Abstract
Background and Purpose
Although myelin-associated neurite outgrowth disinhibitors have shown promise in restoring motor function after stroke, their interactive effects with motor training have rarely been investigated. The present study examined whether a combinatorial treatment (NEP 1–40+motor rehabilitation) is more effective than either treatment alone in promoting motor recovery after focal ischemic injury.
Methods
Adult rats were assigned to one of 3 treatment groups (infarct/NEP 1–40+motor training, infarct/NEP 1–40 only, infarct/motor training only) and 2 control groups (infarct/no treatment, intact/no treatment). A focal ischemic infarct was induced by microinjecting endothelin-1 into the motor cortex. Therapeutic treatments were initiated 1 week postinfarct and included intraventricular infusion of the pharmacological agent NEP 1–40 and motor training (skilled reach task). Behavioral assessments on skilled reach, foot fault, and cylinder tests were conducted before the infarct and for 5 weeks postinfarct.
Results
Rats demonstrated significant forelimb impairment on skilled reach and foot fault tests after the infarct. Although all infarct groups improved over time, motor training alone and NEP 1–40 alone facilitated recovery on the skilled reach task at the end of treatment Weeks 2 and 4, respectively. However, only NEP 1–40 paired with motor training facilitated recovery after 1 week of treatment in addition to treatment at Weeks 2 and 4. Finally, only the NEP 1–40+motor training group maintained a performance level equivalent to that of the intact group over the entire period of posttreatment assessment.
Conclusions
This study suggests that behavioral training interacts with the effects of the axonal growth promoter, NEP 1–40, and may accelerate behavioral recovery after focal cortical ischemia.
Keywords: cerebral infarct, motor cortex, rehabilitation, recovery, regeneration
It is widely recognized that functional recovery after stroke can be aided by rehabilitative training. Motor training induces neurophysiological and neuroanatomical plasticity in the motor cortex of intact brains as well as after stroke.1,2 Postinfarct training induces map reorganization, synaptogenesis, and dendritogenesis and promotes recovery.3 Functional reorganization of the cerebral cortex also occurs in human stroke survivors.4 Furthermore, clinical trials have demonstrated improvement in motor performance in chronic stroke survivors who underwent constraint-induced movement therapy.5 The underlying assumption is that motor recovery is related to adaptive cortical plasticity.
Pharmacological agents can serve as adjuvant therapies to enhance the effects of rehabilitative motor training. For example, administration of D-amphetamine combined with training results in improved recovery compared with D-amphetamine treatment alone.6 Other agents that improve recovery by completely different mechanisms might also benefit from an interactive effect with behavioral experience. One popular approach is to suppress myelin-associated inhibitors, NOGO-A, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein, presumably increasing neurite outgrowth and axonal regeneration.7 Each of the 3 myelin-derived inhibitors act independently through the neuronal receptors NgR18 or PirB.9 Genetic deletion of NOGO-A or NgR1 expression in mice leads to enhanced recovery from stroke.10 Furthermore, administration of anti-NOGO-A antibodies can improve behavioral recovery after stroke11 or spinal cord injury.12 With the greater effectiveness achieved by blocking all 3 ligands, a soluble NgR decoy improves functional recovery from rodent stroke10 and spinal cord injury.13,14 A specific antagonist of NOGO-66 action at NgR1 is NEP 1–40 (NOGO extracellular peptide, residues 1 to 40), the administration of which increases locomotor recovery after rodent spinal cord injury.15,16
Although the use of NOGO antagonists is a potentially efficacious approach to recovery after stroke, the interactive effects of these agents with behavioral training have only been investigated in a single study in a spinal cord injury model.17 In that study, although anti-NOGO-A antibody and treadmill training applied individually improved locomotor performance, the combination treatment resulted in poorer motor performance. It is important to determine if this negative effect of combining neurite outgrowth disinhibitors with behavioral training extends to other central nervous system injury models.
