Abstract
Background
Mutations in the LMNA gene, which encodes all A-type lamins, result in a variety of human diseases termed laminopathies. Lmna-/- mice appear normal at birth but become runted as early as 2 weeks of age and develop multiple tissue defects that mimic some aspects of human laminopathies. Lmna-/- mice also display smaller spleens and thymuses. In this study, we investigated whether altered lymphoid organ sizes are correlated with specific defects in lymphocyte development.
Principal Findings
Lmna-/- mice displayed severe age-dependent defects in T and B cell development which coincided with runting. Lmna-/- bone marrow reconstituted normal T and B cell development in irradiated wild-type recipients, driving generation of functional and self-MHC restricted CD4+ and CD8+ T cells. Transplantation of Lmna-/- neonatal thymus lobes into syngeneic wild-type recipients resulted in good engraftment of thymic tissue and normal thymocyte development.
Conclusions
Collectively, these data demonstrate that the severe defects in lymphocyte development that characterize Lmna-/- mice do not result directly from the loss of A-type lamin function in lymphocytes or thymic stroma. Instead, the immune defects in Lmna -/- mice likely reflect indirect damage, perhaps resulting from prolonged stress due to the striated muscle dystrophies that occur in these mice.
Introduction
Nuclear lamins are intermediate filament proteins and have roles in various nuclear processes, including DNA replication, chromatin organization, and gene transcription [1]–[4]. One gene, LMNA, encodes the A-type nuclear lamins, which produce the predominant A-type lamin proteins A and C. Mutations in LMNA are associated with more than 13 different tissue-specific diseases, collectively termed laminopathies, which include muscular dystrophies, cardiomyopathies, and more recently, a series of progeroid diseases that resemble some aspects of premature aging (for review, see Cohen et al.) [5]. Lmna-/- mice are characterized by a variety of tissue-specific defects consistent with those observed in human laminopathies, but additionally display growth defects as early as 2 weeks of age, reduced thymus and spleen size, defective spermatogenesis, and death by 6–8 weeks of age [6], [7].
A-type lamins have limited expression in the hematopoietic system [8]. Previous studies describing the presence of lamin A/C proteins in lymphocyte lineages are mixed, but largely suggest limited to no presence of A-type lamin proteins in early stages of B and T cell development, with increasing protein abundance in mature B and T cells and stimulated lymphocytes [9]–[13]. Furthermore, A-type lamin proteins are found only in a minority of cells from the bone marrow, thymus, spleen, and lymph nodes, which may represent cell types of the stroma such as epithelial cells, pericytes, inflammatory cells, fibroblasts, endothelial cells, and smooth muscle cells [8], [13]–[15].
Although no immune disease to date has been linked to mutations in LMNA, Lmna-/- mice display reduced thymus and spleen size, suggesting a potential role for A-type lamins in the postnatal development and/or homeostasis of lymphocyte lineages. Here, we investigate the reduced thymus and spleen size in Lmna-/- mice and identify a progressive, age-dependent impairment in T and B cell development. These defects are not cell-autonomous, because transplanted Lmna-/- bone marrow can reconstitute irradiated recipient Lmna+/+ immune tissues. Transplanted Lmna -/- thymus lobes show good engraftment in wild-type hosts and drive normal thymocyte development. Therefore, the altered immune cell development is not specific to loss of Lmna expression in developing lymphocytes or thymic epithelial cells, but likely an indirect effect of loss of lamin expression in other non-lymphoid tissues.
Methods
Mice
Lmna+/- mice were obtained from Colin Stewart (Institute of Medical Biology, Immunos Singapore) [7] and sibling mating was used to generate Lmna+/+ and Lmna-/- mice. This line was also backcrossed to C57BL/6J (B6) mice for 9 additional generations and the resulting mice, B6.129S1(Cg)-Lmnatm1Stw/BkknJ, were used for thymus transplantation studies. B6.SJL (B6.SJL-PtprcaPepcb/BoyJ) Ly5.1+ and C57BL/6J Ly5.2+ mice were crossed to generate F1 Ly5.1+Ly5.2+ mice used as recipients in bone marrow chimera experiments. Mice were bred and maintained under specific-pathogen free conditions. All experiments were conducted in accordance with the review board of the University of Washington Institutional Animal Care and Use Committee, who approved this study.
Antibodies and flow cytometry
Single-cell suspensions were prepared from thymus, spleen, bone marrow, and a pool of brachial, axillary, and inguinal lymph nodes. Red blood cells were removed from spleen cells by water lysis. For flow cytometry, Fc receptors were blocked using anti-CD16/32 (2.4G2; BD Pharmingen). Cells were surface stained in BSS containing 1% BSA with FITC, phycoerythrin- (PE), Peridinin-Chlorophyll-Protein-Cy5.5, PE-Cy7, allophycocyanin- (APC), and APC-AlexaFluor 750 flourochrome-conjugated antibodies and, in some cases, biotinylated antibodies followed by FITC- or APC-conjugated streptavidin. Antibodies were purchased from BD Pharmingen or eBioscience and included monoclonal antibodies recognizing mouse CD4 (RM4-5), CD8α (53–6.7), CD19 (1D3), CD25 (PC61), CD44 (IM7), CD45R/B220 (RA3-6B2), CD62L (MEL-14), CD69 (H1.2F3), panTCRβ (H57-597.13), IgM (II/41), and IgD (11–26c). For intracellular cytokine staining, splenocytes were incubated for 5 hours at 37°C in RP10 (RPMI with 10% FBS, 10 mM HEPES, 4 mM L-Glutamine, 100 U/ml penicilin, 100 µg/mL streptomycin, 50 µM β-mercaptoethanol, and 50 µg/mL Gentamycin) in the presence of GolgiPlug (BD Pharmingen) either in the absence or the presence of 1 µg/mL GP61–80 I-Ab-restricted peptide (GLKGPDIYKGVYQFKSVEFD) for detection of Lymphocytic Choriomeningitis Virus (LCMV)-specific CD4+ T cells or 0.1 µg/mL GP33–41 H-2Db-restricted peptide (KAVYNFATC) (Invitrogen) for detection of LCMV-specific CD8+ T cells [16]. Cells were surface-stained first, and then stained for intracellular cytokines with APC-conjugated anti-IFNγ (XMG1.2) and PE-conjugated anti-IL-2 (JES6-54H4) or anti-TNFα (MP6-XT22), using the BD Cytofix/Cytoperm kit and protocol. Flow cytometry data were collected using a FACS Canto (Becton Dickenson) and analyzed with FlowJo software (Treestar; Ashland, OR).
