2-isoprostanes) (1–5) (Fig. 1). One feature of IsoKs that drew immediate interest was their extreme reactivity toward the lysyl residues of proteins, with reaction rates several orders of magnitude faster than the α, β-unsaturated aldehyde 4-hydroxynonenal, perhaps the most widely studied lipid peroxidation product (5, 6). This increased reactivity derives from the facile formation of stable pyrrole adducts on primary amines. In the presence of oxygen, these pyrrole adducts convert over time into highly stable lactam adducts, as well as hydroxylactam adducts (5, 7). Increased concentrations of IsoK protein adducts have been observed in vivo during a wide variety of conditions associated with inflammation and oxidative stress, including atherosclerosis, end stage kidney disease, myocardial infarction, Alzheimer's Disease, glaucoma, hyperoxia, allergic inflammation, and experimental sepsis (8–14).
Fig. 1.
Pathways leading to the formation of γ-ketoaldehyde isomers and adducts. In vivo, arachidonic acid is converted to bicyclic endoperoxides either enzymatically by the action of cyclooxygenase working on free arachidonic acid (forming prostaglandin H2) or nonenzymatically by free radical mediated lipid peroxidation of esterified arachidonic acid (forming H2-isoprostanes). Nonenzymatic rearrangement of these bicyclic endoperoxide generates IsoKs, termed levuglandins and isolevuglandins (alternatively named isoketals). IsoKs react with the lysyl residues of proteins, and potentially other primary amines including the ethanolamine head group of PE, to rapidly form pyrrole adducts which convert to lactam and hydroxylactam adducts in the presence of oxygen. IsoK, γ-ketoaldehyde isomer; PE, phosphatidylethanolamine.
Whether IsoK adducts contribute to pathogenesis of these diseases or are simply byproducts of the disease remains an active area of investigation. Evidence supporting a potential role in pathogenesis comes from treatment of cultured cells with exogenous IsoK, which induces a variety of responses relevant to disease, including increased macrophage uptake of LDL, activation of platelet aggregation via p38-MAP kinase, inhibition of sodium and potassium channels, inhibition of proteasome function, and cytotoxicity (9, 15–19).
The mechanism by which IsoK induces these effects has been presumed to be its adduction to cellular proteins, because of the high concentration of lysyl residues in cells. However, these lysyl residues are not the only potential target of IsoK present in cells. In vitro, IsoK can form adducts with a variety of other primary amines including the aminophospholipid phosphatidylethanolamine (PE) (20–22). In phosphate buffer, the reaction rate of γ-ketoaldehydes to form pyrrole adducts with ethanolamine, the head group of the PE, is 4.4-fold faster than with lysine (20). Oxidation of multilaminar vesicles containing PE or of isolated high density lipoprotein in vitro produces pyrrolized phospholipids (measured by reactivity with Ehrlich reagent), suggesting the robust modification of PE by pyrrole forming lipoxidation products under these conditions (23).
Although the biological effects of IsoK-modified PE have not been studied, modification of the ethanolamine headgroup of PE by IsoK is likely to dramatically affect the physical properties of PE as it converts the neutral PE to an acidic phospholipid and significantly increases headgroup bulk. PE modified by IsoK might also activate receptor-mediated signaling in a manner analogous to N-acyl-PE, which suppresses food intake via cFOS expression in neuropeptide Y neurons, and suppresses phagocytosis via Rac1 and Cdc42 in macrophages (24, 25). We therefore sought to determine the extent to which IsoK adducts to PE compared with proteins and whether IsoK-PE could mediate IsoK-induced cytotoxicity.
MATERIALS AND METHODS
Materials
L-α-phosphatidylethanolamine (PE), transphosphatidylated from chicken egg phosphatidylcholine was purchased from Avanti Polar lipids (Alabaster, AL). According to the manufacturer and our LC/MS analysis, the major species of PE in this product is 1-palmitoyl-2-oleolyl-sn-glycerophosphotidylethanolamine (C34:1 PE). All solvents were HPLC grade and were purchased from EMD Biosciences. Streptomyces chromofuscus phospholipase D (PLD) was purchased from Enzo Life Sciences International (Plymouth Meeting, PA), and Proteinase K was purchased from Clontech (Mountain View, CA). Human embryonic kidney (HEK293) cells were purchased from ATCC (Manassas, VA). Endothelial cells derived from donated human umbilical cords (HUVEC) were a kind gift from Dr. Matthew Duvernay. Endothelial cell basal medium was purchased from Lonza Walkersville Inc. (Walkersville, MD). [2H4]ethanolamine was purchased from Cambridge Isotopes (Cambridge, MA). All other chemicals were from Sigma-Aldrich.
Synthesis of 15-E2-isoketal and [3H4]15-E2-isoketal methyl ester
15-E2-isoketal (IsoK) was prepared from dimethoxyacetal precursor (DMA) in methylene chloride as previously described (26) and a stock of 10 mM IsoK prepared in DMSO by evaporation of methylene chloride under nitrogen. For synthesis of [3H4]15-E2-IsoK methyl ester, oct-1-yn-3-ol in acetone was oxidized with chromic acid (room temperature, 1 h) to oct-1-yn-3-one. The ketone was purified by distillation (20–25°C/1 Torr) and reduced with NaB3H4 to [3-3H4]oct-1-yn-3-ol. The latter was then incorporated into IsoK methyl ester as described (26).
Treatment and fractionation of HEK293 cells
HEK293 cells were grown on 100mm dishes to >90% confluence. Cells were then scraped, transferred, washed twice in Dulbecco Phosphate Buffer Saline (DBPS), and then resuspended in 2 ml DPBS. [3H4]15-E2-IsoK methyl ester in DMSO (final concentration 1 μM) was added to the resuspended cells and incubated for 2 h while gently rocking. To separate protein adducts from phospholipid adducts, 10 ml of chloroform/methanol (2:1) was added, and the lower (chloroform) phase removed for counting by liquid scintillation. The protein pellet at the interface between the aqueous and chloroform phase was then also removed and counted. To measure the amount of DNA adducts, replicate HEK293 cells were treated with [3H4]15-E2-IsoK methyl ester, and after the 2 h incubation, the cells were pelleted by centrifugation and resuspended in 0.5 ml lysis buffer (100 mM Tris HCl, pH 8.5, 5 mM EDTA, 0.2% SDS, 200 mM NaCl, 100 ug Proteinase K/ml). The resulting lysate was precipitated with isopropanol, and the supernatant discarded. The DNA precipitant was then counted by liquid scintillation.
Synthesis of modified PE and ethanolamine
Synthesis of IsoK-PE was performed by two methods. IsoK-PE was initially synthesized as described previously (21). In brief, 1.25 μmol PE in CHCl3 was dried down under nitrogen and resuspended in 1ml of a two phase mixture of ether/PBS (4:1). 125 nmol IsoK (0.1 molar equivalents) in 12.5 μl DMSO was added to the sample and incubated for 2 h at 37°C. After incubation, the ether layer was dried off under nitrogen and 1ml chloroform/methanol (2:1) added. The resulting chloroform (lower) phase was transferred, dried under nitrogen, resuspended in 0.5 ml methanol/chloroform (9:1) and stored at −70°C until use. Subsequent experiment used PE in a single phase mixture of 1 M triethylammonium acetate/chloroform/ethanol (1:1:3) in place of the two phase ether/PBS solvent. IsoK-PE synthesized in this manner was used without further purification. The reaction of DMA with PE was performed with PBS/ether in a similar manner as the reaction of IsoK with PE, using 125 nmol DMA (0.1 molar equivalents) in 12.5 μl DMSO instead of IsoK. For the vehicle reaction, 12.5 μl DMSO was added to the PE.
To synthesize IsoK-Etn, 20 μl neat ethanolamine was added to 20 nmol IsoK in 200 μl PBS and incubated for 2 h at 37°C. IsoK-[2H4]Etn was synthesized in a similar manner using 20 μl neat [2H4]ethanolamine. Triethylammonium acetate (1M) was used in some reactions in place of PBS. IsoK-Etn was then extracted with 3 vols chloroform/methanol (2:1), and the resulting product analyzed by mass spectrometry. For quantification of resulting pyrrole product, the reaction mixture was treated with Ehrlich reagent and the absorbance at 580 nm measure by spectrometer.
