Abstract
Background/Aims
We determined the effects of age and sex on the blood pressure (BP) response to angiotensin II (Ang II) infusion and evaluated the potential mechanistic role of the thiazide-sensitive NaCl cotransporter (NCC) and the epithelial sodium channel (ENaC).
Methods
Male and female mice (∼3 or 21 months of age) were infused with Ang II or control for 7 days.
Results
Males had a greater BP response to Ang II, somewhat enhanced by aging. Mean systolic BPs (at 7 days) were (mm Hg): 161, 143, 172, and 157 in young male, young female, old male, and old female mice, respectively. Immunoblotting changes in the whole kidney that supported this BP profile included a 51 and 52% increase in NCC band density in the old females and old males (as compared to sex-respective controls) with Ang II infusion, while the young males and young females showed an increase of 40 and 0%, respectively. Young males also had a greater reduction in major bands of β- and γ-ENaC, than did young female mice. The natriuretic response to hydrochlorothiazide supported an increase in activity of NCC with Ang II in aged mice only.
Conclusions
Increased sensitivity to Ang II in aging and male mice may involve overactivity of NCC.
Key Words: Hypertension, Gender differences, Epithelial sodium channel, NaCl cotransporter
Introduction
On a day-to-day basis, sodium homeostasis is finely tuned by regulated sodium reabsorption in the post-macula densa portion of the renal tubule, including the distal convoluted tubule (DCT), connecting tubule, and the collecting duct [1,2,3]. Angiotensin II (Ang II) is a major hormonal regulator of this portion of the renal tubule, primarily via its activity at Ang II (type I) AT1 receptors [4]. Furthermore, as compared to their male counterparts, female rodents have been shown to respond to Ang II infusions in a differential fashion, with reduced increases in blood pressure (BP), for example [5]. The mechanisms underlying these differential responses are incompletely understood. We have previously reported increased protein levels of the thiazide-sensitive NaCl cotransporter (NCC) of the DCT and all 3 of the subunits of the amiloride-sensitive epithelial sodium channel (ENaC) of the late DCT, connecting tubule, and collecting duct, in female, as opposed to male, Zucker rats fed a high-NaCl diet [6]. A subcutaneous Ang II infusion into young male rats at a non-pressor dose (24.4 ng/min) has been shown to increase renal abundance of the α-subunit of ENaC, with either a decrease or no change in β- or γ-ENaC [2]. These changes have been thought to be activating changes as they are also observed in low-NaCl-fed or aldosterone-infused rodents [7,8]. However, how sex influences the response to Ang II is not known, nor whether aging impacts on the response. Furthermore, the regulation of NCC by Ang II infusion has not been described in rats or mice.
In the current set of studies, we determined the impact of sex and age on the regulation of NCC and ENaC subunits during Ang II infusion in mice. Male and female mice of 2 distinct ages, i.e., approximately 3 or 21 months of age, were infused with a pressor dose of Ang II (800 ng/kg·body weight/min) for 7 days. Semiquantitative Western blotting was used to evaluate differences in band densities for the 3 subunits of ENaC and NCC. The relative activity of NCC and ENaC was estimated using selected antagonists, i.e., hydrochlorothiazide (HCTZ) and benzamil (BNZ), respectively. BP was measured by radiotelemetry.
Methods
Animals and Study Design
Three studies were performed in which mice of a mixed genetic background (C57Bl/6 × CBA × 129) were obtained from our own breeding colonies (Georgetown University). In study 1, male and female mice (3 months of age) were anesthetized with isoflurane and either implanted with osmotic minipumps (Alzet®, model 2002; Durect Corp., Cupertino, Calif., USA) to infuse Ang II (Bachem, Torrance, Calif., USA) at 800 ng/kg·body weight/min or received sham surgeries (n = 6/sex/treatment). Mice were singly housed with a normal 12-hour light/dark cycle for 7 days in plastic cages with microfilter tops according to protocols approved by the Georgetown Animal Care and Use Committee, an American Association for Accreditation of Laboratory Animal Care-approved facility. Study 2 was identical to study 1, except that mice were 18–24 (average 21) months of age (n = 6/sex/treatment). In study 3, a third set of young and old, male and female mice (n = 4–8/group) were implanted with radiotelemetry devices to measure BP (as described below). After 5–7 days, baseline natriuretic tests (see Estimation of ENaC and NCC activity, below) were conducted and baseline BP recorded. Next, all mice were implanted with Ang II-infusing osmotic minipumps, as described above. BP (diastolic and systolic) was recorded again after 7 days of infusion. On the 8th and 10th days of infusion, the natriuretic tests were repeated. Pelleted chow (Rodent Diet 5001, LabDiet®; Purina Mills, St. Louis, Mo., USA) and water were offered ad libitum. Mice in all 3 studies were euthanized by cardiac puncture and exsanguination under sodium pentobarbital anesthesia. Both kidneys were rapidly removed.
