Abstract
Background
In most models of experimental thrombosis, healthy blood vessels are damaged. This results in the formation of a platelet thrombus that is stabilized by ADP signaling via P2Y12 receptors. However, such models do not predict involvement of P2Y12 in the clinically relevant situation of thrombosis upon rupture of atherosclerotic plaques. We investigated the role of P2Y12 in thrombus formation on (collagen-containing) atherosclerotic plaques in vitro and in vivo, by using a novel mouse model of atherothrombosis.
Methodology
Plaques in the carotid arteries from Apoe −/− mice were acutely ruptured by ultrasound treatment, and the thrombotic process was monitored via intravital fluorescence microscopy. Thrombus formation in vitro was assessed in mouse and human blood perfused over collagen or plaque material under variable conditions of shear rate and coagulation. Effects of two reversible P2Y12 blockers, ticagrelor (AZD6140) and cangrelor (AR-C69931MX), were investigated.
Principal Findings
Acute plaque rupture by ultrasound treatment provoked rapid formation of non-occlusive thrombi, which were smaller in size and unstable in the presence of P2Y12 blockers. In vitro, when mouse or human blood was perfused over collagen or atherosclerotic plaque material, blockage or deficiency of P2Y12 reduced the thrombi and increased embolization events. These P2Y12 effects were present at shear rates >500 s−1, and they persisted in the presence of coagulation. P2Y12-dependent thrombus stabilization was accompanied by increased fibrin(ogen) binding.
Conclusions/Significance
Platelet P2Y12 receptors play a crucial role in the stabilization of thrombi formed on atherosclerotic plaques. This P2Y12 function is restricted to high shear flow conditions, and is preserved in the presence of coagulation.
Introduction
Rupture of an atherosclerotic plaque and occlusive arterial thrombus formation is the major cause of acute cardiovascular incidents and deaths in the Western countries [1]. The development of a thrombus is a complex and dynamic process, involving platelet aggregation, generation of thrombin and formation of a network of fibrin, to strengthen the platelet aggregate and stabilize the thrombus [2], [3]. In vivo mouse models of experimental thrombosis have indicated a major role of platelet receptors in the process of thrombus formation and stabilization [4]. However, the studies have been carried out by damaging healthy mouse arteries in an artificial way, e.g. by laser-induced tissue ablation, free radical-generating agents or mechanical disruption. Thrombus formation in diseased, atherosclerotic arteries following plaque rupture has hardly been investigated.
Ruptured atherosclerotic plaques expose several platelet-adhesive and -activating components, such as collagen types I and III, von Willebrand factor (VWF), lysophosphatidic acid, thrombospondin, fibronectin, vitronectin, fibrin/fibrinogen and oxidized low density lipoprotein [5], [6], [7], [8], [9], [10]. In addition, plaques contain tissue factor which, upon de-encryption, activates the extrinsic coagulation system [5], while the intrinsic system of factor XII activation is triggered via collagen [11]. To which extent each of these plaque components contribute to thrombus formation is unclear, though the platelet-activating roles of VWF and collagen are well-described [1]. Collagen-bound VWF mediates the initial tethering and transient adhesion of platelets via glycoprotein (GP)Ib-V-IX. Stable platelet adhesion to VWF is achieved via integrin αIIbβ3 and adhesion to collagen via the platelet receptors, GPVI and integrin α2β1 [2], [12], [13]. Recent in vitro studies suggest that these receptors also mediate platelet adhesion to collagen in damaged plaques [5], [6]. Subsequent activation responses of adhered platelets include mobilization of cytosolic Ca2+, secretion of autocoids like ADP, activation of integrin αIIbβ3 (fibrinogen receptor) and formation of pseudopods, all of which events help to recruit circulating platelets into a thrombus [2], [14]. Part of the activated platelets become procoagulant by Ca2+-dependent exposure of phosphatidylserine on their surface, which is required for local thrombin generation [15].
The autocrine agent ADP activates platelets via two receptors, P2Y1 and P2Y12; the first of which mediates shape change and initiates platelet aggregation, while the latter is required for complete aggregation [16]. The importance of P2Y12 was revealed by the observation that the damage of arteries in P2Y12-deficient mice resulted in markedly delayed and unstable thrombus formation [17]. Mechanistically, we and others have shown that continuous signaling via P2Y12 is required to prevent platelet disaggregation and to maintain αIIbβ3 in its active conformation [18], [19]. The P2Y12 receptor also has a role in platelet procoagulant activity by potentiating tissue factor-induced thrombin generation via sustained Ca2+ mobilization [20], [21].
In the present paper, we used a recently developed mouse model of thrombus formation on acutely ruptured plaques to study the role of P2Y12 receptors in atherothrombosis. We investigated effects of the reversible P2Y12 antagonists, ticagrelor and cangrelor, not only using this in vivo model, but also in perfusion studies of whole (P2Y12-deficient) mouse or human blood perfused over collagen-containing plaque material. The results point to a P2Y12-dependent stabilization of thrombus formation at high shear, that is maintained under conditions of coagulation. This observation may have important implications for antithrombotic treatment in patients.
Materials and Methods
Materials
Fibrillar Horm collagen (type I) was purchased from Nycomed Pharma (Munich, Germany). Native fibrillar type I collagen was prepared from bovine tendon with minimal protease treatment, as described [22]. Fibrinogen labeled with Oregon Green (OG) 488 was from Invitrogen (Carlsbad, CA, USA). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was from Molecular Probes (Leiden, the Netherlands). Rat-anti-mouse CD62 labeled with FITC (Wug.E9) and rat-anti-mouse GPIIbIIIa (JON/A) labeled with PE were from Emfret Analytics (Würzburg, Germany). Cangrelor (AR-C69931MX) and ticagrelor (AZD6140) were kindly provided by AstraZeneca (Mölndahl, Sweden). Ketamine and xylazine were from Eurovet (Bladel, the Netherlands). Sources of other materials are described elsewhere [23].
Animals
Wild type C57BL/6 mice (12 weeks old) and Apoe −/− mice on C57BL/6 background (4 weeks old) were obtained from Charles River (Maastricht, The Netherlands). The Apoe −/− mice were fed a Western-type diet with 0.15% cholesterol for 18–20 weeks, i.e. until plaques had developed in the carotid arteries near the bifurcation. Mice homozygously deficient in P2Y12 receptors [24] and corresponding wild types (C57BL/6 background) were bred and housed, as described previously [25]. Animal experiments were approved by the research ethics committees of the universities of Maastricht (The Netherlands) and Sheffield (UK).
In vivo plaque rupture and measurement of thrombus formation
Acute rupture of plaques in the carotid arteries was provoked by targeted ultrasound treatment, as described before [26]. Briefly, Apoe −/− mice fed with cholesterol-containing diet were anesthetized by subcutaneous injection of ketamine and xylazine (0.1 mg/g and 0.02 mg/g body weight, respectively). The carotid arteries were carefully dissected free from surrounding tissue, and the anesthetized animals were injected intravenously with 10% CFSE-labeled platelets, which were obtained from a donor mouse of the same genotype. The mice were then injected with vehicle solution, ticagrelor or cangrelor, as described below. Body temperature was held at 37°C during all procedures.
