Abstract
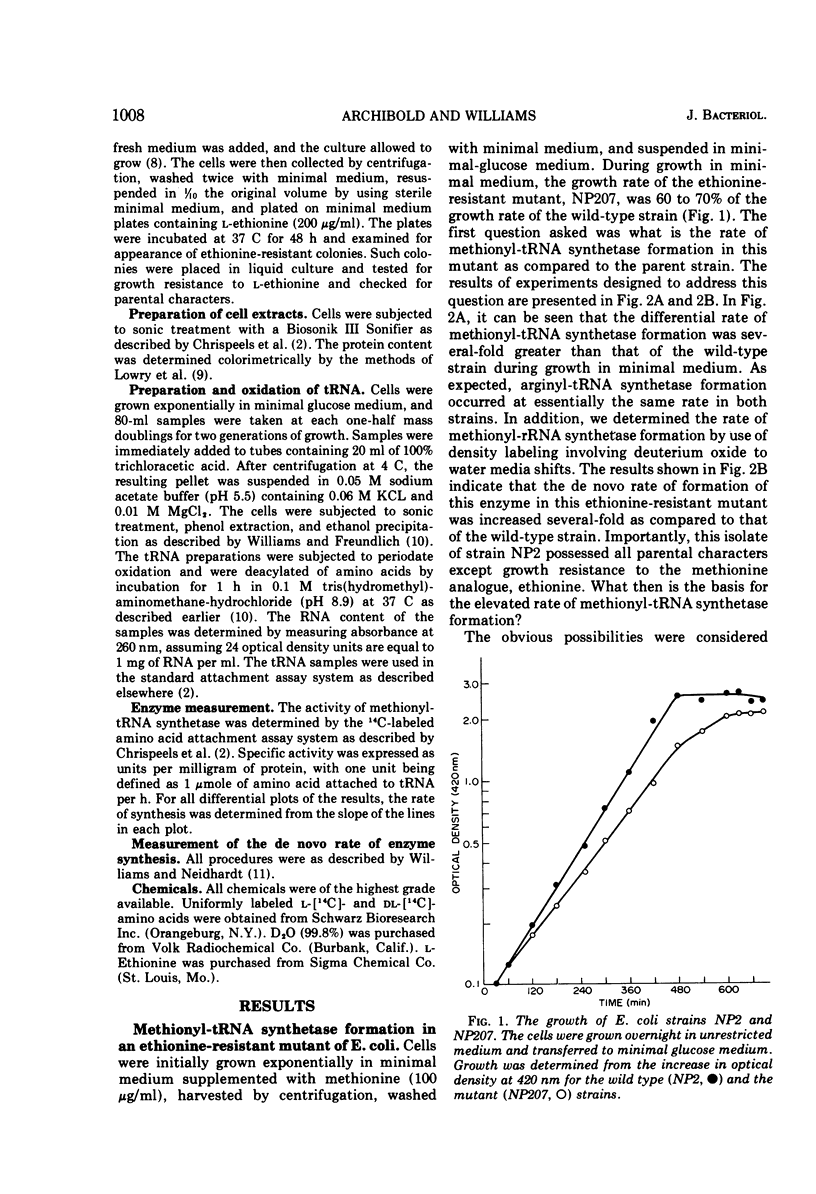
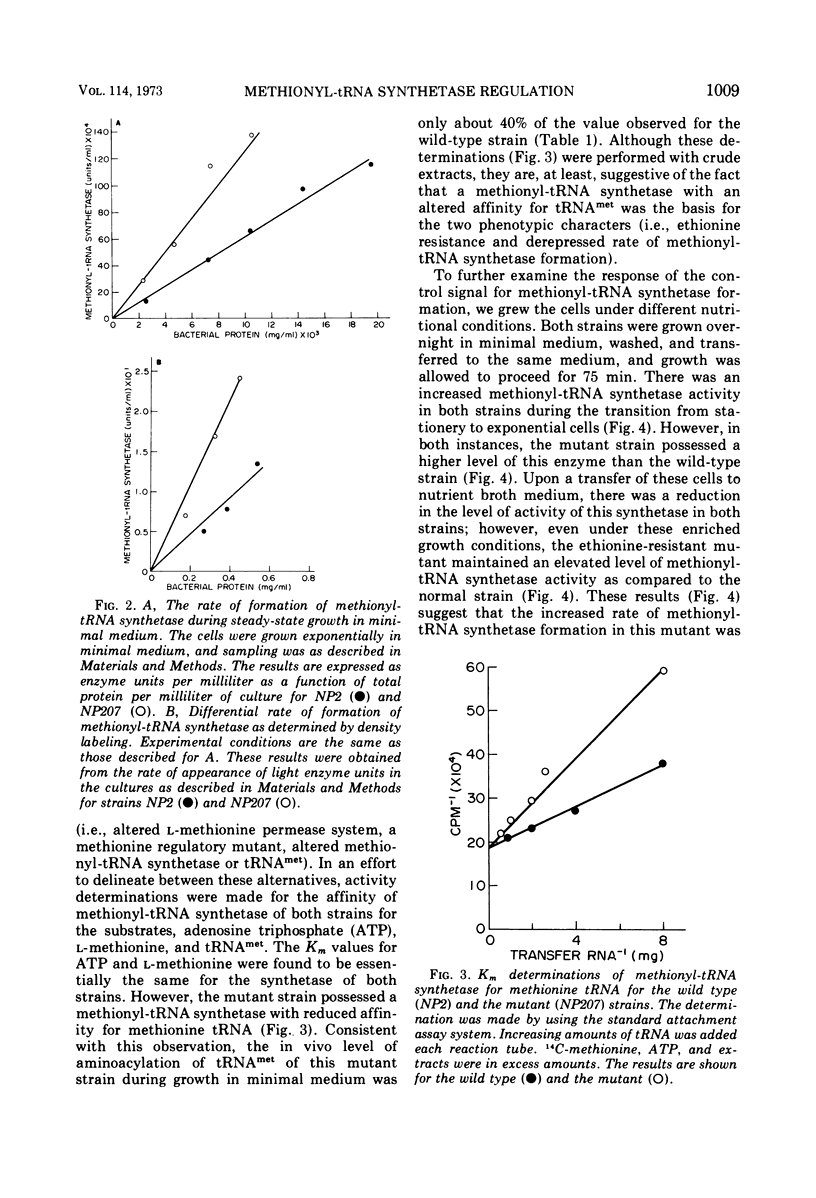
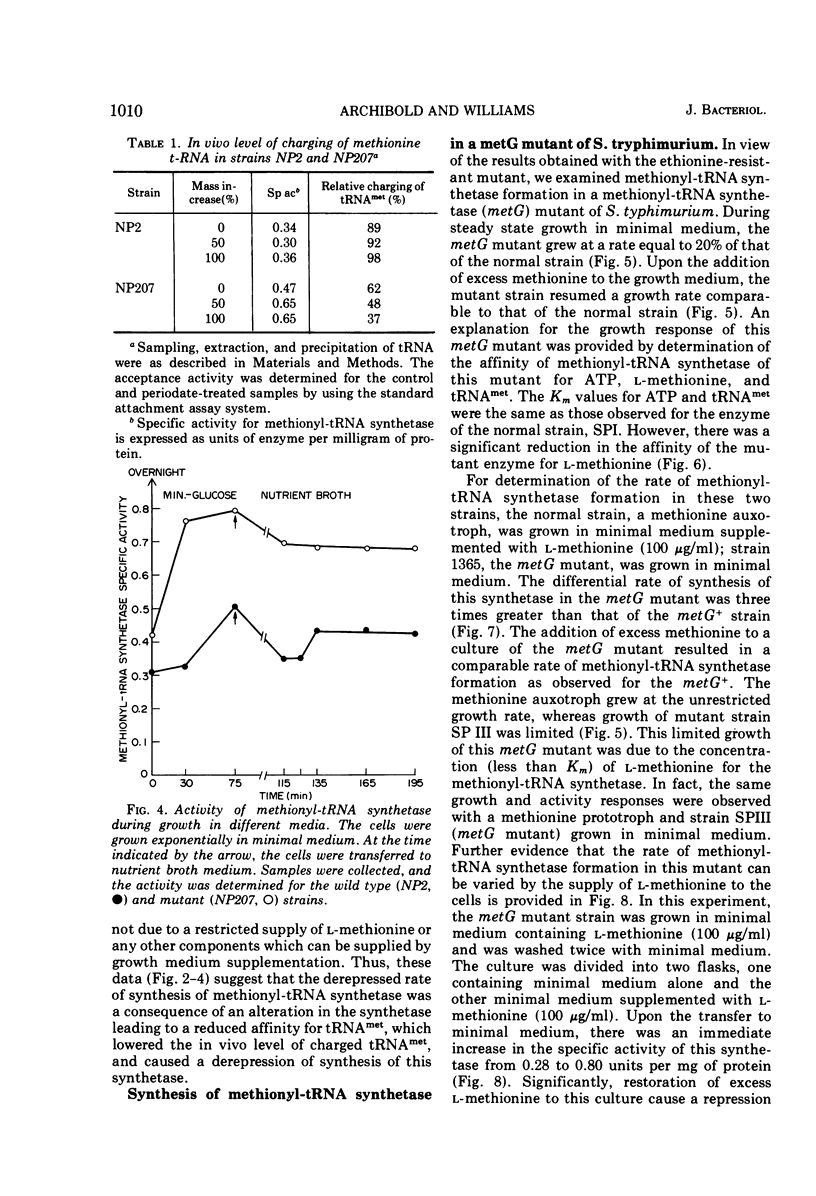
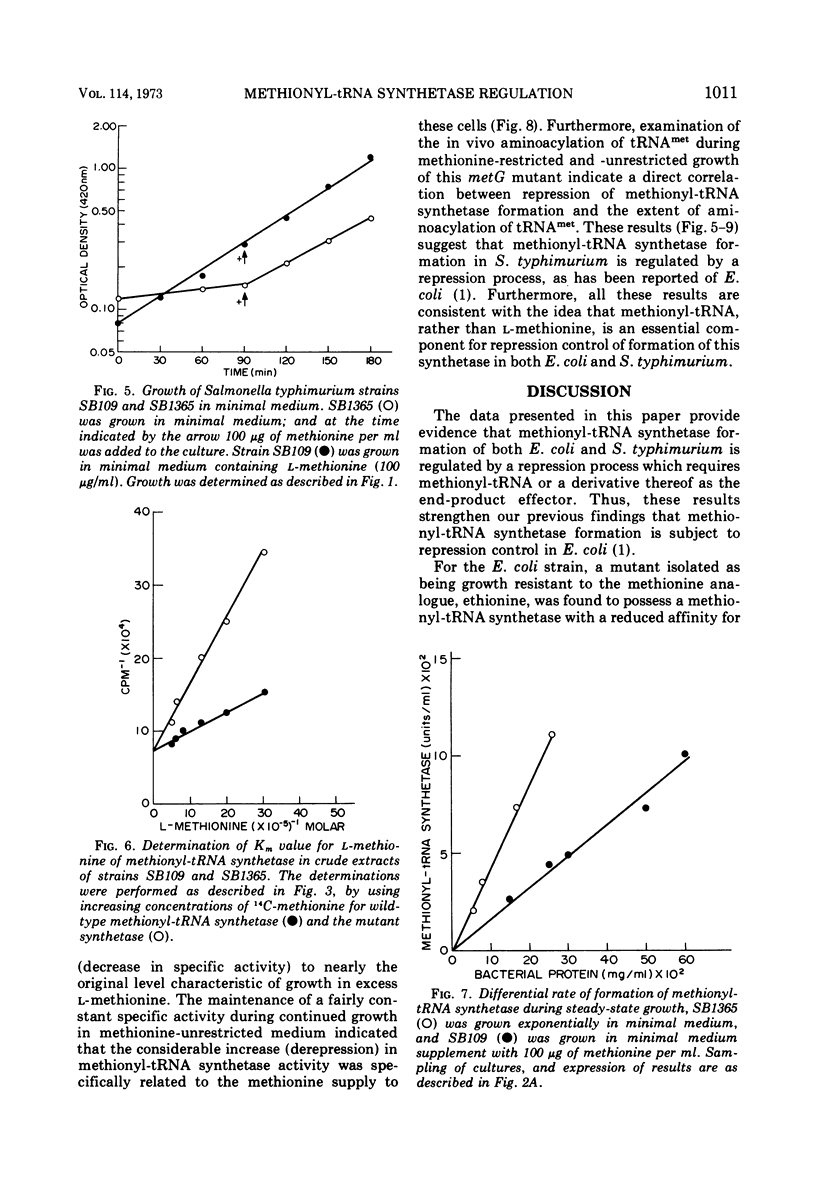
The control of methionyl-transfer ribonucleic acid (tRNA) synthetase (l-methionine: soluble RNA ligase [adenosine monophosphate]) was studied in methionyl-tRNA synthetase mutants of Escherichia coli and Salmonella typhimurium. The results of activity determinations with crude extracts indicate that this enzyme of the E. coli mutant strain possessed a reduced affinity for methionine-tRNA, whereas this enzyme of the S. typhimurium mutant exhibited a decreased affinity for l-methionine. The differential rate of methionyl-tRNA synthetase