Radiotelemetry Blood Pressure Determinations
BP was measured in study 3 using radiotransmitters (Data Sciences Inc.). Mice were anesthetized with pentobarbital, and a ventral incision was made near the sternum. The pressure-sensitive tip of the catheter attached to the transmitter was inserted and secured in the carotid artery, while the transmitter body was subcutaneously placed in a pocket. Mice were housed singly and the cages were placed on top of radioreceivers. After a recovery time of 7 days, BP was recorded every 3–10 min using Data Sciences Acquisition Software (Data Sciences Inc.) as described previously [9,10].
Estimation of NCC and ENaC Activity
In study 3, in order to estimate NCC and ENaC relative activity in young and old mice, we administered single intraperitoneal injections of 2 antagonists of these proteins, i.e., HCTZ (7.5 mg/kg·body weight) or BNZ (1.4 mg/kg·body weight) under normal (control) conditions and after 8 (BNZ) and 10 (HCTZ) days of Ang II infusion. Urine was collected from 0–4 h following the injections. Sodium was measured in urine by an Easylyte Na/K analyzer (Medica Corp., Bedford, Mass., USA).
Immunoblotting
The left kidneys from studies 1 and 2 were homogenized whole (saw-tooth generator, PowerGen 700; Fisher Scientific, Waltham, Mass., USA) and prepared for immunoblotting as previously described [11]. Initially, Coomassie-stained ‘loading gels’ were done in order to assess the quality of the protein by the sharpness of the bands and to confirm or correct protein determinations, as previously described [11,12]. For immunoblotting, 20–30 μg of protein from each sample was loaded into individual lanes of minigels of 7 or 10% polyacrylamide (precast; BioRad, Hercules, Calif., USA). Blots were probed with our own polyclonal antibodies against the thiazide-sensitive NCC and the 3 subunits of ENaC (α, β, and γ). These antibodies were designed using sequences already published [7,13,14,15] and previously characterized [16]. β-Actin reprobe confirmed loading equality.
Statistics
In each study, data were analyzed by both 2- and 1-way analysis of variance (ANOVA) using Sigma Stat® software (Chicago, Ill., USA). For 2-way ANOVA, the main effects of sex and treatment, as well as their interactions, were determined. One-way ANOVA followed by the Holm-Sidak multiple comparisons test was used to determine differences between single groups when more than 2 groups were represented. To determine the effect of aging (within each sex) on the response to Ang II, data were first normalized to their sex- and age-respective controls. The deltas for each sex in response to Ang II were calculated for young and old mice separately. These deltas were compared by unpaired t test. p < 0.05 was considered significant for all analyses.
Results
Blood Pressure and Natriuretic Tests to Assess Relative NCC and ENaC Activity
Ang II infusion did not affect body weight, kidney weight, or food intake (data not shown). However, there were some differences in response to the Ang II with regard to BP and natriuretic responses to BNZ and HCTZ (table 1). Baseline diastolic BP was not different due to age or sex; however, baseline systolic BP was significantly higher in the old mice. With Ang II infusion, female mice had a blunted rise in both systolic and diastolic BP. Age, on the other hand, resulted in a strong trend (p = 0.06) for increased Ang II BP rise. Ang II led to a mean rise in diastolic BP of 33 ± 6, 21 ± 4, 45 ± 4, and 31 ± 4 mm Hg for young males, young females, old males, and old females, respectively (p = 0.028 for sex and 0.06 for age).
Table 1.