Using brightfield illumination and intravital microscopy, a plaque was selected in one of the carotid arteries near the bifurcation. The tip (0.5 mm diameter) of a titanium ultrasound probe was then placed at the plaque shoulder region. Rupture of the plaque was induced by ultrasound application during 10 s at 6 kHz, using a VibraCell VCX130 processor (Sonics, Newtown, CT, USA). Thrombus formation was recorded in real time by capturing 12-bit fluorescence images at 33 Hz during at least 10 min, using a back-thinned electron multiplier C9100-12 EM-CCD camera (Hamamatsu, Japan) at fixed gain settings. Local rupture of the plaques was verified by two-photon laser scanning microscopy and histological staining of sections of the carotid arteries [26].
Time-series of fluorescence images were analyzed using Wasabi (Hamamatsu) software. Per region of interest (ROI), corresponding to the site of thrombus formation, total pixel intensity was calculated and corrected for background intensity. To quantify thrombus size, digital images taken at specific time points were processed using ImagePro software (Media Cybernetics, Silverspring, MD, USA). Within the carotid artery, two similar ROIs were defined, one representing the thrombus area and an adjacent ROI representing the background. A threshold level was set by eliminating all pixels with intensity lower than 99.0% of the pixels of the background ROI. Intensities (gray levels) of all pixels in the thrombus ROI were then integrated. No image processing was applied.
In vivo administration of P2Y12 antagonist and blood collection
Anesthetized mice were injected intravenously with vehicle solution (5% mannitol in saline), ticagrelor (AZD6140) or cangrelor (AR-C69931MX). Ticagrelor was infused at a dose of 420 µg/kg for 1 min, followed by 60 µg/kg/min for 15 min; cangrelor was infused at 3 µg/kg/min for 15 min, and infusions were continued during the experiment. Mice for control experiments were bled retro-orbitally under anesthetics after 15 min of infusion of vehicle solution, ticagrelor or cangrelor. Mouse blood was collected into 40 µM PPACK and 5 U/ml heparin or trisodium citrate, as indicated [13]. Platelet-rich plasma (PRP) was isolated and used for platelet activation analysis. Human blood taken by venipuncture, was collected into 40 µM PPACK or 12.9 mM trisodium citrate, as indicated.
Flow-cytometric evaluation of platelets with active αIIbβ3 or P-selectin exposure was performed by activation with ADP (40 µM) or convulxin (100 ng/ml) in 20x diluted whole mouse blood, followed by labeling with JON/A mAb or anti-CD62 mAb, respectively. Detection of fluorescence was with a FACScan flow cytometer, equipped with an argon laser (Becton-Dickinson, Franklin Lakes, NJ). The percentage of positive cells was analyzed using WinMDI 2.8 software (http://facs.scripps.edu).
Whole blood perfusion experiments
Human and mouse blood samples were perfused through a parallel-plate transparent flow chamber containing a coverslip, coated with either plaque material or collagen, as described [5]. PPACK-anticoagulated blood was used for perfusion experiments without coagulation, respectively; ADP (10 µM) was co-perfused, as indicated [18]. Coagulation was introduced by co-perfusion of human or mouse citrate-anticoagulated blood with 0.1 volume of CaCl2 (75 mM), MgCl2 (37.5 mM) and tissue factor (20 pM) in Hepes buffer pH 7.45 (NaCl 110 mM, glucose 10 mM, Hepes 5 mM, KCl 2.7 mM and 0.1% bovine serum albumin). Wall shear rate was 300–1000 s−1, as indicated. After blood perfusion, thrombi on coverslips were rinsed with Hepes buffer supplemented with 2 mM CaCl2, 2 mM MgCl2 and heparin (1 U/ml). Recording of real-time videos and capturing of phase-contrast images was with a non-confocal microscopic system [13]. Videos were analyzed offline for embolizing events (single or clustered platelets) per aggregate [18]. Randomly captured images were analyzed for surface area coverage, area distribution of individual segmented features or integrated fluorescence intensity with Image-Pro (ImagePro, Silver Spring MD, USA) or Metamorph software (MDS Analytical Technologies, Downingtown, PA, USA) [27]. In specific experiments, OG488-fibrinogen (25 µg/ml) was added to human or (P2Y12-deficient) mouse blood and, after perfusion over collagen or plaque material, fluorescence accumulation was detected by confocal laser scanning microscopy. Confocal images were recorded off-line with a BioRad 2100 multiphoton system at fixed settings of argon laser power, scanning rate and photomultiplier gain [15].
Coating of plaque material
Human atherosclerotic plaques were collected at autopsy from a carotid artery and used in compliance with institutional guidelines (Department of Pathology, Maastricht University). Permission was obtained from the local Medical Ethics Committee. Murine atherosclerotic plaques were collected from the aortic arches of 6 Apoe −/− mice, fed with a Western-type diet with 0.15% cholesterol for 18–20 weeks. After resection, atherosclerotic specimens were frozen into liquid nitrogen and stored at −80°C. Thawed human and mouse atherosclerotic tissues were homogenized in phosphate-buffered saline. Plaque homogenates were pooled at 165 mg wet tissue weight/ml, as described [5]. Coverslips were coated with plaque material at a density of 170 µg wet weight/cm2.
Cone and plate(let) experiments
An Impact-R cone and plate(let) analyzer (CPA, DiaMed, Cressier, Switzerland) was used to evaluate platelet adhesion and aggregation on a surface under defined flow conditions [28]. Citrate-anticoagulated blood (130 µl) was placed in a polystyrene well and subjected to a shear of 500–5000 s−1 for 2 min. The platelet aggregates were washed and stained by May-Grünwald stain, according to the manufacturer's instructions (DiaMed). Platelet deposition was evaluated by an image analyzer system connected to the microscope, measuring the average size of the aggregates in µm2.
Thrombin generation experiments
Thrombin generation was measured in citrate-anticoagulated human or mouse PRP (1×108 platelets/ml), as before [29]. Briefly, PRP was preincubated with inhibitors, and then treated with ADP (20 µM) or vehicle for 10 min. Samples (4 volumes) were pipetted into a polystyrene 96-wells plate (Immulon 2HB, Dynex Technologies, Chantilly, VA, USA), already containing 1 volume of buffer A (20 mM Hepes, 140 mM NaCl and 0.5% bovine serum albumin) and tissue factor (6 pM). Coagulation was started by adding 1 volume of buffer B (2.5 mM Z-GGR-AMC, 20 mM Hepes, 140 mM NaCl, 100 mM CaCl2 and 6% bovine serum albumin). Samples were run at least in duplicate. First-derivative curves of accumulation of fluorescence in human plasma were converted into nM thrombin using a human calibrator.
