Abstract
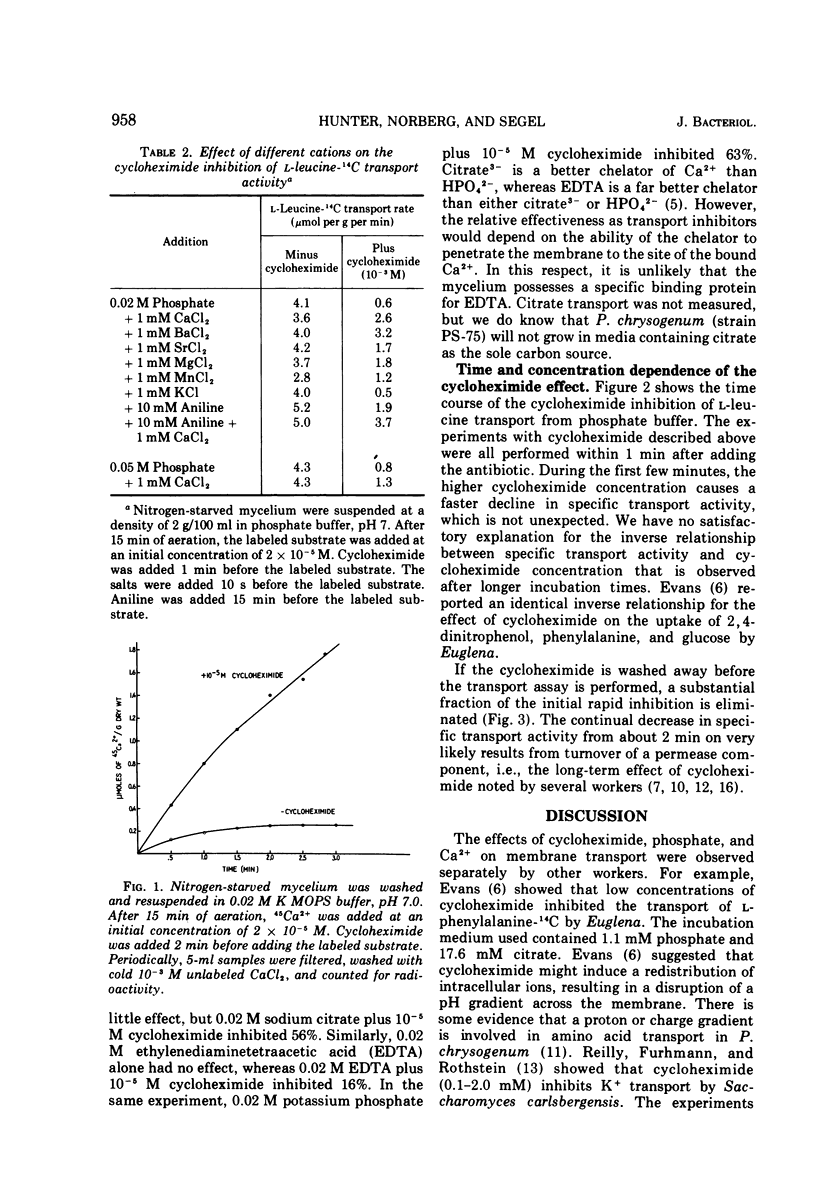
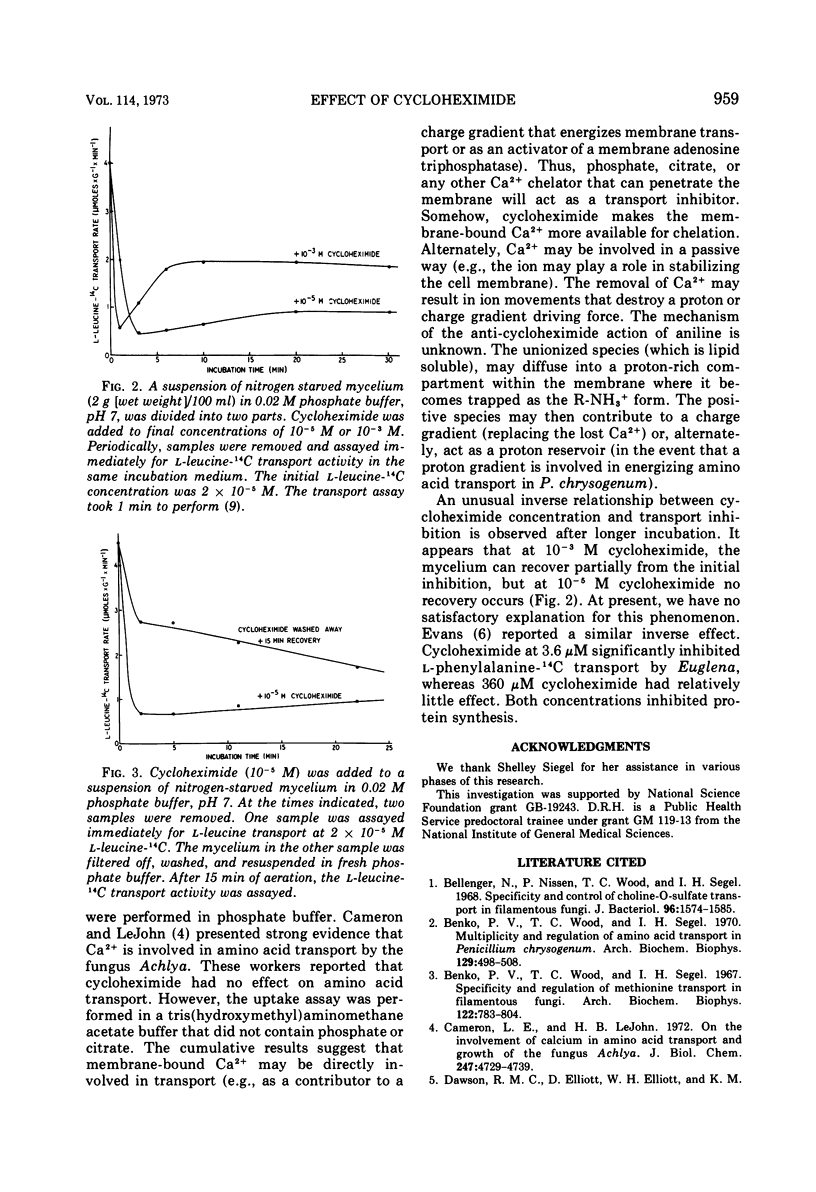
Cycloheximide (actidione) has an immediate inhibitory effect on amino acid transport by nitrogen-starved or carbon-starved mycelium suspended in phosphate buffer. High concentrations of phosphate alone are slightly inhibitory; cycloheximide appears to potentiate the effect of phosphate. Ca2+ reverses the inhibition of transport caused by phosphate plus cycloheximide. Ca2+ did not relieve the inhibition of protein synthesis. Cycloheximide promotes a continual uptake of 45Ca2+ by the mycelium. The cumulative results suggest that (i) membrane-bound Ca2+ is involved in amino acid transport, (ii) cycloheximide labilizes the membrane-bound Ca2+, and (iii) phosphate forms a complex with Ca2+ making it unavailable for its role in transport. The effect of cycloheximide described above is observed within 1 to 2 min after addition of the antibiotic. This initial inhibition occurs more rapidly with 10−3 M cycloheximide than with 10−5 M cycloheximide. However, after a longer preincubation time, a curious inverse relationship between cycloheximide concentration and amino acid transport is observed. The mycelium incubated with 10−5 M cycloheximide remains strongly inhibited (unless the antibiotic is washed away). The mycelium incubated with 10−3 M cycloheximide recovers about 40% of the transport activity lost during the rapid initial phase. We have no obvious explanation for the inverse effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellenger N., Nissen P., Wood T. C., Segel I. H. Specificity and control of choline-O-sulfate transport in filamentous fungi. J Bacteriol. 1968 Nov;96(5):1574–1585. doi: 10.1128/jb.96.5.1574-1585.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko P. V., Wood T. C., Segel I. H. Multiplicity and regulation of amino acid transport in Penicillium chrysogenum. Arch Biochem Biophys. 1969 Feb;129(2):498–508. doi: 10.1016/0003-9861(69)90207-0. [DOI] [PubMed] [Google Scholar]
- Cameron L. E., LéJohn H. B. On the involvement of calcium in amino acid transport and growth of the fungus Achlya. J Biol Chem. 1972 Aug 10;247(15):4729–4739. [PubMed] [Google Scholar]
- Evans W. R. The effect of cycloheximide on membrane transport in Euglena. A comparative study with nigericin. J Biol Chem. 1971 Oct 25;246(20):6144–6151. [PubMed] [Google Scholar]