Bone marrow chimeras
Single-cell suspensions of hind leg bone marrow from Lmna +/+ and Lmna -/- mice were T cell- depleted by staining with PE-conjugated anti-CD4 and anti-CD8 followed by removal of PE-labeled cells using the EasySep PE selection separation kit (Stem Cell Technologies; Vancouver, Canada). Alternatively, T cells were depleted using anti-Thy1 (13.4.6), anti-CD4 (RL172), and anti-CD8 (3.168.8) followed by lysis using rabbit complement (Cedarlane Laboratories Limited; Hornby, Canada). Sex-matched recipient B6 Ly5.1+Ly5.2+ mice were irradiated with 1000 rads 6–8 hours prior to bone marrow injection, and maintained on antibiotic water (polymixin B sulfate and neomycin sulfate from Invitrogen), for 2 days prior to 2 weeks following irradiation. Recipient mice were injected with 3−5×106 T cell-depleted bone marrow cells into the lateral tail vein.
LCMV infection
Mice were infected by intraperitoneal injection with 2×105 PFU of LCMV Armstrong (provided by M.K. Kaja; University of Washington). Spleens were harvested 8 days later for analysis of LCMV-specific CD4+ and CD8+ T cells by intracellular cytokine staining and flow cytometry.
Thymic lobe transplantation
Thymic lobes from 1-day-old Lmna+/+ and Lmna-/- neonates were transplanted into 10- to 16-week-old female Lmna+/+ host mice by engraftment underneath the kidney capsule. Briefly, 2 days prior through 3 days after surgery, host mice were given medicated water (ibuprofen 0.2 mg/mL). Neonatal thymic lobes were placed on sterile nitrocellulose pads and suspended at the culture medium interface to ensure high oxygen transfer and cell survival. Thymuses were cultured at 37°C, 5% CO2 for less than 24 hours prior to transplantation while genotypes of extracted neonatal thymuses were being determined. The culture medium consisted of DMEM, 10% fetal calf serum, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 0.1 mM non-essential amino acids, 0.3% sodium bicarbonate, 25 mM HEPES, and 50 µM β-mercaptoethanol. Host mice were anesthetized with ketamine and xylazine (130 mg/kg and 8.8 mg/kg respectively) and a laparotomy was performed after hair removal and sterilization of the surgical area. Thymic lobes were transferred underneath the left kidney capsule. Host mice were then sutured and monitored until full recovery from anesthesia. Transplanted thymuses were allowed to engraft for 6 weeks prior to analysis.
Results
Runting of Lmna -/- mice is accompanied by progressive thymic and splenic atrophy
It has been previously reported that Lmna -/- have mice small lymphoid organs, including the spleen and thymus [7]. We analyzed lymphoid organs from Lmna -/- mice to better understand the effects of Lmna deficiency on the immune system. Neonatal Lmna -/- mice were indistinguishable from Lmna +/+ littermates 1 week after birth. By 4 weeks of age and older, Lmna -/- mice were severely runted compared to Lmna +/+ mice (Figure 1A). The thymus and spleen from 6-week old Lmna -/- mice were reduced in size (Figure 1B). We observed that while thymic cellularity in neonatal Lmna +/+ and Lmna -/- mice was similar, 4-week old Lmna -/- mice displayed decreased thymic cellularity compared to Lmna +/+ littermates that became more striking with age (Figure 1C). Similarly, splenic cellularity was unaffected in neonatal Lmna -/- mice, but was severely reduced at 4 and 9 weeks of age (Figure 1D). Thymic and splenic cellularity were reduced even when normalized to the reduced body weight of Lmna -/- mice (Figure S1).
Figure 1. Age-dependent progressive decrease in body weight and spleen and thymus size in Lmna -/- mice.
Body weight and splenic and thymic cellularity from Lmna+/+ and Lmna-/- mice were determined at 1, 4, and 9 weeks of age. A. Body weight of mice from indicated age groups. B. Representative thymus and spleen from 6-week-old mice. C. Absolute numbers of thymocytes from mice of the indicated age groups. D. Absolute numbers of splenocytes from mice of the indicated age groups. Bars show the mean value with error bars indicating the standard deviation (Lmna+/+ N = 3; Lmna-/- N = 3 for each indicated age group). P values were calculated using an unpaired two-tailed Student's t test (** p<0.001).