Statistical analysis
Data are presented as means ± SE. Groups were compared using the non-parametric Mann-Whitney U test (one-tailed) using the statistical package for social sciences (SPSS 15.0, Chicago, IL, USA). Size distribution of platelet aggregates was evaluated by χ2 analysis [27]. A p-value below 0.05 was considered significant.
Results
Inhibition of mouse P2Y12 receptors causes unstable thrombus formation after rupture of an atherosclerotic plaque
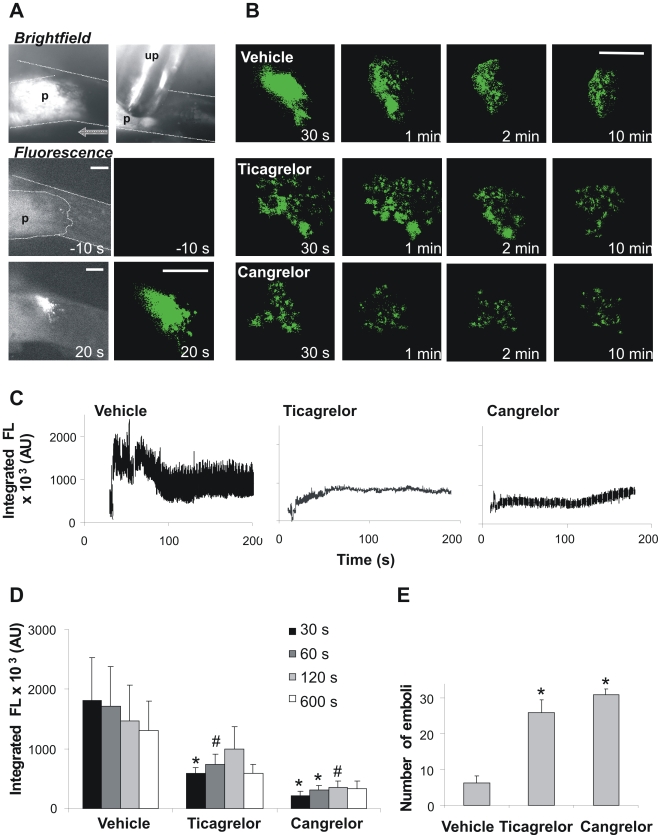
To study the role of platelet P2Y12 in atherothrombosis, we used a recently established mouse model of acute plaque rupture [26]. Mice deficient in ApoE were fed a cholesterol-enriched diet for 18–20 weeks. Plaque-containing carotid arteries were dissected free from surrounding tissue, and CFSE-labeled Apoe −/− platelets were injected to enable measurement of fluorescence by intravital microscopy. In control animals infused with vehicle solution, targeted treatment with an ultrasound probe resulted in acute rupture and subsequent non-occlusive thrombus formation (Fig. 1A). As described, this thrombotic process relies on GPVI-induced platelet activation as well as on thrombin generation and coagulation [26]. Mice were infused with effective concentrations of one of the reversible P2Y12 antagonists, ticagrelor or cangrelor. In blood samples taken from some of these mice, it was checked that the ticagrelor or cangrelor interventions abolished ADP- and collagen-induced platelet aggregation (data not shown). In other mice, the infusion of either P2Y12 antagonist greatly suppressed platelet accumulation induced by carotid plaque rupture, in that only loose thrombi appeared, which consisted of single-layered fluorescent platelets, in contrast to the compact bright fluorescent thrombi in vehicle-treated mice (Fig. 1B). The reduced thrombus formation was also apparent from time-courses of integrated fluorescence intensity after plaque rupture (Fig. 1C). Similarly as described [26], in vehicle-treated mice, plaque rupture caused a biphasic pattern of rapid increase and slower decline in fluorescence accumulation. Only few single platelets were seen to leave the thrombus in the declining phase. Since fluorescence bleaching was relatively low, the declining phase had a different cause, likely contraction of the platelet-fibrin thrombus.
Figure 1. Inhibition of P2Y12 receptors causes unstable thrombus formation on acutely ruptured plaques in mice.
Apoe −/− mice were infused with vehicle solution as a control. Other mice were infused with ticagrelor (210 µg/kg for 1 min, followed by 30 µg/kg/min during the experiment). In the third group of mice, cangrelor was continuously infused at 3 µg/kg/min. Infusion of ticagrelor and cangrelor started at 15 min before ultrasound treatment. (A) Brightfield image of a carotid plaque (p) with ultrasound probe (up). Further, raw fluorescence images (left) and threshold masked images (right) of CFSE-labeled platelets before and after ultrasound treatment. Dotted area indicates location of carotid artery (bars, 100 µm). Time stamps point to images at baseline (−10 s) or after plaque rupture (20 s). (B) Representative threshold masked images of thrombi on ruptured plaques of mice infused with vehicle, ticagrelor or cangrelor (bar, 100 µm). Note the microthrombi with P2Y12 antagonist formed within 1 min of ultrasound treatment. (C) Time-courses of integrated CFSE fluorescence intensity above background (arbitrary units, AU) of representative thrombi formed. (D) Quantification of thrombus size at various time points after ultrasound treatment. Data are integrated fluorescence intensities from threshold masked images. (E) Number of fluorescent emboli shed during 3 min after plaque rupture. Data are means ± SE (n = 3–8), *p<0.05 and # p<0.1 compared to vehicle.
Off-line quantification of images showed that P2Y12 inhibition with either antagonist reduced the size of thrombi compared to the vehicle control (Fig. 1D). Video analysis further revealed that the thrombi formed in the presence of P2Y12 inhibitor were relatively unstable. With ticagrelor or cangrelor present, 4–5 fold more emboli were shed from thrombi in comparison to control mice (Fig. 1E). Together, these data pointed to a marked inhibiting effect of the reversible P2Y12 antagonists on in vivo thrombus formation following plaque rupture.
Absence of functional P2Y12 receptors suppresses thrombus formation and thrombin generation, but increases disaggregation
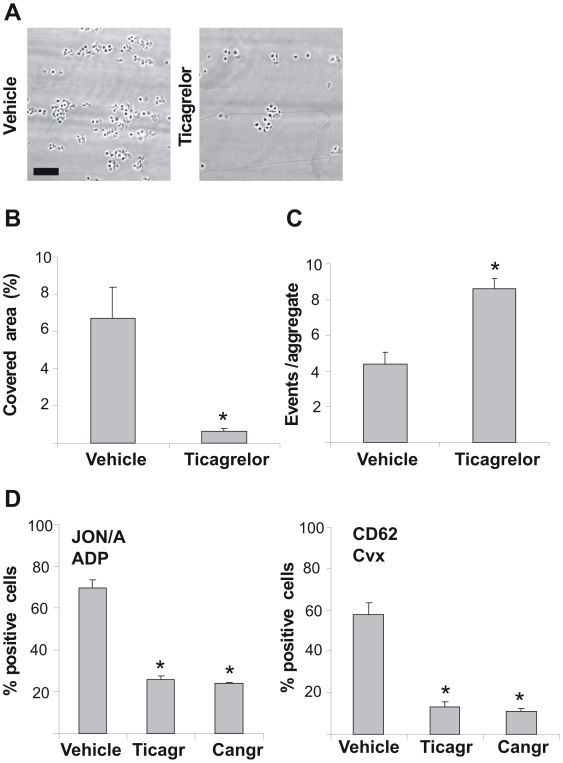
To study the mechanism by which P2Y12 contributes to thrombus stabilization, we performed flow chamber experiments over native type I collagen with blood from Apoe −/− mice which were infused with ticagrelor. Perfusion of blood from ticagrelor-treated mice resulted in diminished thrombus formation on collagen (Fig. 2A). The treatment significantly reduced platelet deposition, while it increased the disaggregation events (Fig. 2B, C). Flow cytometry using whole blood from mice infused with ticagrelor demonstrated near complete inhibition of αIIbβ3 activation in response to ADP and of P-selectin expression in response to low convulxin (Fig. 2D).