Materials and Methods
Subjects and Group Assignment
A total of 37 adult male Long-Evans hooded rats (Harlan, Ind) were used. Preinfarct behavioral training and assessment were begun at 3 months of age and continued for 2 to 3 months. Each animal was singly housed in a standard animal room with a 12/12-hour light/dark cycle. Animals were allowed food and water ad libitum, although moderate food restriction was enforced before reach training and assessment. At 5 to 6 months of age, animals that met behavioral criteria were randomly assigned to one of 3 treatment groups or one of 2 control groups: NEP+TR: lesion+drug treatment+motor training (n=8); NEP: lesion+drug treatment (n=8); or TR: lesion+vehicle+motor training (n=8). Control groups consisted of: LC: lesion+vehicle (n=7); or NC: no lesion, vehicle (ie, intact motor cortex; n=6). Although there were 8 possible groups that could have been compared (including no lesion+treatment groups), the goal was to examine the effects of treatment on postinjury recovery. Thus, the important comparisons were made between the treatment groups and LC. NC was added to control for variation over time due to repeated testing. All procedures followed the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the University of Kansas Medical Center Animal Care and Use Committee.
Behavioral Training and Assessment
Forelimb motor performance was evaluated using 3 commonly used assessments of motor performance: skilled reach, foot fault, and cylinder tests. After infarct, rats were assessed 2 times per week during Week 1 and then once per week between postinfarct Weeks 2 and 5 (ie, after initiation of treatment; Figure 1).
Figure 1.
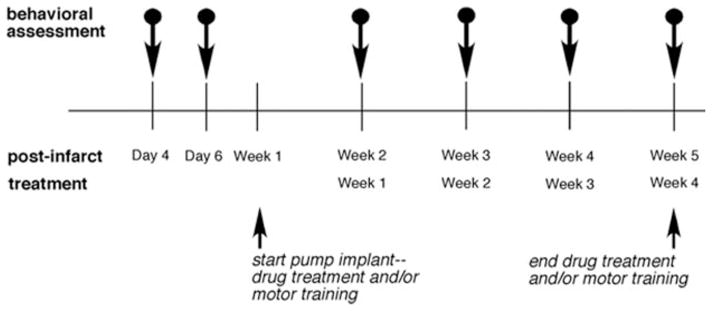
Experimental timeline showing the drug treatment (pump implant) and/or motor training started on postinfarct Week 1 and continued for 4 weeks. Behavioral assessments were performed on postinfarct Days 4 and 6 (Week 1 data are the means of Days 4 and 6) and weekly during postinfarct Weeks 2 to 5.
Skilled Reach Test and Motor Training
Each animal was placed in a Plexiglas chamber (26 cm long×25 cm high×15 cm wide) designed to restrict the use of either the right or left forelimb.18 The external shelf used to present food pellets was 5 cm from the bottom of the chamber and 2 distances were used to vary difficulty of the reach at 1 cm and 2 cm from the front of the chamber. A single food pellet (45 mg, BioServ) was delivered on each trial. Forelimb preference was determined during a preinfarct shaping period. Preinfarct training sessions (60 trials per session) were conducted until the rat obtained a success score of 70% with its preferred limb. Rats that did not attain this score were excluded from the study. Baseline performance was assessed over 2 to 3 weeks until the day of infarct. Postinfarct performance was assessed before treatment on Days 4 and 6 and then during treatment at the end of Weeks 2, 3, 4, and 5. Importantly, the skilled reach task was also used for rehabilitative training that began at postinfarct Week 2 (5 sessions/week, 60 trials/session) and continued for 28 days. Training began on the near well and was gradually titrated to the far well.
Foot Fault Test
Each rat was placed onto an elevated grid (57 cm×44 cm with 3.5 cm×3.5-cm grid opening) and allowed to explore for 2 minutes to assess forelimb use during locomotion.19 The number of times each forelimb was used and the number of slips made with each forelimb were recorded. Foot fault=(total number of slips made with impaired forelimb–intact forelimb)/total number of movements made with both forelimbs. A total of 7 foot fault assessments (one or 2 sessions/week) was conducted before the infarct.
Cylinder Test
Each rat was placed in a transparent cylinder (20 cm diameter, 30 cm height).20 Asymmetry scores were defined as the percentage of intact forelimb touches+½the percentage of bilateral forelimb touches for postural support over the first 60 touches. Sessions lasted 3 to 10 minutes.