Hydrolysis of IsoK-PE by PLD
Twenty-five nmol each of IsoK-PE reaction mixture was added to microfuge tubes, along with 784 pmol of IsoK-[2H4]Etn internal standard and dried under nitrogen. Additional samples containing internal standard only were also prepared. The lipids were resuspended in 50 μl methanol, vortexed, 450 μl HBSS added, and then sonicated for 5 min. 10 μl of PLD (>50,000 U/ml) was added to the appropriate tubes and all samples were incubated overnight at 37°C. Then 1.5 ml of chloroform/methanol (2:1) was added to extract IsoK-Etn, the chloroform layer dried down under nitrogen, resuspended in 50 μl methanol, and 10 μl injected unto LC/MS/MS for analysis.
Mass spectrometry analysis
For limited mass scans of the products of the reaction between IsoK and the PE preparation, samples were injected into the ThermoFinnigan Quantum electrospray ionization triple quadrapole mass spectrometer operating in negative ion mode and scanning between m/z 600 and 1200. For collision induced disassociation (CID) spectrum, product ion scanning was performed on the m/z 1032.7 precursor ion, using argon gas in the collision cell. Collision energy was set at 50 eV. Similar analysis was performed on the PE preparation alone, with scanning performed at m/z 600 to m/z 1200, and a CID spectrum obtained for the precursor ion at m/z 716.5.
For LC/MS/MS analysis of IsoK-PE, gradient HPLC was performed using a Agilent Zorbax XDB-C8 2.1 × 50 mm 5 μm column at a flow rate of 250 μl/min starting at 99% Solvent A (methanol/acetonitrile/1 mM ammonium acetate (60:20:20)) for 1 min, then a gradient ramp to 99% Solvent B (1 mM ammonium acetate in ethanol) at 7 min, and then holding at 99% Solvent B for 1 additional min before returning to the starting conditions. The HPLC eluant was directly connected to the mass spectrometer operating in multiple reaction monitoring (MRM) mode for m/z 1032.7→255.1@50eV and 1032.7→255.1@50eV for IsoK-C34:1 PE and m/z 716.6→255.1@50eV for C34:1 PE.
For limited mass scans of the products of the reaction between IsoK and Etn or IsoK and [2H4]Etn, samples were injected into the mass spectrometer operating in positive ion mode and scanning between m/z 350 and 430. For CID spectrum, product ion scanning was performed on the m/z 378.3, 382.3, 394.3, 398.3, 410.3, and 414.3 ions using argon gas in the collision cell. Collision energy was set at 25 eV. For LC/MS/MS analysis of IsoK-Etn, gradient HPLC was performed using a XDB-C8 column at a flow rate of 500 μl/min starting at 100% Solvent A (water with 0.1% acetic acid) with a gradient ramp to 100% Solvent B (methanol with 0.1% acetic acid) at 3 min, and then holding at 100% Solvent B for 1 additional min before returning to the starting conditions. The HPLC eluant was directly connected to the mass spectrometer operating in multiple reaction monitoring mode for m/z 378.3→152.2@-25eV (IsoK-Etn pyrrole), m/z 394.3→178.2@-25eV (IsoK-Etn lactam), m/z 382.3→156.1@-25eV (IsoK-[2H4]Etn pyrrole), and m/z 398.3→182.2@-25eV (IsoK-[2H4]Etn lactam).
Quantification of IsoK protein and IsoK-PE adducts in IsoK treated HEK293 and HUVEC cells
IsoK (0–10 μM) was incubated with human embryonic kidney (HEK293) plated in duplicate wells of 6 well dishes containing HBSS for two h. The treatment media was then removed carefully to avoid disturbing the cell layer, and any unreacted IsoK in the treatment media extracted with hexane and measured by negative ion electrospray LC/MS/MS by monitoring MRM transitions m/z 351→m/z 271. To measure IsoK adducts in cells, 2 ml HBSS was immediately added to the plated cells after removing incubation media, the cell scraped, and cell and wash media transferred to clean centrifuge tubes. Six ml of chloroform/methanol (2:1) was added to each sample to extract the phospholipids, and the lower chloroform layer containing phospholipids was transferred to a new tube. The protein pellet at the interphase was also transferred to a separate tube. After addition of 1 nmol of IsoK-[2H4]Etn as the internal standard, the chloroform layer was dried down under nitrogen gas and resuspended in 50 μl methanol, vortexed, and then 450 μl HBSS added. The entire mixture was then sonicated for 5 min. Fifteen uL PLD was added to the samples and incubated overnight at 37°C. The samples were extracted with 1.5 ml chloroform/methanol (2:1). The chloroform (lower) layer was transferred, dried down under nitrogen, and resuspended in 50 μl methanol for LC/MS/MS analysis. Analysis of IsoK-protein adducts as IsoK-lysyl-lactam adduct after protease digestion was performed on the protein pellet as previously described after addition of 10 pmol IsoK-[13C615N2]lysyl-lactam the internal standard.
Human umbilical vein endothelial cells (HUVEC; passage 6-8) were plated in 6 well dishes coated with 0.2% gelatin and allowed to grow to >90% confluence. The EBM medium was removed and the cells washed three times with HBSS and then duplicate wells treated with 0-1 μM IsoK. Analysis was then performed in similar manner as for HEK293 cells, except that 2 ml 0.25% Trypsin EDTA solution was added to plated cells, allowed to incubate for 3 min, and the cells and wash media removed and analyzed.
HUVEC cytotoxicity
HUVEC (passage 6-8) in EBM basal medium were plated into 96 well plates coated with 0.2% gelatin and allowed to grow to >90% confluence. The EBM medium was removed and the cells washed three times with HBSS. PE treated with 0.1 molar equivalent IsoK, DMA, or vehicle was prepared as described above, evaporated under nitrogen and resuspended in HBSS containing 0.1% BSA (HBSS/A). To determine the concentration dependence of IsoK-PE induced HUVEC cytotoxicity, the concentration of IsoK, DMA, or vehicle treated PE were calculated from the amount of IsoK or DMA added to PE, respectively. Therefore, for each stated treated PE concentration, the total PE concentration (modified and unmodified PE) was equivalent for each type of treatment and was exactly 10- fold higher than the stated treated PE concentration. IsoK-, DMA-, or vehicle-treated PE (0.1–30 μM final concentration) were each added to six replicate wells of HUVEC. Untreated control wells were used to determine maximum viability. Cells were incubated with their respective treated PE for 22 h, and then 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) added to each well for an additional two h, the culture media replaced with isopropanol and the extent of MTT conversion measured by the absorbance at 570 nm as previously described. Viability was normalized to the HBSS/A only treated cells.
To determine the amount of IsoK-PE that entered the cells during the incubation process to that amount of IsoK-PE formed when IsoK was directly incubated with cells, replicate plates of HUVEC cells were treated with 3 μM IsoK-PE for 2 h, the incubation media removed, and IsoK-PE measured after PLD incubation as described for IsoK treated HUVEC cells.