BP and natriuretic tests in young and old mice (mean ± SEM)
| Group | BP (baseline) | BP (with Ang II1) | BNZ test (baseline) UNa (0–4 h) mmol/kg·bw | BNZ test (with Ang II2) UNa (0–4 h) mmol/kg·bw | HCTZ test (baseline) UNa (0–4 h) mmol/kg·bw | HCTZ test (with Ang II3), UNa (0–4 h) mmol/kg·bw | ||
|---|---|---|---|---|---|---|---|---|
| diastolic mm Hg | systolic mm Hg | diastolic mm Hg | systolic mm Hg | |||||
| YM | 99 ± 4 | 121 ± 3 | 132 ± 7 | 161 ± 7 | 2.40 ± 0.40 | 3.31 ± 1.1 | 3.62 ± 0.49 | 3.18 ± 0.47 |
| YF | 97 ± 3 | 120 ± 3 | 118 ± 7 | 143 ± 9 | 1.89 ± 0.35 | 1.61 ± 0.28 | 2.52 ± 0.20 | 2.45 ± 0.59 |
| OM | 97 ± 2 | 129 ± 6 | 141 ± 4 | 172 ± 6 | 1.05 ± 0.37 | 1.19 ± 0.45 | 1.58 ± 0.48 ∗ | 2.44 ± 0.38 |
| OF | 90 ± 2 | 130 ± 3 | 122 ± 4 | 157 ± 3 | 1.35 ± 0.61 | 1.23 ± 0.33 | 1.68 ± 0.26∗ | 3.32 ± 0.82 |
| Results of 2-way ANOVA (sex × age) | ||||||||
| Sex | 0.24 | 1.00 | 0.013 | 0.038 | 0.804 | 0.183 | 0.205 | 0.894 |
| Age | 0.28 | 0.014 | 0.27 | 0.11 | 0.045 | 0.040 | 0.001 | 0.910 |
| Interaction | 0.55 | 0.74 | 0.66 | 0.86 | 0.371 | 0.170 | 0.131 | 0.105 |
BNZ = Benzamil; HCTZ = hydrochlorothiazide; UNa = urinary sodium; bw = body weight; YM = young males; YF = young females; OM = old males; OF = old females. For natriuretic tests, n = 6; for BP, n = 8, 6, 4, and 6 for YM, YF, OM, and OF, respectively.
Significantly different (p < 0.05) by one-way ANOVA from YM.
24-Hour mean by radiotelemetry days 6–7 of Ang II infusion.
After 8 days of Ang II infusion.
After 10 days of Ang II infusion.
As an estimate of in vivo activity of ENaC and NCC, respectively, we measured the natriuretic response to BNZ and HCTZ. In the untreated (baseline) state, natriuretic responses to BNZ and HCTZ were lower in old mice (table 1) and not significantly different due to sex (although in all 4 tests the trend in young males was higher than in young females). Ang II infusion did not affect natriuretic response to BNZ in any group of mice, but increased the HCTZ response selectively in the old mice, so that it was no longer significantly lower than in young mice. In old female mice, Ang II infusion increased natriuretic response to HCTZ by 98%.
NCC Response to Angiotensin II Infusion in Young Mice
A representative immunoblot of NCC in the whole kidney homogenates from the young mice is shown in figure 1A. The densitometry summary is shown in figure 1B. Two-way ANOVA statistics are shown below the bar graph. There was a significant effect of sex on NCC abundance (p = 0.021). In the untreated control mice, NCC was significantly higher in females; however, Ang II increased band density in the male mice so that there was no longer a sex difference. Ang II infusion did not result in a significant increase in NCC in the female mice.
Fig. 1.
NCC in young mice. A Representative immunoblot of whole kidney homogenates from young male (M) and female (F) control (C) and Ang II-infused (A) mice probed with NCC antibody. Equal amounts of total protein were loaded in each lane, confirmed by β-actin (not shown), and each lane is loaded with a sample from a different mouse. B Densitometry summary (n = 6 mice/group). Results of 2-way ANOVA (sex × treatment) are given below the graph. * Significantly different (p < 0.05) from MC (1-way ANOVA).