Defective thymocyte maturation in the atrophic Lmna -/- thymus
Given the striking decrease in thymic cellularity in Lmna -/- mice, we predicted a decrease in the proportion of the predominant CD4+CD8+ double-positive (DP) thymocyte population. Surprisingly, flow cytometric analysis revealed no perturbation in the frequency of DP thymocytes (Figure 2A and 2B). Slight reductions were noted in the percent of CD4 single-positive (SP) and CD8 SP thymocytes, starting at 4 weeks of age. There was also a significant increase in the proportion of double-negative (DN) thymocytes at 9 weeks of age in Lmna -/- mice, possibly indicating that cells are partially blocked in their developmental progress at one of the DN stages. To further explore this possibility, we analyzed DN thymocytes, and found that the percent of DN cells that were DN1 (least mature), DN2, DN3, and DN4 (most mature) remained unaltered in Lmna -/- mice, indicating that the relative increase in the percent of DN thymocytes at 9 weeks does not result from a developmental block at one of the DN stages (Figure S2).
Figure 2. Lmna -/- mice display an age-dependent defect in thymic T cell development.
Thymocytes were stained for CD4, CD8, CD69, and panTCRβ surface expression. A. Representative flow cytometry plots show CD4/CD8 analysis of live-gated thymocytes. B. Charts show the percent of thymocytes that are DN, DP, CD4 SP, and CD8 SP for each indicated age group compiled from all mice analyzed. C. CD69/TCRβ analysis of CD4lowCD8low gated DP dull cells from 9-week-old mice with a gate indicating the percent of post-selection TCRβhighCD69+ cells. D. The percent of CD4lowCD8low DP dull cells that are TCRβhighCD69+ for 4- and 9-week-old mice. E. Histograms of gated CD4 SP and CD8 SP cells showing TCRβ expression. Colored numbers (Blue: Lmna +/+; Red: Lmna -/-) in histogram indicate the percent of SP cells that are TCRβ+. F. CD4 SP and CD8 SP gated thymocytes were analyzed for the percent that are post-positive selection TCRβ+ mature SP thymocytes. Charts show the mean percent with error bars indicating the standard deviation (Lmna+/+ N = 3; Lmna-/- N = 3 for each indicated age group). P values were calculated using an unpaired two-tailed Student's t test (* p<0.05, ** p<0.001, *** p<0.0001).
After completion of TCRβ rearrangement in the thymus, cells proliferate at the DN3 to DN4 stage and upregulate CD4 and CD8 coreceptors to become CD4+CD8+ DP thymocytes. At the DP thymocyte stage, cells undergo TCRα rearrangement, and test the newly generated TCRα chain paired with the already available TCRβ chain for the ability to interact with self-MHC class I and class II molecules. Useful TCR interactions will result in positive selection of the cell, upregulation of TCR and CD69 surface expression, and lineage commitment of CD4 SP and CD8 SP thymocytes. Failure to be selected results in additional TCRα rearrangement, and eventually, if no positive selection takes place, the cell undergoes apoptosis (for review, see Starr et al.)[17]. We observed that the DPdull (CD4lowCD8low) thymocytes, a sub-population of DP thymocytes that are normally enriched for selected TCRβ+ cells, lacked positively-selected TCRβhighCD69+ cells in 9-week-old Lmna -/- mice (Figure 2C and 2D). Although there was not a significant decrease in the percent of CD4 SP and CD8 SP gated thymocytes from 9-week-old Lmna -/- mice, these cells displayed clear signs of altered development, including decreased cell surface TCRβ expression for both CD4 SP and CD8 SP thymocytes, and decreased frequency of post-positive selection TCRβ+ mature CD8 SP thymocytes (Figure 2E and 2F). Thus, the development of TCRβ+ mature SP thymocytes is defective in Lmna -/- mice, suggesting that positive selection is impaired.
Age-dependent progressive T and B cell lymphopenia in Lmna -/- mice
The apparent defect in positive selection in the thymus, compounded with the reduced thymic size in Lmna-/- mice, suggested the size of the peripheral T cell compartment in these mice would be diminished. To investigate this possibility, we analyzed splenic CD4+ and CD8+ T cells. While Lmna -/- mice exhibit an increased percent of splenic CD4+ and CD8+ T cells compared to Lmna +/+ mice (Figure 3A), the absolute number of CD4+ and CD8+ T cells is dramatically decreased in Lmna -/- mice at both 4 and 9 weeks of age (Figure 3B). These reduced T cell numbers are a reflection of the loss in absolute numbers of total splenocytes in the Lmna-/- mice at these ages (Figure 1D). Given this severe T cell lymphopenia, we predicted that the remaining T cells in Lmna-/- mice would be driven to an activated/memory CD62LlowCD44high phenotype through lymphopenia-induced homeostatic proliferation. Surprisingly, there was no change in the percent of CD4+ and CD8+ cells that bear an activated/memory phenotype in 4-week-old (not shown) and 9-week-old Lmna -/- spleens (Figure 3C). Although there were subtle increases in the proportion of T cells that are CD44high and either CD62low or CD62Lhigh in the lymph nodes, these remained dramatically lower than other mouse systems characterized by T cell lymphopenia [18], [19]. In 9-week-old Lmna -/- spleens, we also observed an alteration in the percent of B cells (Figure 3D). This alteration coincided with a severe reduction in B cell numbers in the spleen (Figure 3E). Taken together, these data indicate that the development and/or survival of both B and T lymphocytes is defective in Lmna -/- mice.
Figure 3. T cell and B cell numbers show an age-dependent decline in the lymphoid periphery of Lmna-/- mice.