RESULTS
Potential formation of nonprotein adducts in IsoK treated cells
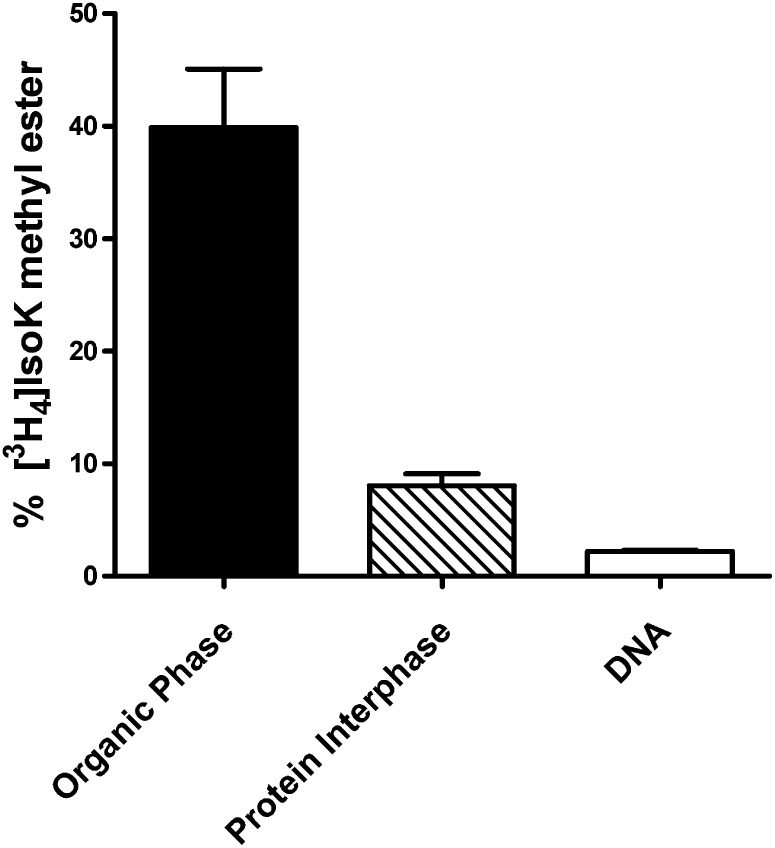
To identify significant cellular targets of IsoKs, a radiolabeled methyl ester ([3H4]IsoK-methyl ester) of 15-E2-IsoK, a representative IsoK, was incubated with HEK293 cells and the resulting adduct species separated by chloroform/methanol extraction. HEK293 cells were chosen for initial cellular studies because of their rapid growth characteristic and ease of maintenance. We found that only 8% of the radiolabel associated with the protein interphase, while 40% of the radiolabel associated with the chloroform lower phase that includes phospholipids (Fig. 2). The remaining radiolabel associated with the upper aqueous phase potentially containing adducts with small peptides, polyamines, nucleic acids, and other polar amines. When DNA was isolated directly from replicate treated cells, 2% of the radiolabel was associated with DNA. The distribution of radiolabel into the lower chloroform layer is consistent with the formation of substantial amounts of adducts with aminophospholipids such as PE; however, [3H4]IsoK methyl ester is itself quite lipophilic, so that the radiolabel in the lower chloroform layer could also represent unreacted IsoK or metabolites. Therefore, we sought definitive evidence for the formation of IsoK-aminophospholipid adducts in treated cells.
Fig. 2.
Distribution of IsoK adducts in cells. [3H]IsoK methyl ester (1 μM) was added to HEK293 cells for two h, and the distribution of the resulting adducts measured by liquid scintillation counting of the [3H] extracted into lower chloroform organic phase that includes phospholipids, into the protein interphase, and into isolated DNA. IsoK, γ-ketoaldehyde isomer.
Characterization of the reaction of IsoK with phosphatidylethanolamine
The amount of PE adduct formed in cells should depend both on the relative amount of PE and the reaction rate of PE versus other primary amines. The model γ-ketoaldehyde, 4-oxopentanal (OPA) reacts with ethanolamine 4.4 times faster than the reaction rate with N-acetyl lysine (20). We determined the second order reaction rate of OPA and PE using methods similar to our previous study, except that we used a more lipophilic solvent system (1 M triethylammonium acetate/chloroform/ethanol 1:1:3 v/v/v) to maintain the solubility of the final product. In this lipophilic system, the reaction rate of OPA with PE was 4.5 × 10−3 M−1 s−1, which was 25 times faster than the reaction rate of OPA with N-acetyl lysine (0.18 × 10−3 M−1 s−1) that we had previously measured.
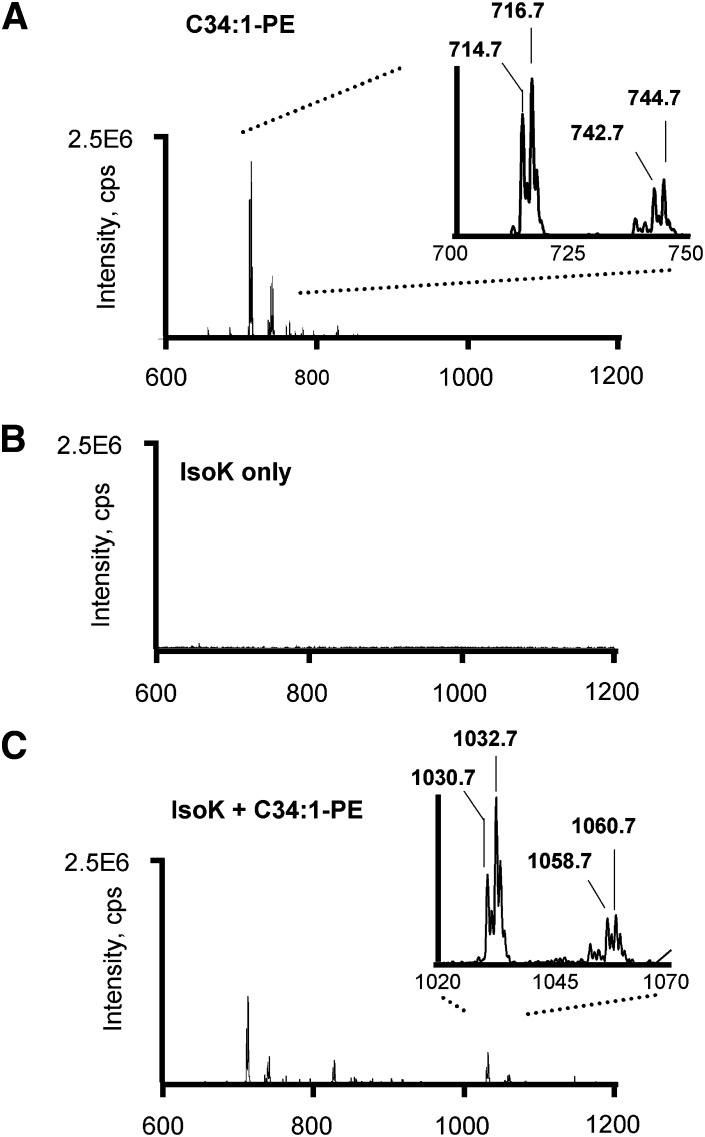
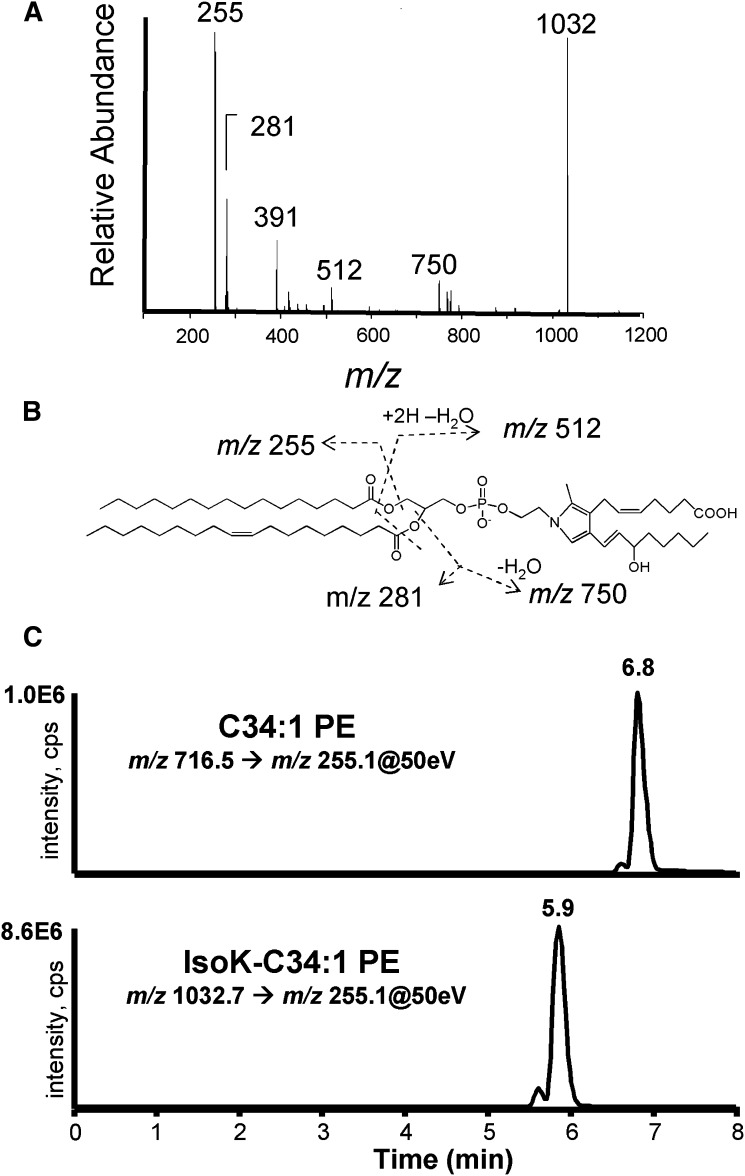
To characterize the IsoK-PE adduct and develop an analytical method to quantify these adducts, we reacted a commercial preparation of PE primarily containing 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (C34:1 PE) and lesser amounts of C34:2 PE, C36:1 PE, and C36:2 PE (Fig. 3A) with a representative IsoK, 15-E2-IsoK (Fig. 3B) and compared the resulting products (Fig. 3C) by negative ion mass spectrometry. Addition of IsoK decreased the amount of signals at m/z 716.7 (C34:1PE), m/z 714.7 (C34:2 PE), m/z 744.7 (C36:1 PE), and m/z 742.7 (C36:2 PE) and led to the formation of new signals at m/z 1032.7, 1030.7, 1060.7, and 1058.7. The +316 amu mass shift of these novel peaks from the starting PE is consistent with formation of IsoK-pyrrole adducts for C34:1, C34:2, C36:1, and C36:2 PE, respectively. Under our reaction conditions, we did not observe formation of oxidized pyrrole adducts (lactam or hydroxylactam) even after overnight incubations, in contrast to our previous observations with IsoK protein adducts, probably because the organic solvents used for the PE reaction tend to exclude oxygen to a greater extent than aqueous buffers used for protein reactions. However, when the final PE product was dried down and left exposed to room air for several h, we observed a peak at 1048.7, consistent with the formation of a lactam adduct (not shown.) The CID spectrum of m/z 1032.7 includes major product ions at m/z 255, 281, 391, 512, and 750 (Fig. 4A). The product ions at m/z 255 and m/z 281 are consistent with the two fatty acid side chains (Fig. 4B), while the product at m/z 391 is consistent with a dehydrated C16:0 lysoPA product. The product ion at m/z 512 is consistent with the neutral loss of the two acyl chains from IsoK-PE, and the product ion at m/z 750 is consistent with the neutral loss of the sn-2 acyl chain. Given the characteristic fragmentation of IsoK-C34:1 PE, the expected product ions formed by other diacyl PE modified by IsoK can be readily predicted. Therefore, we used IsoK-C34:1 PE to develop an appropriate LC/MS/MS assay to measure the amount of IsoK-PE and PE. Modification of the HPLC method previously described for the separation of N-acyl-PE from PE, achieved significant separation of IsoK-C34:1 PE from unmodified C34:1 PE (Fig. 4C).