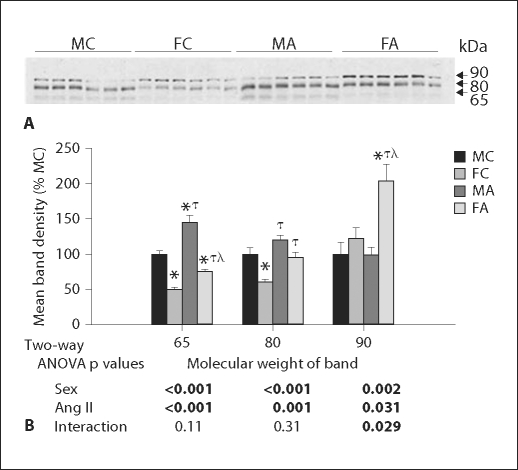
α-ENaC Response
Ang II infusion significantly increased the density of all 3 bands associated with α-ENaC (fig. 2); however, the increase in the 90-kDa band was only apparent in the female mice (thus, the significant interactive term). Moreover, young male and female mice had some fairly robust differences in banding patterns in response to Ang II. In general, male mice had increased expression of the 65- and 85-kDa bands, while females had an increase in the 90-kDa band (by 2-way ANOVA), relative to male mice.
Fig. 2.
α-ENaC in young mice. A Representative immunoblot of whole kidney homogenates from young male (M) and female (F) control (C) and Ang II-infused (A) mice probed with α-ENaC antibody. Equal amounts of total protein were loaded in each lane, confirmed by β-actin (not shown), and each lane is loaded with a sample from a different mouse. B Densitometry summary (n = 6 mice/group). Significantly different (p < 0.05) from * MC, τ FC, and λ MA (1-way ANOVA).
β-ENaC Response
A representative immunoblot for the single 90-kDa band associated with β-ENaC in young mice is shown in figure 3A. β-ENaC band density was substantially decreased by Ang II infusion (fig. 3). The decrease was greater in male mice, resulting in a significant interactive term in the 2-way ANOVA.
Fig. 3.
β-ENaC in young mice. A Representative immunoblot of whole kidney homogenates from young male (M) and female (F) control (C) and Ang II-infused (A) mice probed with β-ENaC antibody. Equal amounts of total protein were loaded in each lane, confirmed by β-actin (not shown), and each lane is loaded with a sample from a different mouse. B Densitometry summary (n = 6 mice/group). Significantly different (p < 0.05) from * MC and τ FC (1-way ANOVA).
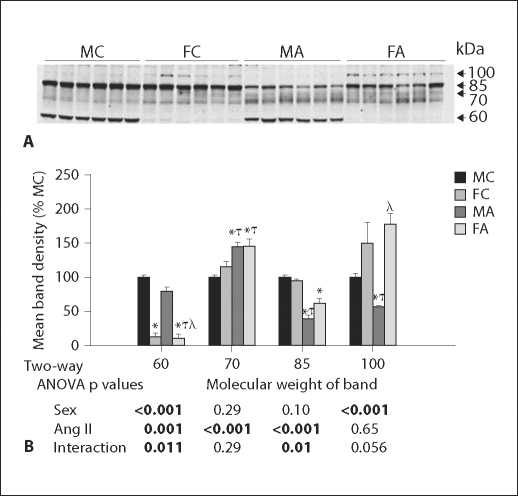
γ-ENaC Response
A representative immunoblot for γ-ENaC in young mice, and band densities for the 4 bands is shown in figure 4. Similar to what others have observed with feeding of a low-NaCl diet or aldosterone infusion [7,8], we found, with Ang II infusion, an increase in the 70- and a decrease in the 85-kDa bands of γ-ENaC. These ‘conventional’ bands are hypothesized to be due to an activating cleavage of the 85-kDa band [7] into the 70-kDa form [7,8]. The 70-kDa band was regulated similarly in males and females by Ang II; however, the decrease in the 85-kDa band with Ang II was greater in males. With regard to the novel 60- and 100-kDa bands, there were large sex differences. Females had less than 20% band density of the 60-kDa band, as compared to the males. This band was reduced modestly, but significantly by Ang II and to a greater extent in males. In contrast, females had greater band density for the 100-kDa band (approximately 150–200% of males); this band was not influenced significantly by Ang II.
Fig. 4.
γ-ENaC in young mice. A Representative immunoblot of whole kidney homogenates from young male (M) and female (F) control (C) and Ang II-infused (A) mice probed with γ-ENaC antibody. Equal amounts of total protein were loaded in each lane, confirmed by β-actin (not shown), and each lane is loaded with a sample from a different mouse. B Densitometry summary (n = 6 mice/group). Significantly different (p < 0.05) from * MC, τ FC, and λ MA (1-way ANOVA).