Splenocytes were surface stained for CD4, CD8, CD19, CD62L, CD44, and panTCRβ. A. Representative flow cytometry plots show the percent of CD4+ and CD8+ T cells. B. Absolute numbers of splenic CD4+ and CD8+ T cells for each indicated age group. C. Gated CD4+ and CD8+ splenocytes were analyzed for CD62L and CD44 expression. D. Representative histograms showing the percent of CD19+ B cells in the spleen. E. Absolute numbers of CD19+ B cells per spleen compiled for each indicated age group. Numbers in plots and histograms indicate the percentage of each gated population. Charts show the mean cell number with error bars indicating the standard deviation (Lmna+/+ N = 3; Lmna-/- N = 3 for each indicated age group). P values were calculated using an unpaired two-tailed Student's t test (* p<0.05, ** p<0.001).
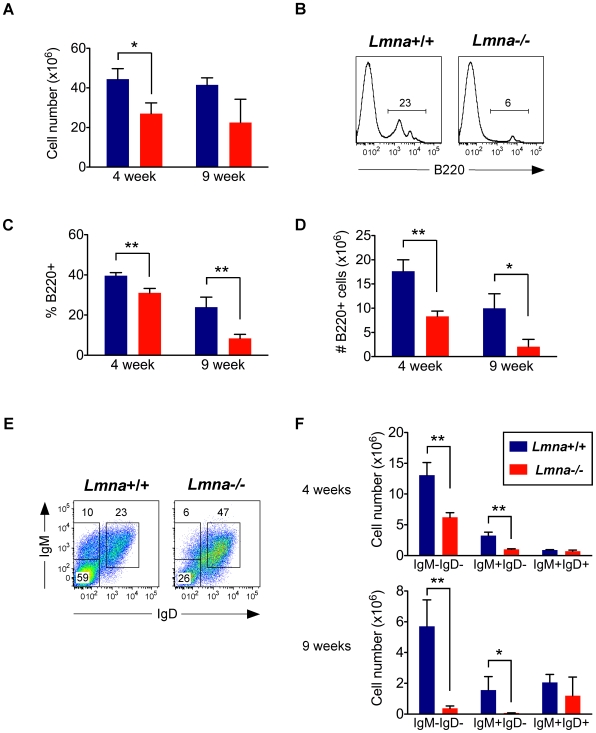
B cell development is interrupted in Lmna-/- mice
The cellularity of bone marrow from Lmna -/- mice was decreased in 4-week-old mice (Figure 4A). Because splenic B cell numbers were dramatically reduced in 4- and 9-week-old Lmna -/- mice compared to Lmna +/+ mice, we analyzed bone marrow to determine whether B cell development was altered. Indeed, the population of developing B220+ cells committed to the B cell lineage was severely reduced in Lmna-/- mice, and composed primarily of a B220high subpopulation (Figure 4B). This reduction occurred as early as 4 weeks of age in Lmna -/- mice (Figure 4C and 4D). Of the remaining B220+ cells in the bone marrow of Lmna -/- mice, there was a reduced percent and number of IgM-IgD- cells and IgM+IgD- immature B cells, and an increased percent but normal number of IgM+IgD+ mature B cells (Figure 4E and 4F). Because the developing B220low IgM-IgD- cells and IgM+IgD- immature B cells are so severely affected in Lmna -/- mice, it is likely that these B220high IgM+IgD+ mature B cells reached maturity before these mice experienced the precipitous decline in B cell development. Therefore, B cell development is impaired in Lmna -/- mice with age.
Figure 4. B cell development is impaired in Lmna -/- mice.
Bone marrow single-cell suspensions were stained for surface B220, IgM, and IgD expression. A. Absolute number of bone marrow cells. B. Representative histograms from 9-week-old mice showing the percent of bone marrow cells that are developing B220+ cells. C. The percent of bone marrow cells that are B220+ for each indicated age group. D. Absolute number of B220+ bone marrow cells for each indicated age group. E. Representative IgM/IgD plots of gated B220+ bone marrow cells from 9-week-old mice. F. Absolute number of B220+ bone marrow cells that are IgM-IgD-, IgM+IgD- (immature B cells), and IgM+IgD+ (mature B cells). Numbers in plots and histograms indicate the percentage of each gated population. Charts show the mean value with error bars indicating the standard deviation (Lmna+/+ N = 3; Lmna-/- N = 3 for each indicated age group). P values were calculated using an unpaired two-tailed Student's t test (* p<0.05, ** p<0.001).
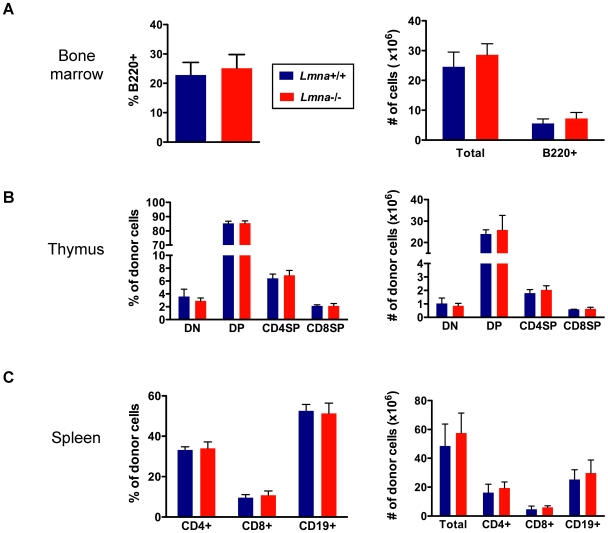
Lmna-/- B and T cell defects are not cell-autonomous
The alteration in lymphocyte development and homeostasis in Lmna -/- mice could result from cell-intrinsic defects caused by Lmna gene deficiency, from the contribution of A-type lamin function in non-hematopoietic cells that impact lymphocyte development, or instead, from the severe pathology and poor health of the Lmna -/- mouse. We generated bone marrow chimeras by reconstituting lethally irradiated Lmna +/+ mice with Lmna +/+ or Lmna -/- bone marrow to determine whether the defects in T and B cell development and homeostasis were cell-autonomous. Because thymocyte and splenocyte defects in Lmna -/- mice were age-dependent, we waited >40 weeks after irradiation and reconstitution to analyze the number of Lmna +/+ and Lmna -/- populations in bone marrow, thymus, and spleen of chimeras. Bone marrow chimeric mice reconstituted with either Lmna -/- or Lmna +/+ bone marrow had similar frequencies and numbers of total bone marrow cells and B220+ bone marrow cells (Figure 5A). Bone marrow chimeras that received either Lmna -/- or Lmna +/+ bone marrow had identical relative and absolute numbers of donor-derived DN, DP, and SP thymocyte populations (Figure 5B). Additionally, the relative and absolute number of donor-derived B and T cells in the spleen were the same in chimeric mice reconstituted with either Lmna -/- or Lmna +/+ bone marrow (Figure 5C).