Fig. 3.
IsoK reacts with PE to form pyrrole adduct. IsoK was incubated with a commercial preparation of PE and negative ion limited mass scanning from m/z 600 to 1200 performed on the starting reactants and reaction mixture to identify novel ions in the reaction mixture. A: Limited mass scans of the commercial PE preparation containing primarily C34:1 PE (m/z 716.7) as well as some C34:2 PE, C36:1 PE, and C36:2 PE. B: Limited mass scans of the IsoK preparation. C: Limited mass scans of the reaction mixture of PE and IsoK showing novel peaks with +316 amu mass shifts from the PE preparation, consistent with the formation of IsoK-PE pyrrole adducts. IsoK, γ-ketoaldehyde isomer; PE, phosphatidylethanolamine.
Fig. 4.
Development of IsoK-PE LC/MS/MS assay. A: CID spectrum of m/z 1032.7 ion. B: Interpretation of CID spectrum. C: MRM ion chromatographs from LC/MS/MS analysis of IsoK-PE preparation for C34:1 PE and IsoK-34:1 PE pyrrole. IsoK, γ-ketoaldehyde isomer. CID, collision induced disassociation; IsoK, γ-ketoaldehyde isomer; MRM, multiple reaction monitoring; PE, phosphatidylethanolamine.
We then treated HEK293 cells with vehicle or 10 μM IsoK and monitored the formation of IsoK-PE using MRM of transitions between the predicted precursor ion of various IsoK-PE species containing C16:0 or C18:0 acyl chains and their appropriate product ions at m/z 255 (C16:0) or m/z 283 (C18:0). In HEK293 cells treated with vehicle (0 μM IsoK), there were virtually no signals above the noise when monitoring predicted IsoK-PE precursor ions (Fig. 5, left panel) . In contrast, addition of 10 μM IsoK to HEK293 resulted in significant signals at the appropriate retention times for a number of potential IsoK-PE species (Fig. 5, right panel). The rank order of peak height for these predicted IsoK-PE species were C34:1 (m/z 1032.7) > C36:1 (m/z 1060.7) > C38:4 (m/z 1082.7) > C34:0 (m/z 1034.7) > C36:0 (m/z 1062.7) > C36:4 (m/z 1054.7). The average ratios of the peak height for IsoK-C34:1 PE (m/z 1032.7→m/z 255) compared with C34:1 PE (m/z 716.7→m/z 255) in cells treated with 10 μM IsoK was 0.013. Although potential differences in ionization efficiency between PE and IsoK-PE preclude exact quantitation based on these peak heights, these results would suggest that 10 μM IsoK potentially modified roughly 1–2% of the C34:1 PE.
Fig. 5.
IsoK treatment of HEK293 cells forms IsoK-PE. HEK293 were treated either with vehicle (0 μM IsoK, left panel) or 10 μM IsoK (right panel) for 2 h. MRM ion chromatographs of the IsoK pyrrole adducts for the most abundant diacyl PE species. IsoK, γ-ketoaldehyde isomer; MRM, multiple reaction monitoring; PE, phosphatidylethanolamine.
IsoK-PE is a substrate for phosholipase D
Although individual PE species modified by IsoK in cells can be measured by this method, relatively high concentrations of IsoK (10 μM) were required to detect significant signals above noise so that this method would not be very useful for cell based studies analyzing lower concentrations of IsoK. Furthermore, accurate quantitation of the total IsoK-PE formed in cells after various stimuli would require monitoring each of the individual diradyl IsoK-PE species and summing their individual abundances. With potentially more than 30 individual diradyl PE species present in cells, accurate measurement of IsoK-PE by this method appeared unwieldy. Therefore, we sought to develop an analytical method that could quantify the entire cellular content of IsoK-PE in a single analysis. One potential strategy to achieve this goal is to enzymatically or chemically hydrolyze the modified ethanolamine (Etn) head group and analyze the resulting IsoK-Etn products.