Angiotensin II Infusion in Old Mice
In figure 5A, B, we show 3 representative lanes per group from immunoblots for NCC, α-, β-, and γ-ENaC in old mice (n = 6/group for statistics). In these older mice, NCC was increased by Ang II to a similar extent in males and females (fig. 5C), with no sex differences in the response. However, unlike in young mice, α-ENaC band densities were not affected by Ang II (fig. 5E). The 65-kDa band for α-ENaC did remain statistically higher in males, relative to females. Furthermore, in contrast to young mice, females had significantly lower expression of the 90-kDa band (as compared to males). Similar to young mice, β-ENaC band density was substantially reduced by Ang II infusion (fig. 5D). However, there was also significantly greater band density for β-ENaC in old females, relative to males (this was not observed in the young). With regard to γ-ENaC (fig. 5F), similar to young mice, Ang II increased the 70-kDa band and decreased the 60- and 85-kDa bands. Also in agreement with what was observed in the young mice, in the old mice there was a significant interaction for the 60-kDa band in that it was reduced to a greater extent by Ang II in males. The 100-kDa band for γ-ENaC was not apparent in the older mice.
Fig. 5.
Ang II effects in old mice. Representative immunoblots for NCC and α-ENaC (A), and β- and γ-ENaC (B) in whole kidney homogenates from old (O) ∼21-month-old male (M) and female (F) control (C) or Ang II-infused (A) mice. Equal amounts of total protein were loaded in each lane, and each lane represents a different mouse (shown are 3 mice per group). C–F Densitometry summaries of NCC, α-, β- and γ-ENaC, respectively (n = 6 mice/group), as indicated. Significantly different (p < 0.05) from * OMC and τ OFC (1-way ANOVA).
Age Differences in the Magnitude of Response to Angiotensin II
Because the 2 studies were done at separate times, we did not feel that it was valid to directly compare band densities of young to old mouse samples. However, we did evaluate the modifying effect of age on the response to Ang II by analyzing and plotting each sex's Ang II infusion data as a percent of ‘age-respective controls’ (fig. 6A, B). In male mice (fig. 6A), there were 2 significant effects of old age on the magnitude of response to Ang II. In the older mice, there was a decrease (rather than increase) in band density for the 65-kDa band of α-ENaC. Second, there was a greater decrease in the 60-kDa band of γ-ENaC. In female mice (fig. 6B), there were 5 significant effects of old age on the magnitude of response to Ang II. Old female mice had a greater rise in band density for NCC with Ang II, as well as a greater fall in band densities for γ-ENaC 60- and 85-kDa bands. They also had falls in the abundance of the 65- and 80-kDa bands for α-ENaC with Ang II, as opposed to a rise, which was observed in the young mice.
Fig. 6.
Comparison of young to old mice with regard to Ang II effects. Normalized band densities from young (Y) and old (O), male (M) and female (F) Ang II-infused (A) mice were divided by the appropriate means of their age- and sex-respective controls. The effects of age on Ang II response are shown in males (A) and females (B). * Significantly different (p < 0.05) from Y (black bars) by unpaired t test.
Discussion
We found increased BP susceptibility to Ang II infusion in male mice relative to female mice and this sex difference persisted in the aged mice. Aging itself, led to significantly higher basal systolic BP and somewhat greater sensitivity of the diastolic BP response to Ang II. We assessed differential NCC and ENaC regulation as potential mechanistic determinants of these sex and age differences. In this regard, we found a variety of fairly marked differences in banding patterns for NCC and ENaC subunits between the sexes with and without Ang II infusion. Many of these changes were modified by aging, and we saw a greater number of age-related modifications in female as compared to male mice. Aged female mice would be expected to have greater changes in circulating sex steroids between the ages of 3 and 21 months. In females estradiol levels fall by around 1 year in most mouse strains, while testosterone levels remain high [17,18,19].
In many respects, changes in NCC regulation most closely matched the BP changes. NCC was increased by Ang II infusion in young male mice, but not in young female mice. This sex difference was abolished with aging, where Ang II increased the abundance of NCC in both old male and female mice. In general, the overall increases were greater in old mice for NCC and this matched our assessment of NCC activity, as determined by HCTZ sensitivity. Nonetheless, NCC levels were higher in young control female relative to young control male mice, which was in agreement with our observation in young rats [6]. Estradiol may be responsible for the higher levels of NCC in young female mice. In support of this, Verlander et al. [20] showed increased apical localization of NCC in the DCT with estrogen replacement to ovariectomized female rats, suggesting regulation of this protein, at least at a trafficking level, by estradiol.