Figure 5. Lmna -/- T and B cell defects are not cell-autonomous.
Bone marrow chimeras were generated by reconstituting irradiated wild-type Ly5.1+Ly5.2+ mice with bone marrow cells from either Lmna +/+ or Lmna -/- Ly5.2+Ly5.1- donor mice. Single-cell suspensions from bone marrow, thymus, and spleen were analyzed 44 weeks following irradiation and bone marrow reconstitution. Cells were stained for surface Ly5.1, Ly5.2, B220, CD4, CD8, TCRβ, and CD19 expression. Donor-derived Ly5.2+Ly5.1- cells were analyzed in thymus and spleen. Total bone marrow cells were analyzed because not all bone marrow cells express Ly5; however, >98% of Ly5+ cells were donor-derived Ly5.2+Ly5.1-, indicating efficient engraftment. A. The percentage of total bone marrow cells that are B220+ (left) and the absolute number of total bone marrow cells and B220+ bone marrow cells (right) in the indicated bone marrow chimeric mice. B. The percent (left) and absolute number (right) of the indicated donor cells that are DN, DP, CD4 SP, and CD8 SP thymocytes. C. The percent (left) and absolute number (right) of the indicated donor cells that are CD4+ T cells, CD8+ T cells, and CD19+ B cells. Charts show the mean percent or number with error bars indicating the standard deviation (Lmna +/+ into wild-type (WT) chimera N = 3; Lmna -/- into WT chimera N = 5). P values were calculated using an unpaired two-tailed Student's t test and none reached significance.
To assess the immunocompetence of Lmna -/- CD4+ and CD8+ T cells that appear to develop normally in Lmna +/+ bone marrow chimeric hosts, we generated Lmna +/+/Lmna -/- mixed bone marrow chimeras by reconstituting lethally-irradiated Lmna +/+ mice with a mix of Lmna +/+ and Lmna -/- bone marrow. Chimeras were infected 18 weeks post-reconstitution with the acute virus LCMV, known to generate a strong virus-specific response from both CD4+ and CD8+ T cells in B6 mice. Eight days later (at the peak of the T cell response), we analyzed Lmna +/+ and Lmna -/- CD4+ and CD8+ T cells for the presence of antigen-specific effector T cells. IFNγ+, IL-2+, and TNFα+ virus-specific effector cells were present at the same frequencies among both Lmna +/+ and Lmna -/- CD4+ T cells within the same mixed bone marrow chimera (Figure 6A and 6B). Similarly, virus-specific cytokine-producing effectors were present at the same frequencies among Lmna +/+ and Lmna -/- CD8+ T cells (Figure 6C and 6D). These data show that Lmna -/- CD4+ and CD8+ T cells that develop in an A-type-lamin-sufficient environment can recognize foreign antigen in the context of self MHC, and proliferate and produce cytokine following antigen encounter. Therefore, in a Lmna +/+ host, Lmna -/- T cells are appropriately selected and functional, even when in direct competition with Lmna +/+ T cells.
Figure 6. Lmna -/- T cells in mixed chimeric mice generate functional antigen-specific responses to viral infection.
Mixed bone marrow chimeras were generated by reconstituting irradiated Ly5.1+Ly5.2+ mice with a mix of bone marrow cells from Ly5.1+Ly5.2− Lmna +/+ and Ly5.2+Ly5.1- Lmna -/- donor mice. Chimeric mice were infected with LCMV. Eight days later, splenocytes were restimulated with the relevant LCMV peptides for 5 hours, stained for cell surface CD4, CD8, Ly5.1, and Ly5.2 expression, and then stained for intracellular IFNγ and IL-2 or TNFα expression. Flow cytometric analysis allowed for clear separation of Lmna +/+ (Ly5.1+Ly5.2-) and Lmna -/- (Ly5.1-Ly5.2+) donor T cells. A. Representative plots of intracellular IFNγ and IL-2 staining of Lmna +/+ and Lmna -/- CD4+ cells within the same mixed bone marrow chimeric mouse. Antigen-specific CD4+ T cells produce cytokine when incubated in the presence of GP61–80 peptide (bottom plots) but not in its absence (top plots). B. The percent LCMV GP61–80-specific cytokine producing cells of donor-derived CD4+ T cells. C. Representative plots of intracellular IFNγ and IL-2 staining of Lmna +/+ and Lmna -/- CD8+ cells within the same mixed bone marrow chimeric mouse. Antigen-specific CD8+ T cells produce cytokine when incubated in the presence of GP33–41 peptide (bottom plots) but not in its absence (top plots). D. The percent LCMV GP33–41-specific cytokine producing cells of donor-derived CD8+ T cells. Numbers in plots indicate the percent of each gated population. Charts show the mean percent with error bars indicating the standard deviation (Lmna +/+/Lmna -/- mixed bone marrow chimeras N = 5). P values were calculated using an unpaired two-tailed Student's t test and none reached significance.