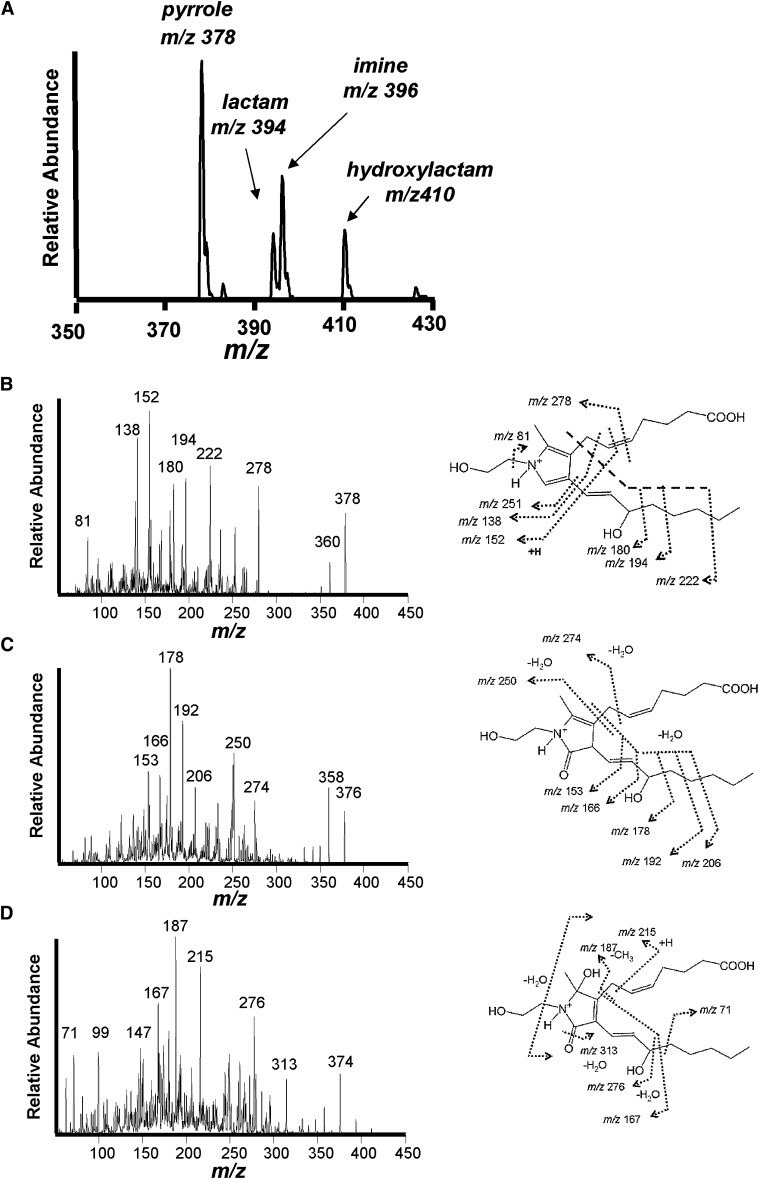
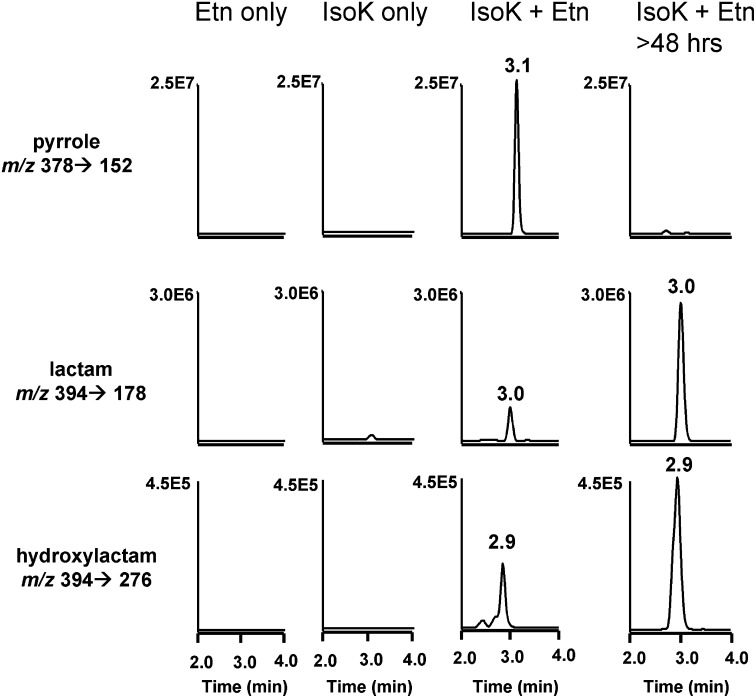
To determine if IsoK-Etn could be quantified by stable isotope dilution LC/MS/MS, we reacted IsoK with an excess of Etn or [2H4]Etn and analyzed the resulting products by limited mass scan positive ion electrospray mass spectrometry. The most abundant product formed by the two h reaction of IsoK with Etn was a m/z 378 ion, consistent with an IsoK-Etn pyrrole adduct (Fig. 6A). Additional products at m/z 394, m/z 396, and m/z 410 are consistent with formation of the expected lactam, imine, and hydroxylactam IsoK-Etn products. The CID spectrum of the m/z 378 ion showed a major product ion at m/z 152, along with ions with m/z 81, 138, 152, 180, 194, 222, 234, 251, 278, and 360 (Fig. 3B). The CID spectrum of the m/z 394 ion showed product ions at m/z 153, 166, 178, 192, 206, 250, 274, 358, and 376. The CID spectrum of the m/z 410 ion showed product ions at m/z 71, 99, 147, 167, 187, 215, 276, 313, and 374. The CID spectrum of the m/z 396 ion showed product ions at m/z 62, 221, 281, 291, 299, 317, 360, and 378 consistent with an imine adduct (not shown), but this product was not studied further because our experience with IsoK protein adducts suggested that the imine is an unstable intermediate that converts relatively rapidly to the pyrrole adduct. The reaction of IsoK with [2H4]Etn generated products at m/z 382, 398, 400, and 414, and the CID spectrum of these individual products showed 4 amu mass shifts from the CID spectrum of their nonisotopically labeled counterparts (not shown). We then utilized these ions to develop a suitable stable isotope dilution LC/MS/MS assay for IsoK-Etn. Under these conditions, the hydroxylactam adduct eluted with a retention time of 2.9 min, the lactam product eluted with a retention time of 3.0 min, and the pyrrole adduct with a retention time of 3.1 min (Fig. 7). When the products of the reaction with either IsoK only, Etn only, or IsoK with Etn were monitored using this method, signal for the pyrrole, lactam, and hydroxylactam adduct were only present in the reaction containing both IsoK and Etn. Analysis of the IsoK with Etn reaction initially showed that the pyrrole product gave the most intense signal, but after 48 h, the predominant signal was that of the lactam adduct. Therefore, both pyrrole and lactam were chosen for monitoring in subsequent experiments.
Fig. 6.
Identification of IsoK ethanolamine (Etn) adducts. IsoK was incubated with Etn and resulting products analyzed by positive ion electrospray ionization mass spectrometry. A: Limited mass scan spectrum of the reaction of IsoK with Etn produces ion consistent with pyrrole (m/z 378), lactam (m/z 394), Schiff base (m/z 396), and hydroxylactam (m/z 410) adducts. B: CID spectrum and interpretation of m/z 378 ion. C: CID spectrum and interpretation of m/z 394 ion. D: CID spectrum and interpretation of m/z 410 ion. CID, collision induced disassociation; IsoK, γ-ketoaldehyde isomer.
Fig. 7.
LC/MS/MS analysis of IsoK-Etn adducts. MRM ion chromatographs for the pyrrole, lactam, and hydroxylactam adducts in reaction mixtures containing Etn only, IsoK only, or both IsoK and Etn. IsoK, γ-ketoaldehyde isomer; MRM, multiple reaction monitoring.