Surgical Procedures and Drug Delivery
Within 1 week after preinfarct baseline assessments were completed, animals underwent a surgical procedure under aseptic conditions to create a focal cortical infarct and to implant an intraventricular cannula. Animals were anesthetized with ketamine (80 mg/kg, intraperitoneally) and xylazine (5 mg/kg, intramuscularly) to produce a surgical level of anesthesia. The scalp was shaved, the head stabilized in a stereotaxic frame, and the skin incised to expose the skull. Supplemental ketamine (20 mg/kg, intramuscularly) was administered as necessary.
Focal Infarct and Cannula Implant
Six 0.7-mm diameter holes were drilled into the skull over the dominant hemisphere (contralateral to the preferred forelimb): anteroposterior +1.5, +0.5, and −0.5 and mediolateral +2.5 and +3.5 from bregma, corresponding to the caudal forelimb area of the motor cortex.21 To induce the cortical infarct, 0.33 μL endothelin-1 (0.3 μg dissolved in 1 μL saline; Peninsula Laboratories, San Carlos, Calif) was injected into each hole (3 nL/s) at a depth of 1.5 mm from the cortical surface through a tapered micropipette (tip size 160 μm outer diameter, barrel size 900 μm outer diameter) attached to a Hamilton syringe.22 Bone wax was used to seal the injection holes.
An additional hole was made over the nondominant hemisphere at anteroposterior −0.9 mm and mediolateral +2.0 mm from bregma. A cannula (0.36 mm outer diameter, Alzet brain infusion kit II; Alzet Scientific Products, Cupertino, Calif) connected to a catheter and filled with either vehicle (0.1 mol/L phosphate-buffered saline+dimethyl sulfoxide) or drug (NEP 1–40) was implanted into the lateral ventricle at a depth of 4.0 mm from the pial surface.10 The other end of the catheter was sealed and secured under the skin. Postoperatively, animals were given penicillin (0.15 mL, subcutaneously), saline (5 mL, subcutaneously), buprenorphine (0.05 mg/kg, subcutaneously), and acetaminophen (20 mg/kg, orally).
Drug Delivery
On postinfarct Day 7, each animal was anesthetized with ketamine/xylazine and the skin over the back was incised. The catheter was connected to an osmotic minipump (Alzet model 2ML4, Alzet Scientific Products) filled with either 1 mg NEP 1–40 in 2 mL vehicle (groups NEP+TR; NEP) or 2 mL of vehicle (97.5% phosphate-buffered saline+2.5% dimethyl sulfoxide; groups TR, LC, and NC).10 The pump continuously delivered the solution at 2.5 μL/h for 28 days.
Histology
On postinfarct Day 47, animals were administered a lethal dose of 1.0 mL Beuthanasia (390 mg sodium pentobarbital) and perfused transcardially with 0.9% sodium chloride and then 4% paraformaldehyde fixative in 0.1 mol/L phosphate buffer. The brain was removed and postfixed with 20% glycerol and 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline and then 20% glycerol plus 2% dimethyl sulfoxide in 0.1 mol/L phosphate-buffered saline. The brain was sectioned in the coronal plane (40 μm). One series of brain sections was processed with a Nissl stain to reveal the cortical cytoarchitecture, infarct size, and location.
Statistical Analysis
Data were analyzed and compared among the groups (between-group comparison; JMP software). Postmortem histology was analyzed with a one-way analysis of variance to compare infarct volume across groups. Behavioral data were also analyzed with analyses of variance. Post hoc comparisons were analyzed with Student t least square mean difference test to control for Type I error (α = 0.05) and simple effects analyses of variance (slice effects) to assess group×time interactions. Percentage data were normalized with an arcsine transformation.
Results
Histological Results
Nissl-stained sections revealed that the infarct destroyed all 6 layers of the caudal forelimb area. There was no obvious evidence that the underlying white matter was damaged. Also, the infarct was continuous throughout the territory of the endothelin-1 injections, ranging from anteroposterior 1.8 to −0.9 mm from bregma (Figure 2A). The lesion was reconstructed through StereoInvestigator software (MicroBrightField, Colchester, Vt) and the volume measured by subtracting injured from intact neocortex with the Cavalieri estimator. A one-way analysis of variance demonstrated no significant difference in lesion volume among groups (F[3,14]=0.36, P=0.79; Figure 2B).