Thus, the splenic and thymic cellularity defects and the apparent deficiencies in lymphocyte development observed in Lmna -/- mice are not cell-autonomous for T or B lymphocytes. This implies that A-type lamin function/expression is not required in these cell types for normal development and homeostasis. Further, it implies the possibility that A-type lamin expression in non-hematopoietic cells influences the function and development of these lymphocytes.
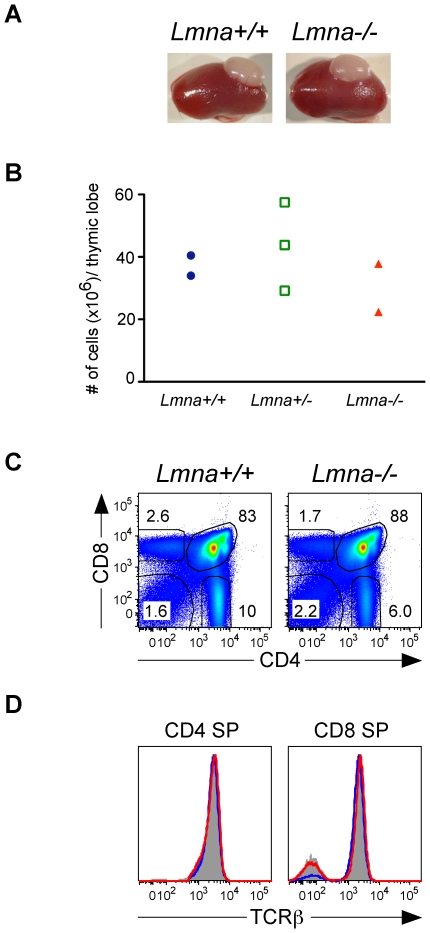
Lmna -/- thymus supports normal T cell development
We examined the possibility that defective T cell development in Lmna -/- mice might result from specific defects in thymic stromal cells, which are required for positive selection of developing DP thymocytes. To determine whether Lmna -/- thymic lobes could foster proper thymocyte development, we transplanted Lmna -/- neonatal thymic lobes into adult syngeneic recipients and analyzed thymic grafts 6 weeks later, after seeding of the lobes with host-derived Lmna+/+ stem cells. Grafted Lmna -/- and Lmna +/+ thymuses were similar in appearance and contained similar numbers of thymocytes (Figure 7A and 7B). Engraftment of both Lmna +/+ and Lmna -/- thymic lobes appeared normal, as both supported a normal distribution of DN, DP, and CD4 SP and CD8 SP developing thymocytes (Figure 7C). Unlike the CD4 SP and CD8 SP thymocytes from Lmna -/- mice (see Figure 2E), CD4 SP and CD8 SP thymocytes within Lmna -/- thymuses grafted into Lmna +/+ hosts had normal TCRβ expression and a similar percent of TCRβ+ mature SP thymocytes (Figure 7D). Therefore, Lmna -/- thymic stromal cells support normal T cell development when they are in a healthy, A-type-lamin-sufficient host.
Figure 7. Lmna -/- thymic lobes support normal T cell development in Lmna +/+ recipients.
Thymic lobes from one-day-old Lmna +/+, Lmna +/-, and Lmna -/- neonates were transplanted under the kidney capsule of syngenic wild-type recipient mice. Thymic grafts were analyzed 6 weeks later, allowing sufficient time for seeding by stem cells from the Lmna +/+ recipient, promoting subsequent thymocyte development within the grafted thymus. A. Kidneys of wild-type recipient mice with grafted Lmna +/+ and Lmna -/- thymic lobes. B. The absolute number of thymocytes within the grafted thymic lobes. C. Representative flow cytometry plots show CD4/CD8 analysis of live-gated thymocytes from grafted Lmna +/+ and Lmna -/- thymic lobes. D. Representative histogram overlays of gated CD4 SP and CD8 SP cells comparing TCRβ expression by Lmna +/+ thymocytes (gray filled histogram), thymocytes from the Lmna +/+ graft (blue histogram), and thymocytes from the Lmna -/- graft (red histogram). Data are compiled from two independent experiments.
Discussion
In this study, we sought to determine the nature of lymphoid organ atrophy and lymphocyte population deficiencies in Lmna -/- mice. Young adult Lmna -/- mice exhibit striking decreases in splenic T and B lymphocyte numbers that correlate with reduced populations of developing thymocytes and B220+ B cells in bone marrow. The apparent selective block in T cell positive selection and early B cell development in Lmna -/- mice hinted at specific developmental checkpoints at which A-type lamin function was required. However, global reduction in both developing and mature lymphocytes coincided with the severe runting, as well as cardiac and skeletal muscle disease onset that occurs in Lmna -/- mice [7], [20]. In addition, 1-week-old neonatal Lmna -/- mice are healthy and indistinguishable from wild-type littermates, with comparable thymocyte numbers and splenic T and B cell numbers. These latter observations suggest a more general, perhaps indirect, impact on lymphocyte development resulting from the rapidly degrading health of older Lmna -/- mice.