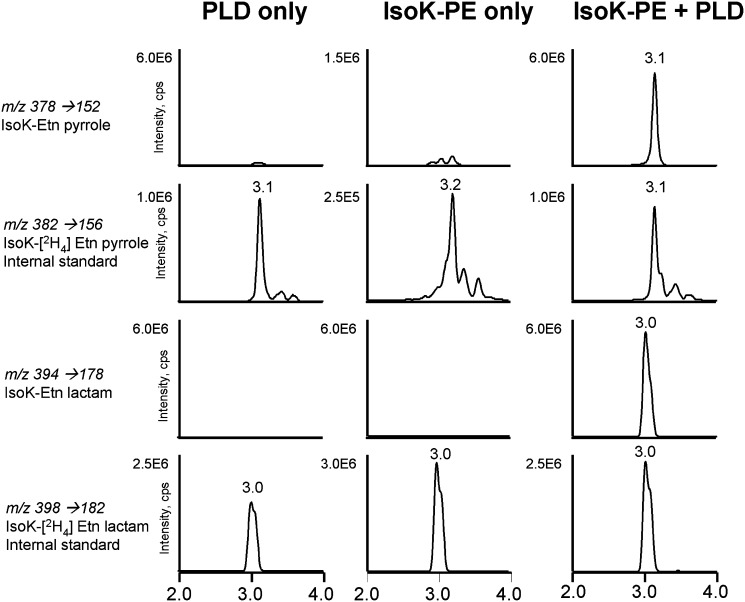
The phospholipase D from S. chromofuscus is able to efficiently hydrolyze PE modified with long chain N-acyl groups, suggesting that this enzyme might be able to recognize IsoK-PE as well. We therefore determined whether IsoK-PE could be converted to IsoK-Etn utilizing treatment with this enzyme. IsoK-[2H4]Etn was added as an internal standard to samples with PLD only, IsoK-PE only, or IsoK-PE and PLD. Sample with S. chromofuscus PLD only did not generate significant IsoK-Etn signal, (Fig. 8, left panel), but gave strong signals for both the IsoK-[2H4]Etn internal standard pyrrole and lactam adducts. The sample with IsoK-PE only gave similar results (Fig. 8, middle panel.) In contrast, the sample with S. chromofuscus PLD treated IsoK-PE gave very strong signals with appropriate retention times for both IsoK-Etn pyrrole and lactam adducts (Fig. 8, right panel.) These results demonstrate that S. chromofuscus PLD converts IsoK-PE to IsoK-Etn. Although the original IsoK-PE preparation contained very little lactam adduct, the final PLD hydrolysis product contains significant amounts of the lactam adduct, suggesting that during the overnight incubation with buffer and lipase the pyrrole adduct undergoes conversion to the lactam adduct.
Fig. 8.
Streptomyces chromofuscus PLD hydrolyzes IsoK-PE to IsoK-Etn. IsoK-PE preparation was treated with vehicle or S. chromofuscus PLD for 2 h, IsoK-[2H4]Etn added as internal standard, and the resulting products analyzed by LC/MS/MS for IsoK-Etn. Left panel: MRM ion chromatographs for reaction with PLD only. Middle panel: MRM ion chromatographs for IsoK-PE treated with vehicle only. Right panel: MRM ion chromatographs for PLD treated IsoK-PE. IsoK, γ-ketoaldehyde isomer; MRM, multiple reaction monitoring; PE, phosphatidylethanolamine; PLD, phospholipase D.
PE is a significant target of IsoK in cells
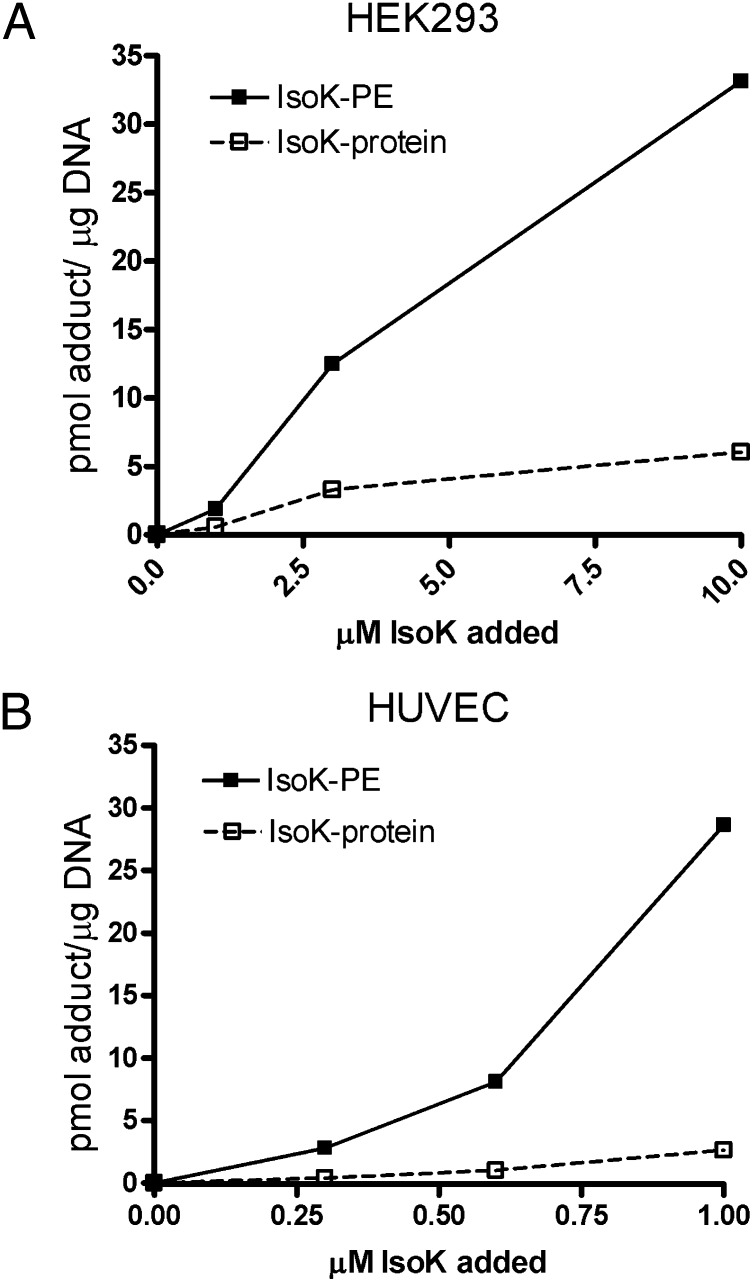
We then utilized this methodology to quantify the total amount of IsoK-PE formed in cells treated with IsoK and compared this to the total amount of IsoK-protein adduct. HEK293 cells were treated with IsoK (0–10 μM) for 2 h, the treatment media removed and analyzed for unreacted IsoK, and the levels of PE and protein adduct measured in the cells. On average, less than half of the added IsoK (47%) was still present in the incubation media after 2 h of incubation at the various IsoK concentrations, suggesting that the majority of IsoK reacted with cellular amines. Levels of both protein and PE adducts in HEK293 cells increased as the concentration of IsoK in the treatment media increased (Fig. 9A). Under these conditions, the average amount of measured PE and protein adducts represented 6.9% and 1.7%, respectively, of the reacted IsoK, consistent with the greater reactivity rate of PE.
Fig. 9.
PE adducts form to a greater extent than protein adducts in cells. IsoK was added for 2 h at increasing concentrations to HEK293 or HUVEC cells plated in six well plates and the amount of PE and protein adduct quantified by LC/MS/MS and normalized to amount of DNA in each well. A: Levels of PE and protein adduct in HEK293 cells incubated with 0-10 μM IsoK. B: Levels of PE and protein adduct in HUVEC cells incubated with 0-1 μM IsoK. IsoK, γ-ketoaldehyde isomer; PE, phosphatidylethanolamine.
To determine whether IsoK-PE adducts were also formed in cell types relevant to disease, we measured the extent of IsoK-PE formation in human umbilical vein endothelial cells treated with IsoK. Treatment of HUVEC with IsoK (0-1 μM) also resulted in the formation of IsoK-PE adducts and protein adducts (Fig. 9B), and again the amount of PE adduct formed was greater than that of protein adduct. These results indicate that PE is a significant target of IsoK in cells, raising the possibility that IsoK-PE might mediate some of the effects of IsoK on cells.