Figure 2. Inhibition of murine P2Y12 receptors diminishes thrombus formation and provokes platelet disaggregation under flow.
Apoe −/− mice were infused with vehicle solution, ticagrelor or cangrelor (see Figure 1). (A–C) PPACK/heparin anticoagulated blood from treated mice was perfused over native type I collagen at a shear rate of 1000 s−1 for 4 min. (A) Representative phase-contrast images after 4 min perfusion (bar = 20 µm). (B) Quantitative effect of ticagrelor on surface area coverage by platelets. (C) Average number of disaggregation events measured from a preformed aggregate during flow. (D) Flow-cytometric evaluation in whole blood of platelets with active αIIbβ3 (JON/A mAb) by activation with ADP (20 µM), and of platelets exposing P-selectin (anti-CD62 mAb) by activation with convulxin (Cvx, 10 ng/ml). Bars give percentages of positive platelets. Data are means ± SE (n = 3–4), *p<0.05.
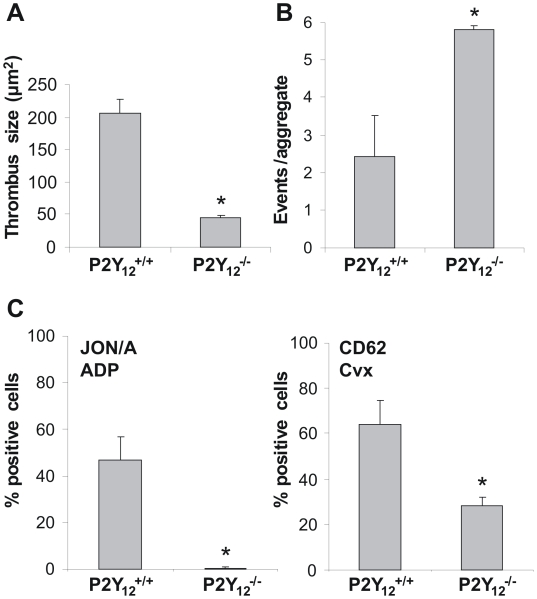
To confirm the role of P2Y12 in these platelet responses, experiments were performed with blood from P2Y12-deficient mice. Perfusion of blood from these mice also resulted in diminished thrombus formation (Fig. 3A), accompanied by increased disaggregation events (Fig. 3B). Furthermore, flow cytometry using whole blood from P2Y12-deficient mice demonstrated complete inhibition of αIIbβ3 activation in response to ADP and of P-selectin expression in response to low convulxin (Fig. 3C). Ticagrelor was without further effect on these responses of P2Y12-deficient platelets (not shown).
Figure 3. Deficiency in mouse P2Y12 receptors diminishes thrombus formation on collagen and provokes disaggregation under flow.
Blood from P2Y12 deficient (P2Y12 −/−) or wild type (P2Y12 +/+) mice was perfused over native type I collagen at 1000 s−1 for 4 min in the presence of 10 µM ADP. (A) Quantitative effect of P2Y12 deficiency on surface area coverage by platelets. (B) Average number of disaggregation events measured from a preformed aggregate during flow (n = 3). (C) Flow-cytometric evaluation of ADP-stimulated platelets with active αIIbβ3 (JON/A mAb) and of convulxin-stimulated platelets exposing P-selectin (anti-CD62 mAb) in whole blood. Experiments were performed as in Fig. 2. Data are means ± SE (n = 5–6), *p<0.05.
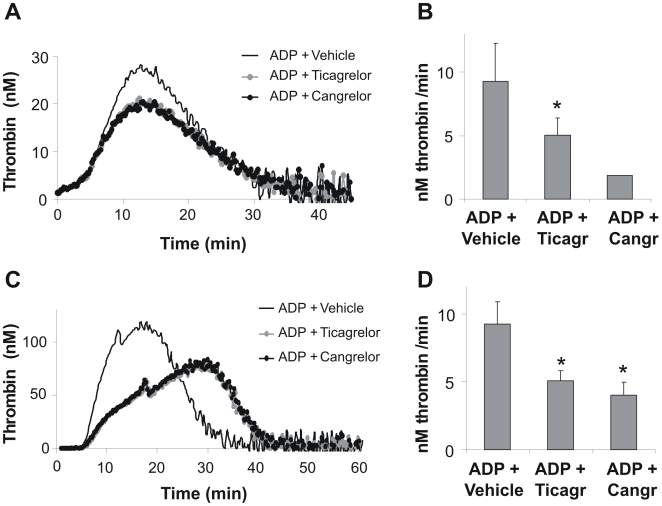
Since enhancement of platelet-dependent thrombin generation is an established outcome of P2Y12 signaling [20], effects of P2Y12 antagonism on thrombin generation were investigated in mouse and human PRP. Dose-response experiments demonstrated that 20 µM ticagrelor or cangrelor were maximally effective (data not shown). At this concentration, either antagonist partly reduced the stimulating effect of ADP on thrombin peak levels in mouse and human PRP (Fig. 4A, C). Since in particular the rate of thrombin generation is an important parameter in flow-dependent thrombus formation, we quantified the slope of the thrombin generation curves for effects of P2Y12 inhibition. both ticagrelor and cangrelor markedly suppressed this slope in mouse and human PRP (Fig. 4B, D). We reasoned that this contribution of P2Y12 may play a role in thrombus stabilization by increasing the availability of thrombin to activate platelets and form fibrin fibers.
Figure 4. Procoagulant role of P2Y12 receptors in platelet-dependent thrombin generation.
Mouse (A, B) or human (C, D) PRP was preincubated with vehicle, ticagrelor (20 µM) or cangrelor (20 µM), and stimulated with ADP (20 µM). Thrombin generation was measured after triggering with tissue factor (1 pM) and CaCl2 (16.7 mM). (A, C) Representative thrombin generation curves of PRP stimulated with ADP alone, or ADP in combination with P2Y12 inhibitor. (B, D) Quantitative effect of ticagrelor or cangrelor on ADP-stimulated rate of thrombin formation. Data are means ± SE (n = 3–6), *p<0.05 compared to ADP + vehicle.