Bone marrow chimeras were generated to test the possibility that lamin A/C expression is required in developing lymphocytes for normal T and B cell maturation. We did not find any evidence for this, as Lmna -/- bone marrow completely reconstituted wild-type recipient mice with normal T and B cell development and peripheral lymphocyte populations for more than 40 weeks following bone marrow transfer. Lmna -/- CD4+ and CD8+ T cell populations in mixed bone marrow chimeras were able to generate self-MHC class II and class I restricted T cell responses to LCMV infection. These antigen-specific and differentiated CD4+IFNγ+ and CD8+IFNγ+ effector cells were generated at the same frequency and demonstrated the same cytokine-producing potential from Lmna -/- and Lmna +/+ cells within the same mixed bone marrow chimeras. These data show that even in differentiated T cell effector lineages, Lmna gene deficiency has no cell-autonomous consequence. Together, these data show that intrinsic lamin A/C expression is not required either for B cell development or for T cell development and function.
A second possible explanation for the lymphocyte developmental defects observed in Lmna -/- mice is that stromal elements within primary lymphoid organs require lamin A/C expression for their function in promoting normal T and B cell development. Thymic epithelial cells play a critical role in the positive selection of developing thymocytes as rearranged TCRα and TCRβ gene products are tested for useful and safe TCR interactions with self-MHC:peptide complexes. We tested whether there were thymic epithelial cell-specific defects in the Lmna -/- thymus by transplanting mutant thymic lobes into wild-type hosts, and allowing them to engraft for sufficient time to be seeded by wild-type hematopoietic stem cells. Within these mice, Lmna -/- thymic lobes maintained normal size and thymocyte cellularity compared to wild-type thymic grafts. Thus, Lmna -/- thymic stroma displayed no cell-intrinsic defects that can explain the reduced cellularity and altered thymocyte development observed in Lmna -/- mice.
However, a formal possibility still exists that defective stromal elements may influence B cell development in the Lmna-/- bone marrow. The stromal and hematopoietic microenvironments in the bone marrow are different than in the thymus and perhaps represent more complex interactions with diverse cell types. Of note, mesenchymal stem cells also inhabit the bone marrow and, in addition to their role in repopulating tissues of mesenchymal origin, have a regulatory role stimulating hematopoietic stem cell growth and differentiation (reviewed in Uccelli, et al.) [21], [22]. Because most tissues affected in laminopathy syndromes are of mesenchymal origin and because recent findings have suggested that altered A-type lamin function affects the maintenance of mesenchymal stem cell identity and ability to differentiate [23], defective communication between these cell types could also lead to altered lymphocyte development. Unfortunately, bone marrow architecture is difficult to preserve in transplantation studies, and future studies to address these possibilities will require conditional deletion of Lmna within select cell types of the bone marrow.
A third possible explanation for the lymphocyte developmental defects observed in Lmna -/- mice is an indirect effect of laminopathies at distal sites. Lmna -/- mice suffer from stunted growth and previously characterized cardiac and skeletal muscle defects contribute to their decline in health and premature death at around 6–8 weeks of age [7], [20], [24], [25]. Given that A-type lamins influence a variety of cell types, it is difficult to distinguish between cell-autonomous defects in specific tissues of Lmna deficiency and possible indirect effects rendered by so many dystrophic tissues. Thus, the atrophic lymphoid organs and accompanying reduced lymphocyte development and maintenance in the Lmna -/- mice may result from the overall poor health of the mice. These defects may be driven by altered stress hormone levels, cytokines from a chronic inflammatory response to striated muscle dysfunction that is present in Lmna-/- mice [7], [20], [26, Frock et al. manuscript in preparation], or decreased metabolic function resulting in nutritional deprivation of lymphocytes. Possibly supporting the argument for a role in nutritional defects, homeostasis of peripheral T cells appears to be impaired in Lmna -/- mice. Despite severe decreases in splenic T cell numbers, there was no upregulation of CD44 or downregulation of CD62L in spleen (Figure 3C), changes that characterize T cells that have undergone lymphopenia-induced homeostatic proliferation.
In summary, we have shown that Lmna -/- mice undergo a progressive decline in the size of their thymus and spleen, a decline of developing B220+ B cells in the bone marrow, a decline of total developing thymocytes, and striking decreases in peripheral B and T cells. All of these changes occurred after 1 week of age, and coincided with runting of Lmna -/- mice and onset of multi-tissue dystrophies. These immune defects were completely rescued when lymphocyte development was uncoupled from Lmna -/- animals through bone marrow chimera and thymic grafting experiments. We conclude that these effects on lymphocyte development in Lmna -/- mice are indirect, and most likely result from an unhealthy animal that is unable to support normal immune system development and homeostasis. Our data indicate that even apparently selective developmental blocks can arise from lymphocyte-extrinsic, indirect effects and underscore the need to analyze lymphocyte development in animals that provide a healthy environment before concluding that a particular gene or cell type of interest impacts development of a functional immune system.
Supporting Information
Progressive cellularity defects in spleen and thymus occur even when organ cellularity is normalized to body weight of Lmna-/- mice. A. Thymic and B. splenic cellularity was normalized to the body weight of each individual mouse of each of the indicated ages. Charts show the mean normalized value with error bars indicating the standard deviation (Lmna+/+ N = 3; Lmna-/- N = 3 for each indicated age group). P values were calculated using an unpaired two-tailed Student's t test (* p<0.05, ** p<0.001).
(0.25 MB TIF)
Normal composition of double-negative DN1-DN4 thymocytes in 9-week old Lmna-/- mice. Thymocytes from 9-week old mice were stained for CD4, CD8, CD25, and CD44 surface expression. DN thymocytes were analyzed for the percent that are DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−). Chart shows the mean percent with error bars indicating the standard deviation (Lmna+/+ N = 3, Lmna-/- N = 3). P values were calculated using an unpaired two-tailed Student's t test and none reached significance.