Figure 2.
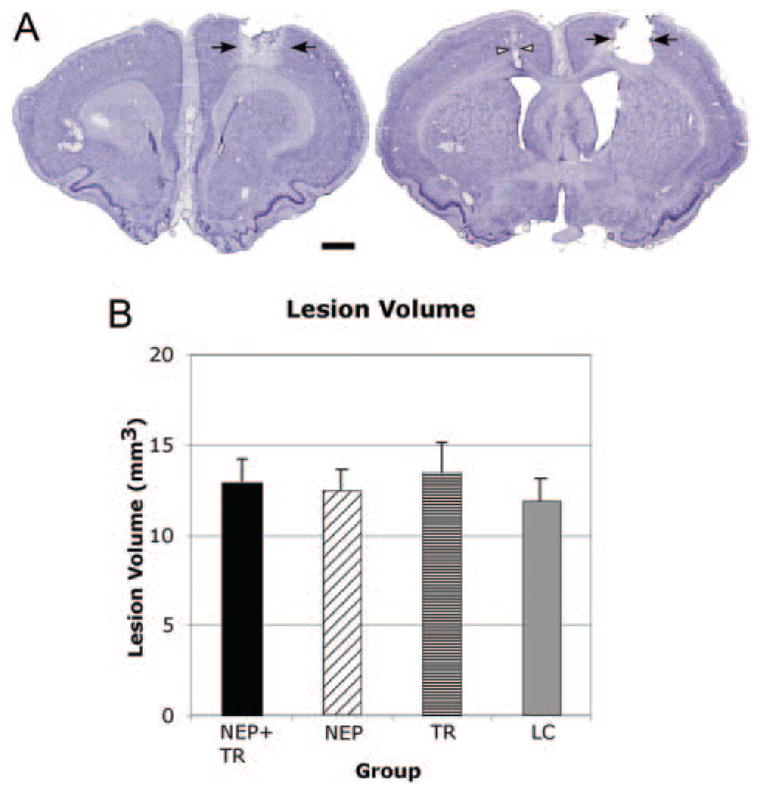
A, Nissl stains showing the infarct (black arrowheads) in the motor cortex in the right hemisphere from one representative rat. Left section is 1.8 mm rostral and right section is 0.6 caudal to bregma. Cannula track (white arrow) shown in the left hemisphere. Scale bar=1 mm. B, There were no differences in lesion volume between groups. NEP+TR, NEP 1–40+motor training; NEP, NEP 1–40; TR, motor training; LC, lesion only, no treatment). Error bar=SEM.
Skilled Reach Test
Pretreatment Performance
Before the endothelin-1 infarct, there were no significant differences among groups in successful reach and retrieval skills (F[4,32]=1.24; P=0.31; Table 1). However, the infarct significantly impaired skilled reach when assessed during Week 1 (F[4,32]=6.36; P=0.0007). Post hoc analysis showed that all infarct groups were significantly impaired compared with NC. There were no significant differences among the infarct groups.
Table 1.
Percentage of Successful Food Pellet Retrievals Over 60 Trials (mean±SEM)
| Group | Baseline | Postinfarct Week 1 | Postinfarct Week 2 (Treatment Week 1) | Postinfarct Week 3 (Treatment Week 2) | Postinfarct Week 4 (Treatment Week 3) | Postinfarct Week 5 (Treatment Week 4) |
|---|---|---|---|---|---|---|
| NC | 74.16±1.45 | 72.33±0.33 | 72.33±3.83 | 75.83±3.68 | 77.17±3.04 | 74.67±4.43 |
| NEP+TR | 78.00±1.69 | 56.71±2.55 | 65.86±3.56 | 80.43±1.73 | 69.86±5.59 | 75.29±3.31 |
| NEP | 76.00±2.15 | 57.13±2.78 | 60.25±3.66 | 59.13±4.77 | 70.50±4.70 | 70.88±3.57 |
| TR | 73.88±1.56 | 52.75±4.83 | 61.75±5.96 | 64.88±4.30 | 62.13±5.26 | 67.38±5.48 |
| LC | 72.86±2.11 | 49.293±3.71 | 55.57±2.82 | 55.29±2.49 | 66.29±3.76 | 61.86±4.79 |
Posttreatment Performance
There was a significant group effect (F[4,32] =17.88, P=0.0001), time effect (F[3,96]=5.37, P=0.0001), and group×time interaction (F[12,96]=2.31, P=0.012; Figure 3). The interaction was analyzed with post hoc simple effects analyses of variance, demonstrating a significant difference among groups for each week of treatment (Table 2). Table 2 also indicates each of the significant post hoc pairwise comparisons with LC and NC.