IsoK-PE decreases the viability of endothelial cells
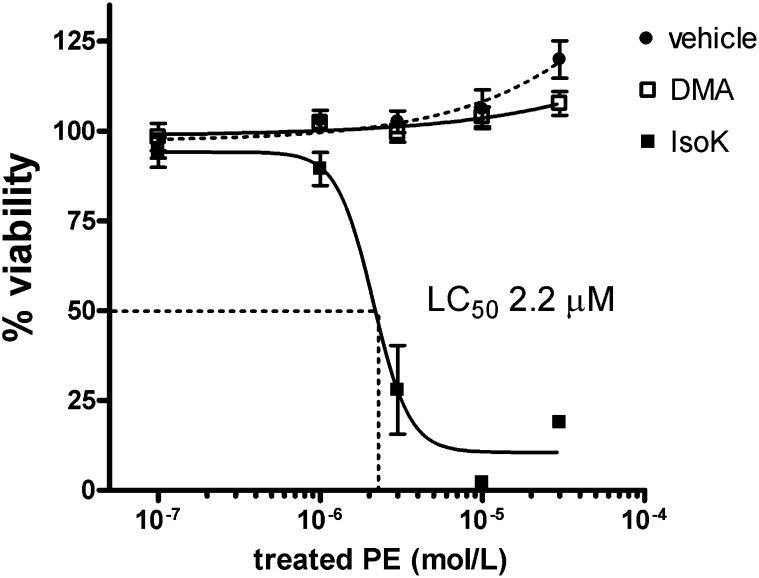
To test whether IsoK-PE could induce similar effects as IsoK, we examined the ability to IsoK-PE to induce endothelial cell death. Endothelial cell injury is a hallmark of atherosclerosis and IsoKs are some of the most potent cytotoxins formed by lipid peroxidation. To test whether IsoK-PE induced endothelial cytotoxicity, we prepared IsoK-PE as before, along with PE treated with the DMSO (vehicle) or with the inactive dimethoxy acetal isoketal precursor (DMA). Human umbilical vein endothelial cells were incubated overnight with 0.1–30 μM IsoK, DMA, or vehicle treated PE and the resulting changes in cell viability measured. Vehicle and DMA treated PE had no significant effect on viability at any of the tested concentrations, but IsoK-PE concentration dependently reduced the viability of HUVEC (LC50 2.2 μM) (Fig. 10). To compare the amount of IsoK-PE that actually entered the cells under these conditions with the amount of IsoK-PE formed when HUVEC where directly treated with IsoK, we incubated HUVEC for 1 h with 3 μM IsoK-PE, removed the treatment media from the cells, and then measured the IsoK-PE adduct/ μg DNA in the HUVEC. We found that treatment with 3 μM IsoK-PE resulted in 1.4 pmol adduct/μg DNA in the treated HUVEC, approximately half the amount of IsoK-PE formed when adding 0.3 μM IsoK to the HUVEC. These results are consistent with the notion that IsoK induces cytotoxicity at least in part by reacting with PE to form a cytotoxic product.
Fig. 10.
IsoK-PE is cytotoxic to HUVEC. PE was treated with either 0.1 molar equivalent of IsoK (IsoK-PE), the inactive dimethoxy acetal synthetic precursor of IsoK (DMA-PE), or the DMSO vehicle (veh-PE) and 0.1 to 30 μM of the resulting PE reaction mixture incubated with HUVEC overnight. Viability was determined by MTT conversion assay and normalized to viability of untreated cells. HUVEC, human umbilical vein endothelial cell; IsoK, γ-ketoaldehyde isomer; PE, phosphatidylethanolamine.
DISCUSSION
While the formation of protein adducts by IsoK has been well established in vivo and presumed to mediate the biological effects of IsoK, the extent of IsoK adduction to other biologically relevant amines in vivo and their biological activity remains poorly characterized. Our findings suggest that at least one other type of adduct, PE adducts, are formed in greater abundance in cells and may mediate some of the effects of IsoK. While our findings were being revised for publication, Li et al. published a report finding IsoK modified PE in human plasma, as well as in the liver of ethanol treated mice (27). Interestingly, the levels of PE adducts that they reported in healthy volunteers (3.4 ng/ml) are substantially higher than the levels of protein adduct than we have previously reported in plasma (0.56 ng/ml) (17). The plasma ratio of PE to protein adducts in these two studies are very similar to the ratio of PE to protein adducts that we found in our cellular studies. Another publication during the revision period by Carrier et al. showing that IsoK formed adducts on deoxycytidine (28) is consistent with our finding that IsoK also formed DNA adduct to a lesser extent than protein adducts.
The finding that PE adducts are formed to a greater extent than protein adducts raises the possibility that IsoK-PE is the active mediator of some of the biological effects of IsoK. Our finding that IsoK-PE alone is sufficient to induce cytotoxicity in HUVEC is consistent with the notion that IsoK induces cytotoxicity at least in part by forming IsoK-PE which then directly induces cell death. While in vitro studies clearly indicate that direct covalent modification of enzymes by IsoK can profoundly alter enzymatic activity, the biological function of membrane associated enzymes and receptors could also be altered by noncovalent interactions with IsoK-PE. Modification of the terminal amine of PE by the bulky and acidic IsoK would be anticipated to significantly disrupt inner leaflet membrane packing and curvature and thereby perturb membrane integrity and membrane protein function. Such potential perturbations of mitochondrial membranes by IsoK-PE might plausibly underlie the cytotoxicity to HUVEC induced by IsoK-PE. IsoK-PE might also act as ligand for receptors, analogous to recent findings that N-acyl-PE suppresses food intake via cFOS expression in neuropeptide Y neurons (24) and suppresses phagocytosis via Rac1 and Cdc42 in macrophages (25). The activation of a membrane receptors linked to cell death by IsoK-PE would be consistent with the previously described activation of p38-MAPK by IsoK in platelets (16). While no receptors for lipid modified PE have been identified to date, several G-protein coupled receptors and nuclear receptors bind lipids as high affinity ligands and the physiological ligands for many receptors remain uncharacterized, so that future studies may well identify specific lipid modified PE receptors.
One general characteristic of receptor ligands is the existence of mechanisms to regulate their levels, particularly degradative enzymes. The finding that the PLD from S. chromofuscus can cleave IsoK-PE in addition to N-acyl-PE suggests that lipases active against N-acyl-PE in other species might also be able to degrade IsoK-PE. Of interest in this regard is the recent finding that in mammalian cells there are at least two enzymes, N-acyl-PE -PLD (29) and α/beta-hydrolase 4 (30), that hydrolyze N-acyl-PE. It would be of great interest to determine if IsoK-PE is a substrate for either of these enzymes and the effect of genetic ablation of these enzymes on endothelial cell viability or inflammatory signaling during oxidative stress and inflammation.
Given the greater abundance of PE compared with protein adducts, it is of interest to consider whether the effects of IsoK scavengers such as salicylamine, which protects cells from hydrogen peroxide induced cytotoxicity (22), might be mediated at least in part by protecting against the formation of IsoK-PE adducts. Future studies will be need to determine both the mechanisms of this cytotoxicity and if other cell types are also vulnerable.
In summary, our finding that IsoK-PE is one of the most abundant adducts of IsoK in cells identifies a new potential mediator of the effects of IsoK and provides methods to examine the extent of its formation in physiologically relevant conditions.
Acknowledgments
The authors appreciate the valuable advice of Dr. Stephen Milne on mass spectrometric analysis of phospholipids and Pablo Darelli for excellent technical assistance.