formation in these two mutants was several-fold greater than that of the respective parental strains. On the other hand, the level of in vivo aminoacylation of methionine-tRNA was only about one-third that of the parent strains. These results suggest that aminoacylation of methionine-tRNA is a necessary step in repression control of methionyl-tRNA synthetase of both E. coli and S. typhimurium strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibold E. R., Williams L. S. Regulation of synthesis of methionyl-, prolyl-, and threonyl-transfer ribonucleic acid synthetases of Escherichia coli. J Bacteriol. 1972 Mar;109(3):1020–1026. doi: 10.1128/jb.109.3.1020-1026.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Boyd R. F., Williams L. S., Neidhardt F. C. Modification of valyl tRNA synthetase by bacteriophage in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):463–475. doi: 10.1016/0022-2836(68)90421-x. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Gross T. S., Rowbury R. J. Methionyl transfer RNA synthetase mutants of Salmonella typhimurium which have normal control of the methionine biosynthetic enzymes. Biochim Biophys Acta. 1969 Jun 17;184(1):233–236. doi: 10.1016/0304-4165(69)90126-3. [DOI] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGinnis E., Williams L. S. Regulation of histidyl-transfer ribonucleic acid synthetase formation in a histidyl-transfer ribonucleic acid synthetase mutant of Salmonella typhimurium. J Bacteriol. 1972 Sep;111(3):739–744. doi: 10.1128/jb.111.3.739-744.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis E., Williams L. S. Regulation of synthesis of the aminoacyl-transfer ribonucleic acid synthetases for the branched-chain amino acids of Escherichia coli. J Bacteriol. 1971 Oct;108(1):254–262. doi: 10.1128/jb.108.1.254-262.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis E., Williams L. S. Role of histidine transfer ribonucleic acid in regulation of synthesis of histidyl-transfer ribonucleic acid synthetase of Salmonella typhimurium. J Bacteriol. 1972 Feb;109(2):505–511. doi: 10.1128/jb.109.2.505-511.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]