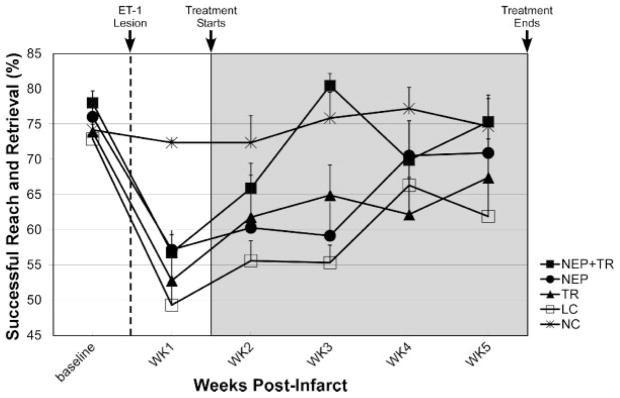
Figure 3.
Skilled reach performance. The infarct significantly impaired use of the forelimb to retrieve food pellets (P<0.001). After the infarct (dashed line), but before treatment initiation (ie, postinfarct Days 4 and 6 combined), there were no differences in performance between groups. During the treatment period (gray region), there was a significant time effect (P=0.0001), group effect (P=0.0001) and group×time interaction (P=0.012). Post hoc simple effects analysis of the interaction showed that there was a significant treatment effect among groups for each week. Performance of the NEP+TR group was significantly improved compared with the lesion only control group (LC) throughout the 4-week treatment period. Error bar=SEM.
Table 2.
Post hoc Pairwise Comparisons
| Group | Week 2 (Treatment Week 1) F=4.03; P=0.005 | Week 3 (Treatment Week 2) F=13.60; P=7.9(10−9) | Week 4 (Treatment Week 3) F=3.59; P=0.01 | Week 5 (Treatment Week 4) F=3.66; P=0.008 | ||||
|---|---|---|---|---|---|---|---|---|
| Versus lesion only group (LC) | ||||||||
| NC | * | * | * | * | ||||
| NEP+TR | * | * | NS | * | ||||
| NEP | NS | NS | NS | * | ||||
| TR | NS | * | NS | NS | ||||
| Versus intact control group (NC) | ||||||||
| NEP+TR | NS | NS | NS | NS | ||||
| NEP | * | * | NS | NS | ||||
| TR | * | * | * | NS | ||||
| LC | * | * | * | * | ||||
F-values (4,96) from post hoc comparisons.
Significant pairwise comparison (P<0.05).
Brackets indicate additional significant pairwise comparisons.
NS indicates nonsignificant.
To summarize effects of the treatment groups compared with LC, TR demonstrated significant improvement only at the end of treatment Week 2, NEP at the end of treatment Week 4, and NEP+TR at the end of treatment Weeks 1, 2 and 4. Furthermore, compared with NC, TR was statistically indistinguishable at the end of treatment Week 4, NEP at the end of treatment Weeks 3 and 4, and NEP+TR at the end of each of the 4 treatment weeks.
Foot Fault Test
Pretreatment Performance
Before the endothelin-1 infarct, there were no significant differences in foot faults between groups (F[4,32]=1.78, P=0.16). During the first week after the lesion (pretreatment), there was a significant impairment in skilled locomotion on the foot fault task (F[4,32]=3.62, P=0.02). Post hoc analysis showed that there were no differences among infarct groups (NEP+TR, NEP, TR, and LC) and that all infarct groups were significantly impaired compared to NC (Figure 4).