Inhibition of P2Y12 impairs thrombus formation and stabilization at high but not low shear rate
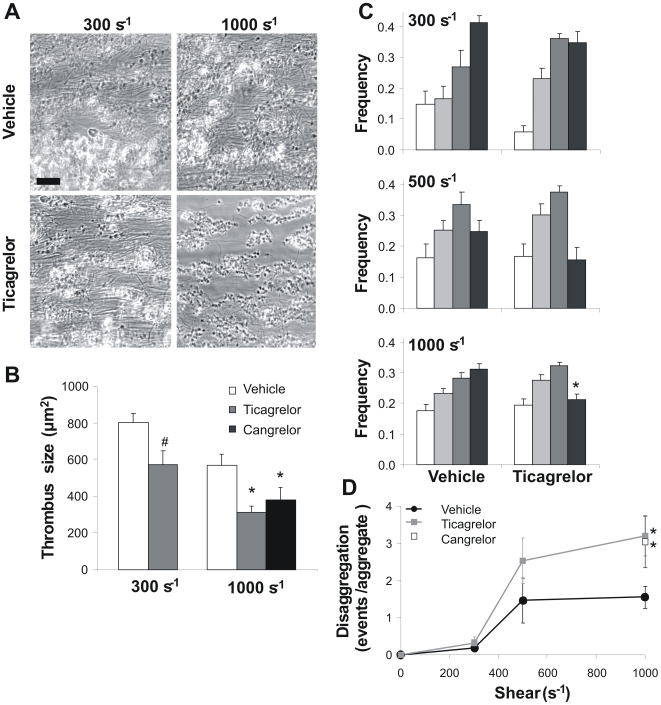
The clinical efficacy of P2Y12 antagonists may depend on the local shear rate, which can vary considerably at arterial sites of vulnerable atherosclerotic plaques. To investigate this, human blood was perfused at low and high shear rate over a collagen-containing surface under physiological conditions allowing coagulation. Under control conditions in the absence of P2Y12 inhibitors, stable thrombi containing platelets and fibrin were formed at shear rates of 300 to 1000 s−1 (Fig. 5A). Fibrin fibers were most clearly visible between adjacent thrombi. In the presence of ticagrelor or cangrelor, much smaller thrombi were formed. Especially at 1000 s−1, fibrin fiber formation and the average thrombus size were drastically reduced with ticagrelor (Fig. 5B). Analysis of the size distribution patterns on coverslip showed that ticagrelor suppressed the formation of larger thrombi (>400 platelets) only at this higher shear rate (Fig. 5C). Interestingly, this was accompanied by a twofold increase in disaggregation events, again only at 1000 s−1 (Fig. 5D). Similar effects of P2Y12 inhibition on thrombus stabilization at high shear were seen in flow studies without coagulation (not shown). Together, these experiments indicated that, under conditions of high shear and coagulation, P2Y12 antagonism suppressed the formation of stable, fibrin-containing thrombi.
Figure 5. Inhibition of P2Y12 receptors affects collagen-dependent thrombus formation at high but not low shear rate.
Citrate-anticoagulated human blood, recalcified with CaCl2/MgCl2 in the presence of 2 pM tissue factor, was perfused over Horm type I collagen at a shear rate of 300 or 1000 s−1. Blood samples were preincubated with vehicle, ticagrelor (20 µM) or cangrelor (10 µM), as indicated. (A) Representative phase-contrast images after 4 min of perfusion (bar = 20 µm). (B) Average size of thrombi formed in treated blood samples at different shear rates. (C) Frequency distribution of feature size on coverslips; estimated numbers of platelets per feature (aggregate) were: 9–24 (white), 24–75 (light gray), 75–400 (dark gray) and >400 (black). (D) Number of disaggregation events from individual aggregates in the 4th minute of blood perfusion at 300 or1000 s−1. Data are means ± SE (n = 3–9), *p<0.05 compared to vehicle.
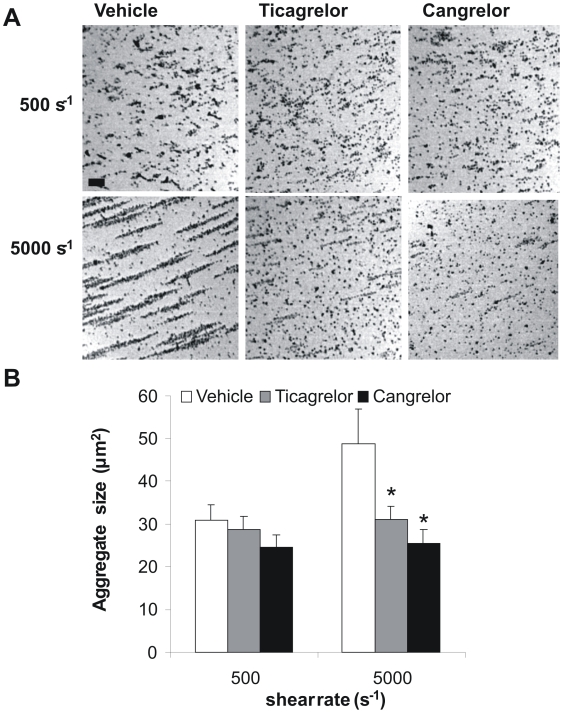
To confirm the shear-dependent effect of P2Y12 inhibition, additional whole blood experiments were performed with a cone and plate(let) analyzer. This test evaluates platelet adhesion and aggregation to a surface under defined shear conditions [28]. At a low shear rate of 500 s−1, the size of the platelet aggregates was not influenced by ticagrelor or cangrelor (Fig. 6A, B). However, at 5000 s−1, the larger size aggregates became greatly sensitive to P2Y12 inhibition. This experiment hence supports the conclusion that P2Y12 contributes to thrombus formation especially at high shear rate.
Figure 6. Inhibition of P2Y12 receptors reduces aggregate size at high but not low shear in cone and plate(let) analyzer.
Citrate-anticoagulated human blood was preincubated with vehicle, ticagrelor (20 µM) or cangrelor (10 µM) for 10 min. Blood samples were subjected to a shear rate of 500 or 5000 s−1 for 2 min in a cone and plate(let) analyzer (CPA). (A) Representative images of platelet aggregates formed on the surface after 2 min (bar = 20 µm). (B) Average aggregate size in µm2. Data are means ± SE (n = 4–6), *p<0.05 compared to vehicle.