(0.10 MB TIF)
Acknowledgments
The authors would like to thank Andrew G. Farr and Stephen D. Hauschka for helpful advice on experimental procedures, and Carmen Lau and Christina Brown for mouse genotyping. We would also like to thank Warren Ladiges for support with propagation of the Lmna-/- mouse colony.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institute on Aging (NIA) grant R01 AG024287 to B.K.K. and National Institutes of Health (NIH) grant R01 AG13078 to P.J.F. R.L.F. is supported by the Cardiovascular and Pathology Training Grant (5T32HL007312-32). J.S.H. is supported by the National Cancer Institute Basic and Cancer Immunology Grant (T32CA09537). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kennedy BK, Barbie DA, Classon M, Dyson N, Harlow E. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 2000;14:2855–2868. doi: 10.1101/gad.842600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DC, Welton KL, Smith ED, Kennedy BK. A-type nuclear lamins act as transcriptional repressors when targeted to promoters. Exp Cell Res. 2009;315:996–1007. doi: 10.1016/j.yexcr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozaki T, Saijo M, Murakami K, Enomoto H, Taya Y, et al. Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene. 1994;9:2649–2653. [PubMed] [Google Scholar]
- 4.Taniura H, Glass C, Gerace L. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol. 1995;131:33–44. doi: 10.1083/jcb.131.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen TV, Hernandez L, Stewart CL. Functions of the nuclear envelope and lamina in development and disease. Biochem Soc Trans. 2008;36:1329–1334. doi: 10.1042/BST0361329. [DOI] [PubMed] [Google Scholar]
- 6.Alsheimer M, Liebe B, Sewell L, Stewart CL, Scherthan H, et al. Disruption of spermatogenesis in mice lacking A-type lamins. J Cell Sci. 2004;117:1173–1178. doi: 10.1242/jcs.00975. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rober RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 9.Bladon T, Brasch K, Brown DL, Setterfield G. Changes in structure and protein composition of bovine lymphocyte nuclear matrix during concanavalin-A-induced mitogenesis. Biochem Cell Biol. 1988;66:40–53. doi: 10.1139/o88-006. [DOI] [PubMed] [Google Scholar]
- 10.Guilly MN, Kolb JP, Gosti F, Godeau F, Courvalin JC. Lamins A and C are not expressed at early stages of human lymphocyte differentiation. Exp Cell Res. 1990;189:145–147. doi: 10.1016/0014-4827(90)90267-e. [DOI] [PubMed] [Google Scholar]
- 11.Stadelmann B, Khandjian E, Hirt A, Luthy A, Weil R, et al. Repression of nuclear lamin A and C gene expression in human acute lymphoblastic leukemia and non-Hodgkin's lymphoma cells. Leuk Res. 1990;14:815–821. doi: 10.1016/0145-2126(90)90076-l. [DOI] [PubMed] [Google Scholar]
- 12.Guilly MN, Bensussan A, Bourge JF, Bornens M, Courvalin JC. A human T lymphoblastic cell line lacks lamins A and C. Embo J. 1987;6:3795–3799. doi: 10.1002/j.1460-2075.1987.tb02715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rober RA, Sauter H, Weber K, Osborn M. Cells of the cellular immune and hemopoietic system of the mouse lack lamins A/C: distinction versus other somatic cells. J Cell Sci. 1990;95 (Pt4):587–598. doi: 10.1242/jcs.95.4.587. [DOI] [PubMed] [Google Scholar]
- 14.Broers JL, Machiels BM, Kuijpers HJ, Smedts F, van den Kieboom R, et al. A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol. 1997;107:505–517. doi: 10.1007/s004180050138. [DOI] [PubMed] [Google Scholar]
- 15.Jansen MP, Machiels BM, Hopman AH, Broers JL, Bot FJ, et al. Comparison of A and B-type lamin expression in reactive lymph nodes and nodular sclerosing Hodgkin's disease. Histopathology. 1997;31:304–312. doi: 10.1046/j.1365-2559.1997.2820881.x. [DOI] [PubMed] [Google Scholar]
- 16.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 17.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 18.Voehringer D, Liang HE, Locksley RM. Homeostasis and effector function of lymphopenia-induced "memory-like" T cells in constitutively T cell-depleted mice. J Immunol. 2008;180:4742–4753. doi: 10.4049/jimmunol.180.7.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendricks DW, Fink PJ. Uneven colonization of the lymphoid periphery by T cells that undergo early TCRα rearrangements. J Immunol. 2009;182:4267–4274. doi: 10.4049/jimmunol.0804180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 22.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 23.Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, et al. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melcon G, Kozlov S, Cutler DA, Sullivan T, Hernandez L, et al. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet. 2006;15:637–651. doi: 10.1093/hmg/ddi479. [DOI] [PubMed] [Google Scholar]
- 26.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Progressive cellularity defects in spleen and thymus occur even when organ cellularity is normalized to body weight of Lmna-/- mice. A. Thymic and B. splenic cellularity was normalized to the body weight of each individual mouse of each of the indicated ages. Charts show the mean normalized value with error bars indicating the standard deviation (Lmna+/+ N = 3; Lmna-/- N = 3 for each indicated age group). P values were calculated using an unpaired two-tailed Student's t test (* p<0.05, ** p<0.001).
(0.25 MB TIF)
Normal composition of double-negative DN1-DN4 thymocytes in 9-week old Lmna-/- mice. Thymocytes from 9-week old mice were stained for CD4, CD8, CD25, and CD44 surface expression. DN thymocytes were analyzed for the percent that are DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−). Chart shows the mean percent with error bars indicating the standard deviation (Lmna+/+ N = 3, Lmna-/- N = 3). P values were calculated using an unpaired two-tailed Student's t test and none reached significance.
(0.10 MB TIF)