Footnotes
Abbreviations:
- CID
- collision induced disassociation
- HEK293
- human embryonic kidney
- HUVEC
- human umbilical vein endothelial cell
- IsoK
- γ-ketoaldehyde isomer
- MRM
- multiple reaction monitoring
- PE
- phosphatidylethanolamine
- PLD
- phospholipase D
This work was supported by Vanderbilt junior faculty start-up funds and the National Institutes of Health Grants HL-079365 and GM-42056. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Salomon R. G., Miller D. B., Zagorski M. G., Coughlin D. J. 1984. Solvent induced fragmentation of prostaglandin endoperoxides. New aldehyde products from PGH2 and novel intramolecular 1,2-hydride shift during endoperoxide fragmentation in aqueous solution. J. Am. Chem. Soc. 106: 6049–6060. [Google Scholar]
- 2.Salomon R. G., Miller D. B. 1985. Levuglandins: isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv. Prostaglandin Thromboxane Leukot. Res. 15: 323–326. [PubMed] [Google Scholar]
- 3.Salomon R. G., Sha W., Brame C., Kaur K., Subbanagounder G., O'Neil J., Hoff H. F., Roberts L. J., II 1999. Protein adducts of iso[4]levuglandin E2, a product of the isoprostane pathway, in oxidized low density lipoprotein. J. Biol. Chem. 274: 20271–20280. [DOI] [PubMed] [Google Scholar]
- 4.Salomon R. G., Subbanagounder G., Singh U., O'Neil J., Hoff H. F. 1997. Oxidation of low-density lipoproteins produces levuglandin-protein adducts. Chem. Res. Toxicol. 10: 750–759. [DOI] [PubMed] [Google Scholar]
- 5.Brame C. J., Salomon R. G., Morrow J. D., Roberts L. J., II 1999. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 274: 13139–13146. [DOI] [PubMed] [Google Scholar]
- 6.Iyer R. S., Ghosh S., Salomon R. G. 1989. Levuglandin E2 crosslinks proteins. Prostaglandins. 37: 471–480. [DOI] [PubMed] [Google Scholar]
- 7.DiFranco E., Subbanagounder G., Kim S., Murthi K., Taneda S., Monnier V. M., Salomon R. G. 1995. Formation and stability of pyrrole adducts in the reaction of levuglandin E2 with proteins. Chem. Res. Toxicol. 8: 61–67. [DOI] [PubMed] [Google Scholar]
- 8.Salomon R. G., Batyreva E., Kaur K., Sprecher D. L., Schreiber M. J., Crabb J. W., Penn M. S., DiCorletoe A. M., Hazen S. L., Podrez E. A. 2000. Isolevuglandin-protein adducts in humans: products of free radical-induced lipid oxidation through the isoprostane pathway. Biochim. Biophys. Acta. 1485: 225–235. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda K., Davies S. S., Nakajima T., Ong B. H., Kupershmidt S., Fessel J., Amarnath V., Anderson M. E., Boyden P. A., Viswanathan P. C., et al. 2005. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ. Res. 97: 1262–1269. [DOI] [PubMed] [Google Scholar]
- 10.Zagol-Ikapitte I., Masterson T. S., Amarnath V., Montine T. J., Andreasson K. I., Boutaud O., Oates J. A. 2005. Prostaglandin H(2)-derived adducts of proteins correlate with Alzheimer's disease severity. J. Neurochem. 94: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 11.Salomon R. G., Subbanagounder G., O'Neil J., Kaur K., Smith M. A., Hoff H. F., Perry G., Monnier V. M. 1997. Levuglandin E2-protein adducts in human plasma and vasculature. Chem. Res. Toxicol. 10: 536–545. [DOI] [PubMed] [Google Scholar]
- 12.Davies S. S., Talati M., Wang X., Mernaugh R. L., Amarnath V., Fessel J., Meyrick B. O., Sheller J., Roberts L. J., II 2004. Localization of isoketal adducts in vivo using a single-chain antibody. Free Radic. Biol. Med. 36: 1163–1174. [DOI] [PubMed] [Google Scholar]
- 13.Talati M., Meyrick B., Peebles R. S., Jr., Davies S. S., Dworski R., Mernaugh R., Mitchell D., Boothby M., Roberts L. J., II, Sheller J. R. 2006. Oxidant stress modulates murine allergic airway responses. Free Radic. Biol. Med. 40: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 14.Poliakov E., Brennan M. L., Macpherson J., Zhang R., Sha W., Narine L., Salomon R. G., Hazen S. L. 2003. Isolevuglandins, a novel class of isoprostenoid derivatives, function as integrated sensors of oxidant stress and are generated by myeloperoxidase in vivo. FASEB J. 17: 2209–2220. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe G., Subbanagounder G., O'Neil J., Salomon R. G., Hoff H. F. 1997. Macrophage recognition of LDL modified by levuglandin E2, an oxidation product of arachidonic acid. Biochim. Biophys. Acta. 1344: 1–5. [DOI] [PubMed] [Google Scholar]
- 16.Bernoud-Hubac N., Alam D. A., Lefils J., Davies S. S., Amarnath V., Guichardant M., Roberts Ii L. J., Lagarde M. 2009. Low concentrations of reactive [gamma]-ketoaldehydes prime thromboxane-dependent human platelet aggregation via p38-MAPK activation. Biochim. Biophys. Acta. 1791: 307–313. [DOI] [PubMed] [Google Scholar]
- 17.Brame C. J., Boutaud O., Davies S. S., Yang T., Oates J. A., Roden D., Roberts L. J., II 2004. Modification of proteins by isoketal-containing oxidized phospholipids. J. Biol. Chem. 279: 13447–13451. [DOI] [PubMed] [Google Scholar]
- 18.Davies S. S., Amarnath V., Montine K. S., Bernoud-Hubac N., Boutaud O., Montine T. J., Roberts L. J., II 2002. Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 16: 715–717. [DOI] [PubMed] [Google Scholar]
- 19.Davies S. S. 2008. Modulation of protein function by isoketals and levuglandins. Subcell. Biochem. 49: 49–70. [DOI] [PubMed] [Google Scholar]
- 20.Amarnath V., Amarnath K., Amarnath K., Davies S., Roberts L. J., II 2004. Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem. Res. Toxicol. 17: 410–415. [DOI] [PubMed] [Google Scholar]
- 21.Bernoud-Hubac N., Fay L. B., Armarnath V., Guichardant M., Bacot S., Davies S. S., Roberts L. J., II, Lagarde M. 2004. Covalent binding of isoketals to ethanolamine phospholipids. Free Radic. Biol. Med. 37: 1604–1611. [DOI] [PubMed] [Google Scholar]
- 22.Davies S. S., Brantley E. J., Voziyan P. A., Amarnath V., Zagol-Ikapitte I., Boutaud O., Hudson B. G., Oates J. A., Roberts L. J., II 2006. Pyridoxamine analogues scavenge lipid-derived gamma-ketoaldehydes and protect against H(2)O(2)-mediated cytotoxicity. Biochemistry. 45: 15756–15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hidalgo F. J., Nogales F., Zamora R. 2004. Determination of pyrrolized phospholipids in oxidized phospholipid vesicles and lipoproteins. Anal. Biochem. 334: 155–163. [DOI] [PubMed] [Google Scholar]
- 24.Gillum M. P., Zhang D., Zhang X-M., Erion D. M., Jamison R. A., Choi C., Dong J., Shanabrough M., Duenas H. R., Frederick D. W., et al. 2008. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 135: 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiratsuchi A., Ichiki M., Okamoto Y., Ueda N., Sugimoto N., Takuwa Y., Nakanishi Y. 2009. Inhibitory effect of N-palmitoylphosphatidylethanolamine on macrophage phagocytosis through inhibition of Rac1 and Cdc42. J Biochem. 145: 43–50. [DOI] [PubMed] [Google Scholar]
- 26.Amarnath V., Amarnath K., Masterson T., Davies S., Roberts L. J. 2005. A simplified synthesis of diastereomers of Levuglandin E2. Synth. Commun. 35: 397–408. [Google Scholar]
- 27.Li W., Laird J. M., Lu L., Roychowdhury S., Nagy L. E., Zhou R., Crabb J. W., Salomon R. G. 2009. Isolevuglandins covalently modify phosphatidylethanolamines in vivo: detection and quantitative analysis of hydroxylactam adducts. Free Radic. Biol. Med. 47: 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrier E. J., Amarnath V., Oates J. A., Boutaud O. 2009. Characterization of covalent adducts of nucleosides and DNA formed by reaction with levuglandin. Biochemistry. 48: 10775–10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto Y., Morishita J., Tsuboi K., Tonai T., Ueda N. 2004. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 279: 5298–5305. [DOI] [PubMed] [Google Scholar]
- 30.Simon G. M., Cravatt B. F. 2006. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J. Biol. Chem. 281: 26465–26472. [DOI] [PubMed] [Google Scholar]