Inhibition or deficiency of P2Y12 suppresses thrombus formation on plaques under high shear flow
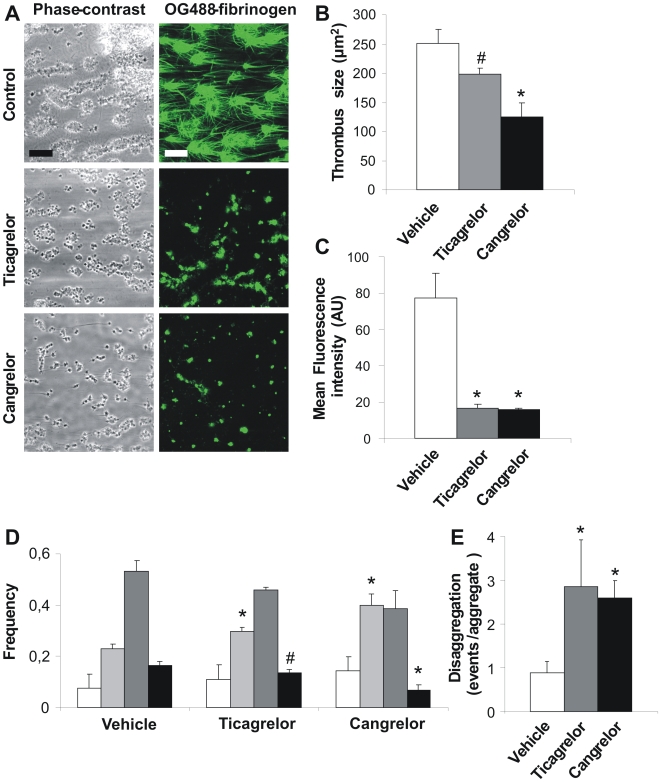
To approach the clinical situation, plaque tissue was isolated from large atherosclerotic vessels obtained by autopsy. Cell-free homogenates were prepared from pools of four plaques [5], and these were used as thrombogenic surface for flow chamber studies. Human blood, supplemented with OG488-labeled fibrinogen, was perfused over the plaque homogenate, again under conditions of high shear and coagulation. This resulted in the assembly of large, fluorescent thrombi, which were interconnected by fluorescent fibrin fibers (Fig. 7A). Preincubation of the blood with ticagrelor or cangrelor substantially reduced thrombus size as well as fibrin fiber formation (Fig. 7B, C). That thrombus size was reduced with either P2Y12 antagonist was further confirmed by morphometric analysis of the platelet aggregates (Fig. 7D). This was in agreement with analysis of the disaggregation events during blood flow, pointing to a marked destabilization of the thrombi formed in the presence of ticagrelor or cangrelor (Fig. 7E).
Figure 7. Inhibition of P2Y12 receptors affects plaque-induced thrombus formation at high shear rate.
Citrate-anticoagulated human blood was supplemented with OG488-fibrinogen (25 µg/ml), recalcified with CaCl2/MgCl2 in the presence of 2 pM tissue factor and perfused over homogenized human plaque material at 1000 s−1 for 8 min. Blood was pre-incubated with ticagrelor (20 µM) or cangrelor (10 µM) as indicated. (A) Representative phase-contrast and fluorescence images (bar = 20 µm). (B) Average size of thrombi after perfusion. (C) Mean fluorescence intensity from fibrin(ogen)-binding platelets and thrombi. (D) Histograms of features on surface; the estimated numbers of platelets per feature were 9–24 (white), 24–75 (light gray), 75–400 (dark gray) and >400 (black). (E) Disaggregation events measured per platelet aggregate per min. Data are means ± SE (n = 3), *p<0.05, # p = 0.06 compared to vehicle.
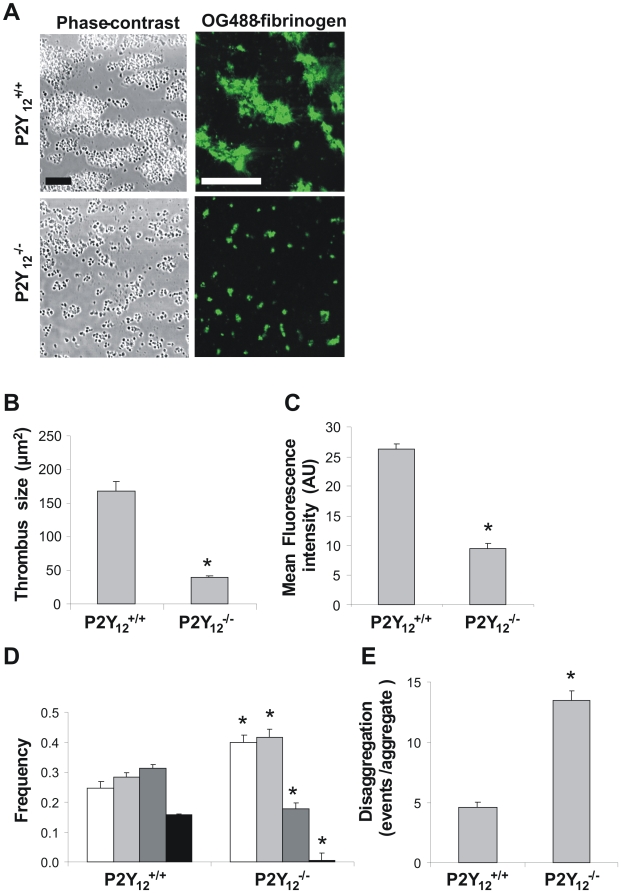
To confirm these results based on pharmacological inhibition of P2Y12, flow experiments were performed using blood from P2Y12-deficient mice. Blood was perfused over homogenized plaque material, which was isolated from atherosclerotic aortic arches of cholesterol-fed Apoe −/− mice. Perfusion of P2Y12-deficient blood over this material resulted in greatly reduced thrombus formation, leaving only a monolayer of platelets at the plaque surface (Fig. 8A). Deficiency in P2Y12 also suppressed the deposition of OG488-fibrinogen. Image analysis pointed to a significant reduction in both overall thrombus size (Fig. 8B) and label accumulation (Fig. 8C). Morphometric analysis showed the formation of more smaller aggregates with P2Y12-deficient blood (Fig. 8D). These thrombi were instable, as appeared from analysis of the platelet disaggregation events during flow (Fig. 8E).
Figure 8. Deficiency of murine P2Y12 receptors affects thrombus formation on plaque at high shear rate.
Citrate-anticoagulated blood from P2Y12 −/− and corresponding wild type (P2Y12 +/+) mice was supplemented with OG488-fibrinogen (25 µg/ml) and co-perfused with ADP (10 µM) over homogenized murine plaque material at 1000 s−1 for 4 min. (A) Representative phase-contrast and fluorescence images (bar = 20 µm). (B) Average size of thrombi after perfusion. (C) Average fluorescence intensity (arbitrary units) from fibrin(ogen)-binding platelets and thrombi. (D) Histograms of features on surface; estimated numbers of platelets per feature were 9–24 (white), 24–75 (light gray), 75–400 (dark gray) and >400 (black). (E) Disaggregation events measured per platelet aggregate per min. Data are means ± SE (n = 3), *p<0.05.