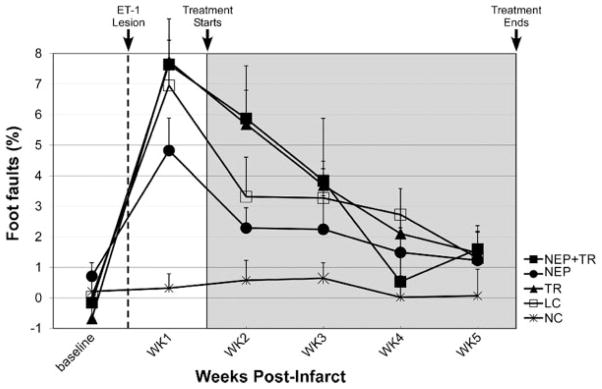
Figure 4.
Foot fault performance. Data represent the difference of foot faults between the preinfarct preferred forelimb (or impaired forelimb) and the nonpreferred forelimb (or intact forelimb) divided by the total number of movements. Negative values represent more foot faults with the intact fore-limb ipsilateral to the infarct. The infarct significantly impaired forelimb coordination and increased foot faults before the treatments (P=0.02). During treatment period, there was no significant group effect or group×time interaction. Error bar=SEM.
Posttreatment Performance
There was no significant group effect (F[4,32]=2.29, P=0.065) nor interaction of group×time (F[12,96]=1.16, P=0.326; Figure 4) during any of the postinfarct weeks.
Cylinder Test
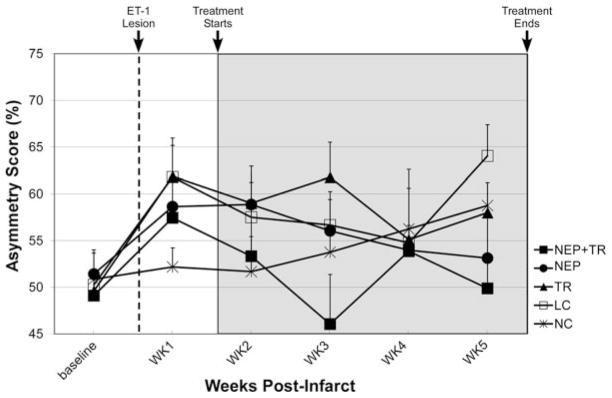
The cylinder test did not demonstrate a deficit in any of the groups (Figure 5; see “Discussion”).
Figure 5.
Cylinder test performance. There were no significant differences in performance among groups either before or after the infarct. Error bar=SEM.
Discussion
The purpose of this study was to determine the efficacy of NEP 1–40 plus motor training on motor recovery after a focal cortical infarct and whether the 2 treatments in combination interact. Our results showed that 2 of the 3 treatments, NEP 1–40 only (NEP) and NEP 1–40 combined with motor training (NEP+TR), facilitated recovery on a skilled reach task in that performance was superior to a lesion only control group (LC) at the end of the 4-week treatment regimen. However, the combination treatment resulted in recovery earlier, because skilled reach performance was statistically superior to LC as early as treatment Week 1. Furthermore, although lack of statistical significance cannot be equated with negative proof, it is important to note that the combination treatment group’s performance on the skilled reach task was statistically indistinguishable from that of intact control animals throughout the treatment period. The effects of the combined treatment were most pronounced at treatment Week 2, when the combination treatment group performed significantly better than all other lesion groups (Table 2).
In a previous study in a spinal cord injury model, the myelin-associated neurite outgrowth disinhibitor anti-NOGO-A antibody was administered either alone or in combination with treadmill training.17 The combinatorial treatment resulted in poorer locomotion performance. Although there are several differences between this study and the present one, including the administration of NEP 1–40 rather than anti-NOGO-A antibody, it is possible that different injury models may be differentially responsive to combinatorial treatments of drug and behavioral training.
Alternatively, the effects of drug interaction with behavioral experience may be task-dependent. In the present study, we found significant drug–behavior interactions examining performance with the skilled reach task but not with the foot fault task. These results are very similar to those of a recent study using virtually an identical lesion model pairing D-amphetamine with skilled reach and grid walking.23 Like in our study, a positive interaction was found for skilled reach but not grid walking. It is possible that grid walking (foot fault task) is not sensitive enough to observe subtle deficits and hence interactive effects with drug treatment. Additionally, it is possible that reach training, presumably requiring more skill, may be a more effective modifier of synaptic plasticity than treadmill training. At least in intact rats, skilled reach training is more effective in modifying motor map organization and synaptic plasticity than unskilled motor activity.1,24 It should also be noted that in the present study, rats were not specifically trained on the foot fault task, although brief assessment was conducted weekly.