- Grenson M., Crabeel M., Wiame J. M., Béchet J. Inhibition of protein synthesis and simulation of permease turnover in yeast. Biochem Biophys Res Commun. 1968 Feb 26;30(4):414–419. doi: 10.1016/0006-291x(68)90760-2. [DOI] [PubMed] [Google Scholar]
- Hackette S. L., Skye G. E., Burton C., Segel I. H. Characterization of an ammonium transport system in filamentous fungi with methylammonium-14C as the substrate. J Biol Chem. 1970 Sep 10;245(17):4241–4250. [PubMed] [Google Scholar]
- Hunter D. R., Segel I. H. Acidic and basic amino acid transport systems of Penicillium chrysogenum. Arch Biochem Biophys. 1971 May;144(1):168–183. doi: 10.1016/0003-9861(71)90466-8. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Segel I. H. Control of the general amino acid permease of Penicillium chrysogenum by transinhibition and turnover. Arch Biochem Biophys. 1973 Jan;154(1):387–399. doi: 10.1016/0003-9861(73)90071-4. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Segel I. H. Effect of weak acids on amino acid transport by Penicillium chrysogenum: evidence for a proton or charge gradient as the driving force. J Bacteriol. 1973 Mar;113(3):1184–1192. doi: 10.1128/jb.113.3.1184-1192.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf G. A. Control of the synthesis, activity, and turnover of enzymes of sulfur metabolism in Neurospora crassa. Arch Biochem Biophys. 1972 Jun;150(2):714–724. doi: 10.1016/0003-9861(72)90090-2. [DOI] [PubMed] [Google Scholar]
- Reilly C., Fuhrmann G. F., Rethstein A. The inhibition of K + and phosphate uptake in yeast by cycloheximide. Biochim Biophys Acta. 1970 Jun 2;203(3):583–585. doi: 10.1016/0005-2736(70)90197-5. [DOI] [PubMed] [Google Scholar]
- SIEGEL M. R., SISLER H. D. INHIBITION OF PROTEIN SYNTHESIS IN VITRO BY CYCLOHEXIMIDE. Nature. 1963 Nov 16;200:675–676. doi: 10.1038/200675a0. [DOI] [PubMed] [Google Scholar]
- Skye G. E., Segel I. H. Independent regulation of cysteine and cystine transport in Penicillium chrysogenum. Arch Biochem Biophys. 1970 May;138(1):306–318. doi: 10.1016/0003-9861(70)90311-5. [DOI] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. II. Metabolic control. J Bacteriol. 1968 Mar;95(3):959–966. doi: 10.1128/jb.95.3.959-966.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