Altogether these results indicate that ADP, via P2Y12, stabilizes thrombi formed under flow on ruptured plaques in the mouse carotid artery in vivo, and on human and mouse plaque material in vitro. Furthermore, they show that the contribution of P2Y12 to thrombus stability increases with shear rate and is maintained in the presence of coagulation.
Discussion
In the present paper, we used a novel in vivo mouse model of thrombus formation on acutely ruptured atherosclerotic plaques, to demonstrate a marked antithrombotic effect of two reversible blocking agents of the platelet P2Y12 receptors, i.e. ticagrelor and cangrelor. Both P2Y12 inhibitors destabilized thrombus formation by increasing the embolization of platelets, with as a result smaller sized thrombi. Our data extend earlier findings, using the same model of acute atherothrombosis, which indicated that thrombus formation is the result of a multi-factorial process involving: platelet interaction with collagen, exposure of tissue factor, generation of thrombin, and formation of fibrin fibers [26]. Recently, other authors have confirmed the importance of collagen and thrombin, using the same model of carotid plaque rupture by ultrasound [30]. Since these in vivo data pointed to collagen as a major thrombogenic component in the carotid plaque, we continued to study thrombus formation by in vitro perfusion studies with immobilized plaque material or native collagen as a surface. However, it should be noted that also other thrombogenic components have been identified in plaque material, including tissue factor, oxidized low density lipoproteins and lysophosphatidate [8], [10], [31].
A key role of platelet P2Y12 receptors in thrombus stabilization was confirmed by in vitro flow studies using either mouse or human blood. Markedly, when coagulation was introduced, blockage or deficiency of P2Y12 suppressed the formation of larger sized platelet aggregates as well as the formation of fibrin fibers, in particular at high shear rate. Furthermore, inhibition or absence of P2Y12 destabilized the thrombi formed on immobilized collagens or plaque material. Also cone-and-plate(let) experiments, where platelets adhered to a surface, showed an enhancing effect of P2Y12 on thrombus formation at high but not low shear rate.
In earlier work, we and others have shown that autocrine, platelet-derived ADP contributes to thrombus growth and stability on collagen-containing surfaces [18], [32], [33]. The present data confirm this and indicate that, in addition, the thrombus-stabilizing role of P2Y12 is maintained in the presence of coagulation. Mechanistically, P2Y12 can contribute to the formation of stable thrombi via two different processes: (i) continuous signaling to phosphoinositide 3-kinases β and γ with as a result sustained αIIbβ3 activation [18]; (ii) enhancement of thrombin/fibrin generation due to increased Gi-mediated Ca2+ mobilization and platelet procoagulant activity [20], [21], [34]. The observations that ticagrelor and cangrelor retards ADP-dependent thrombin generation and fibrin formation relates to the latter function. In conjunction with the present results, others have reported that P2Y12 is implicated in shear-induced platelet aggregation via activation of Syk kinase and phosphoinositide 3-kinase [35]. Interestingly, another study describes no effect of cangrelor of collagen-dependent thrombus formation [36]. However, this study focused short-term processes (<1.5 min), during which thrombi are still in the growing phase and the contribution of adhesive receptors such as GP-Ib-V-IX is relatively high. Our time-dependent analysis however indicates that a role of P2Y12 becomes more prominent at later stages.
The P2Y12-directed prodrug clopidogrel is increasingly used to prevent secondary ischemic events in patients with myocardial infarction or stroke [37], [38]. Recently, clinical studies performed with the new, irreversible P2Y12 inhibitor prasugrel [39], [40] and the reversible P2Y12 inhibitor ticagrelor [41], [42] have shown promising results for the treatment of acute coronary syndrome. Our findings suggest that the success of such anti-P2Y12 interventions relies on the selective abrogation of a platelet response–autocrine activation of P2Y12–that is at least in part dependent on the local, high shear conditions.
In summary, we have shown that ADP, via P2Y12, stabilizes thrombi on ruptured plaques both in vivo and on human plaque material in vitro. Furthermore, this P2Y12 dependency of thrombus stability is maintained in the presence of coagulation, but most pronounced at high shear flow conditions.
Acknowledgments
We thank dr. R. Megens and Laura Jackman for participation in experiments. We also thank Schering Plough Research Institute for providing the P2Y12 deficient mice.
Footnotes
Competing Interests: J.J.J. van Giezen is an employee of AstraZeneca. Partial financial support for the conductance of this study was provided by AstraZeneca who own the patent for ticagrelor. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Funding: Partial financial support for the conductance of this study was provided by AstraZeneca. Support is acknowledged from the Hacettepe University (Ankara, Turkey), the Netherlands Organization for Scientific Research (11.400.0076) and the Netherlands Heart Foundation (2005B079). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 3.Munnix IC, Cosemans JM, Auger JM, Heemskerk JW. Platelet response heterogeneity in thrombus formation. Thromb Haemost. 2009;102:1149–1156. doi: 10.1160/TH09-05-0289. [DOI] [PubMed] [Google Scholar]
- 4.Denis CV, Wagner DD. Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:728–739. doi: 10.1161/01.ATV.0000259359.52265.62. [DOI] [PubMed] [Google Scholar]
- 5.Cosemans JM, Kuijpers MJE, Lecut C, Loubele ST, Heeneman S, et al. Contribution of platelet glycoprotein VI to the thrombogenic effect of collagens in fibrous atherosclerotic lesions. Atherosclerosis. 2005;181:19–27. doi: 10.1016/j.atherosclerosis.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Penz S, Reininger AJ, Brandl R, Goyal P, Rabie T, et al. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. Faseb J. 2005;19:898–909. doi: 10.1096/fj.04-2748com. [DOI] [PubMed] [Google Scholar]
- 7.De Meyer GR, Hoylaerts MF, Kockx MM, Yamamoto H, Herman AG, et al. Intimal deposition of functional von Willebrand factor in atherogenesis. Arterioscler Thromb Vasc Biol. 1999;19:2524–2534. doi: 10.1161/01.atv.19.10.2524. [DOI] [PubMed] [Google Scholar]
- 8.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, et al. Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 9.Guyton JR, Klemp KF. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 2003;10:267–274. doi: 10.5551/jat.10.267. [DOI] [PubMed] [Google Scholar]
- 11.van der Meijden PE, Munnix IC, Auger JM, Govers-Riemslag JW, Cosemans JM, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114:818–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 12.Auger JM, Kuijpers MJE, Senis YA, Watson SP, Heemskerk JW. Adhesion of human and mouse platelets to collagen under shear: a unifying model. Faseb J. 2005;19:825–827. doi: 10.1096/fj.04-1940fje. [DOI] [PubMed] [Google Scholar]