The lack of behavioral deficits using the cylinder test is surprising, because this test has been a reliable indicator of behavioral asymmetry in various infarct models. However, a recent study using endothelin-1 to produce a focal infarct in mice also found no deficit on this task,25 perhaps due to the lack of striatal involvement. However, in an earlier focal cortical infarct study in rats, these same investigators observed deficits on this task.26 Although lesion volume was not reported, the figure suggests that lesions were larger than those in the present study. It would appear that the skilled reach test is a more sensitive measure of forelimb function than the cylinder test, especially after small cortical lesions.
The present study did not specifically address the mechanisms of recovery related to the various treatments. However, other lesion studies have shown that NEP 1–40 promotes axonal outgrowth,16 and rehabilitative training induces synaptogenesis and dendritic arborization.2,27 The outcome in the present study parallels findings on the interactive effects of D-amphetamine and task-dependent motor training in that the combination of these 2 treatments resulted in accelerated recovery compared with single treatment alone.
In summary, this study establishes a positive interactive effect of a treatment agent associated with axonal growth in combination with motor rehabilitation. NEP 1–40 plus motor training maximized motor recovery in that there were no differences between infarcted rats with the combinatorial treatment and normal rats, and combinatorial treatment accelerated the recovery process compared with either treatment alone. This treatment was effective when initiated 1 week after the infarct, suggesting the therapeutic application period for this combinatorial approach is relatively long.
Footnotes
Disclosures
None.
References
- 1.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 2.Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SC, Riley JD. Neuroplasticity and brain repair after stroke. Curr Opin Neurol. 2008;21:76–82. doi: 10.1097/WCO.0b013e3282f36cb6. [DOI] [PubMed] [Google Scholar]
- 5.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 6.Barbay S, Nudo RJ. The effects of amphetamine on recovery of function in animal models of cerebral injury: a critical appraisal. Neurorehabilitation. 2009;25:5–17. doi: 10.3233/NRE-2009-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cafferty WB, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating NOGO-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 9.Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. Pirb is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 10.Lee JK, Kim JE, Sivula M, Strittmatter SM. NOGO receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O’Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 12.Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. NOGO-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed NOGO receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, Worley D, Sah DW, Pepinsky B, Lee D, Relton J, Strittmatter SM. Blockade of NOGO-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble NOGO-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Strittmatter SM. Delayed systemic NOGO-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GrandPre T, Li S, Strittmatter SM. NOGO-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 17.Maier IC, Ichiyama RM, Courtine G, Schnell L, Lavrov I, Edgerton VR, Schwab ME. Differential effects of anti-NOGO-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132:1426–1440. doi: 10.1093/brain/awp085. [DOI] [PubMed] [Google Scholar]
- 18.Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage in female rats: forelimb behavioral effects and dendritic structural plasticity in the contralateral homotopic cortex. Exp Neurol. 2004;190:433–445. doi: 10.1016/j.expneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Barth TM, Stanfield BB. The recovery of forelimb-placing behavior in rats with neonatal unilateral cortical damage involves the remaining hemisphere. J Neurosci. 1990;10:3449–3459. doi: 10.1523/JNEUROSCI.10-10-03449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 21.Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- 22.Gilmour G, Iversen SD, O’Neill MF, Bannerman DM. The effects of intracortical endothelin-1 injections on skilled forelimb use: implications for modelling recovery of function after stroke. Behav Brain Res. 2004;150:171–183. doi: 10.1016/j.bbr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Gilmour G, Iversen SD, O’Neill MF, O’Neill MJ, Ward MA, Bannerman DM. Amphetamine promotes task-dependent recovery following focal cortical ischaemic lesions in the rat. Behav Brain Res. 2005;165:98–109. doi: 10.1016/j.bbr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 25.Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in c57bl/6 mice. J Neurosci Methods. 2009;181:18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]