- 13.Kuijpers MJE, Schulte V, Bergmeier W, Lindhout T, Brakebusch C, et al. Complementary roles of glycoprotein VI and α2β1 integrin in collagen-induced thrombus formation in flowing whole blood ex vivo. Faseb J. 2003;17:685–687. doi: 10.1096/fj.02-0381fje. [DOI] [PubMed] [Google Scholar]
- 14.Cosemans JM, Iserbyt BF, Deckmyn H, Heemskerk JW. Multiple ways to switch platelet integrins on and off. J Thromb Haemost. 2008;6:1253–1261. doi: 10.1111/j.1538-7836.2008.03041.x. [DOI] [PubMed] [Google Scholar]
- 15.Munnix IC, Kuijpers MJE, Auger J, Thomassen CM, Panizzi P, et al. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation. Arterioscler Thromb Vasc Biol. 2007;27:2484–2490. doi: 10.1161/ATVBAHA.107.151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost. 2008;99:466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 17.André P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, et al. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108:3045–3052. doi: 10.1182/blood-2006-03-006338. [DOI] [PubMed] [Google Scholar]
- 19.Goto S, Tamura N, Ishida H, Ruggeri ZM. Dependence of platelet thrombus stability on sustained glycoprotein IIb/IIIa activation through adenosine 5′-diphosphate receptor stimulation and cyclic calcium signaling. J Am Coll Cardiol. 2006;47:155–162. doi: 10.1016/j.jacc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 20.van der Meijden PE, Feijge MA, Giesen PL, Huijberts M, van Raak LP, et al. Platelet P2Y12 receptors enhance signalling towards procoagulant activity and thrombin generation A study with healthy subjects and patients at thrombotic risk. Thromb Haemost. 2005;93:1128–1136. doi: 10.1160/TH04-09-0597. [DOI] [PubMed] [Google Scholar]
- 21.Leon C, Ravanat C, Freund M, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler Thromb Vasc Biol. 2003;23:1941–1947. doi: 10.1161/01.ATV.0000092127.16125.E6. [DOI] [PubMed] [Google Scholar]
- 22.Siljander P, Lassila R. Studies of adhesion-dependent platelet activation: distinct roles for different participating receptors can be dissociated by proteolysis of collagen. Arterioscler Thromb Vasc Biol. 1999;19:3033–3043. doi: 10.1161/01.atv.19.12.3033. [DOI] [PubMed] [Google Scholar]
- 23.Munnix ICA, Strehl A, Kuijpers MJE, Auger JM, van der Meijden PEJ, et al. The glycoprotein VI-phospholipase Cγ2 signaling pathway controls collagen- and tissue factor-induced thrombus formation in vitro and in vivo. Atheroscl Thromb Vasc Biol. 2005;25:2673–2678. doi: 10.1161/01.ATV.0000193568.71980.4a. [DOI] [PubMed] [Google Scholar]
- 24.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans DJ, Jackman LE, Chamberlain J, Crosdale DJ, Judge HM, et al. Platelet P2Y12 receptor influences the vessel wall response to arterial injury and thrombosis. Circulation. 2009;119:116–122. doi: 10.1161/CIRCULATIONAHA.107.762690. [DOI] [PubMed] [Google Scholar]
- 26.Kuijpers MJE, Gilio K, Reitsma S, Nergiz-Unal R, Prinzen L, et al. Complementary roles of platelets and coagulation in thrombus formation on plaques acutely ruptured by targeted ultrasound treatment: a novel intravital model. J Thromb Haemost. 2009;7:152–161. doi: 10.1111/j.1538-7836.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 27.Siljander PR, Munnix IC, Smethurst PA, Deckmyn H, Lindhout T, et al. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood. 2004;103:1333–1341. doi: 10.1182/blood-2003-03-0889. [DOI] [PubMed] [Google Scholar]
- 28.Varon D, Savion N. Cone and Plate(let) Analyzer. In: Michelson AD, editor. Platelets. San Diego, USA: Academic Press; 2002. pp. 337–345. [Google Scholar]
- 29.Vanschoonbeek K, Feijge MA, Van Kampen RJ, Kenis H, Hemker HC, et al. Initiating and potentiating role of platelets in tissue factor-induced thrombin generation in the presence of plasma: subject-dependent variation in thrombogram characteristics. J Thromb Haemost. 2004;2:476–484. doi: 10.1111/j.1538-7933.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 30.Hechler B, Eckly A, Magnenat S, Freund M, Cazenave JP, et al. Localized arterial thrombosis on ruptured atherosclerotic plaques of Apoe −/− mice after vascular injury with ultrasound. J Thromb Haemost. 2009;7:Abstract OC-TH-033. [Google Scholar]
- 31.Haseruck N, Erl W, Pandey D, Tigyi G, Ohlmann P, et al. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 2004;103:2585–2592. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 32.Penz SM, Reininger AJ, Toth O, Deckmyn H, Brandl R, et al. Glycoprotein Ibalpha inhibition and ADP receptor antagonists, but not aspirin, reduce platelet thrombus formation in flowing blood exposed to atherosclerotic plaques. Thromb Haemost. 2007;97:435–443. [PubMed] [Google Scholar]
- 33.Remijn JA, Wu YP, Jeninga EH, Ijsseldijk MJW, van Willigen G, et al. Role of ADP receptor P2Y12 in platelet adhesion and thrombus formation in flowing blood. Arterioscler Thromb Vasc Biol. 2002;22:686–691. doi: 10.1161/01.atv.0000012805.49079.23. [DOI] [PubMed] [Google Scholar]
- 34.van der Meijden PE, Schoenwaelder SM, Feijge MA, Cosemans JM, Munnix IC, et al. Dual P2Y 12 receptor signaling in thrombin-stimulated platelets - involvement of phosphoinositide 3-kinase beta but not gamma isoform in Ca2+ mobilization and procoagulant activity. Febs J. 2008;275:371–385. doi: 10.1111/j.1742-4658.2007.06207.x. [DOI] [PubMed] [Google Scholar]
- 35.Resendiz JC, Feng S, Ji G, Francis KA, Berndt MC, et al. Purinergic P2Y12 receptor blockade inhibits shear-induced platelet phosphatidylinositol 3-kinase activation. Mol Pharmacol. 2003;63:639–645. doi: 10.1124/mol.63.3.639. [DOI] [PubMed] [Google Scholar]
- 36.Turner NA, Moake JL, McIntire LV. Blockade of adenosine diphosphate receptors P2Y12 and P2Y1 is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001;98:3340–3345. doi: 10.1182/blood.v98.12.3340. [DOI] [PubMed] [Google Scholar]
- 37.Fintel DJ. Antiplatelet therapy in cerebrovascular disease: implications of Management of Artherothrombosis with Clopidogrel in High-risk Patients and the Clopidogrel for High Artherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance studies' results for cardiologists. Clin Cardiol. 2007;30:604–614. doi: 10.1002/clc.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vande Griend JP, Saseen JJ. Combination antiplatelet agents for secondary prevention of ischemic stroke. Pharmacotherapy. 2008;28:1233–1242. doi: 10.1592/phco.28.10.1233. [DOI] [PubMed] [Google Scholar]
- 39.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 40.Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25:357–374. doi: 10.1111/j.1527-3466.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 41.van Giezen JJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, et al. Ticagrelor binds to the human P2Y receptor independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009 doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 42.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]