A blunted rise in band density for NCC in the young female mice with Ang II infusion suggests a reduced sensitivity to Ang II. Glomerular Ang II (AT1) receptor binding has been reported to be reduced in female rats as compared to males [21]. However, a similar finding has not been reported for the distal tubule. In fact, Oestreicher et al. [22] demonstrated that estradiol-replaced ovariectomized female rats had a greater increase in renal AT1a receptor protein and mRNA when infused with Ang II plus N(ω)-nitro-L-arginine methyl ester, an nitric oxide synthase inhibitor, than did intact female controls or ovariectomized females which were not replaced. This suggested, at least when nitric oxide production was inhibited, that estradiol increased AT1a-binding capacity. Nevertheless, we did not detect any sex differences in the natriuretic response to HCTZ in young or old mice, in either the basal state or after Ang II infusion.
With regard to ENaC, in agreement with several other studies [7,11,12,23], we showed that the 3 subunits were not regulated in sync. Nonetheless, the response we found with Ang II infusion agreed, in general, with what has been previously reported in the literature for other models with predicted increased activity of the whole channel, i.e., increased α-ENaC and 70-kDa γ-ENaC and decreased β-ENaC and 85-kDa γ-ENaC [7,12,15]. This ‘pattern’ has been observed with aldosterone infusion [7,15], low-NaCl diet [8], and vasopressin analog infusion coupled to high-water intake (vasopressin escape) [12]. Furthermore, rats fed a low-NaCl diet with the AT1 receptor blocked by candesartan had the reverse pattern, i.e., decreased α-ENaC and increased β- and γ-ENaC (85 kDa) [2].
In addition, we have described several novel bands for both α- and γ-ENaC which were clearly differentially regulated between the sexes. Young male mice had greater evidence of bands at 65- and 60-kDa, for α- and γ-ENaC, respectively, than did their female counterparts. In fact in female mice, the 60-kDa band of γ-ENaC was barely perceptible, whereas in male mice it was a dominant band. On the other hand, young female mice had greater evidence of 2 higher MW bands at 90- and 100-kDa for α- and γ-ENaC, respectively. The presence of a variety of different MW bands for α-ENaC has been previously observed in rodent kidneys using a similarly designed antibody against a short sequence in the NH2 end of the molecule [24]. Ergonul et al. [24] reported the presence of an ∼60-kDa band (along with other bands around 30- and 90-kDa) for α-ENaC in male rat whole kidney samples. However, they did not report any novel bands for γ-ENaC (in addition to the 70- and 85-kDa bands which have been previously described [7]). In agreement with our studies, they found β-ENaC ran as a single band [24]. Furthermore, Ergonal et al. [24] suggest that higher MW bands for the ENaC subunits may represent immature, uncleaved forms of the protein. On the other hand, lower bands may be active cleaved forms of the protein [24,25]. This might suggest more ‘active’ ENaC in the male mice, while female mice have more immature forms. Nonetheless, other possibilities exist which might affect how the protein runs on a gel including alterations in glycosylation [24] and phosphorylation [26]. Nevertheless, we believe that it is likely that these unique banding patterns are due to differential activation by sex steroids or the sex chromosomal complement. Nonetheless, additional studies are clearly warranted to confirm specificity and define the chemical nature and effect on transport of these bands. Furthermore, we found that age resulted in a significantly lower response to BNZ, which would suggest reduced activity of the channel with aging. Sex and Ang II infusion did not affect BNZ responsiveness; however, there was a strong trend for young males to have a greater BNZ-natriuretic response as compared to young females after Ang II infusion (105% greater response). The natriuretic tests, in general, had high variability associated with them, and further examination using these tests with larger numbers of animals is clearly desirable.
In summary, differential expression profiles for ENaC and NCC may be an important determinant of sex differences in BP and extracellular fluid volume. Alteration in this pattern with aging may provide one possible or facilitatory mechanism for the loss of the female ‘protective advantage’ with age with regard to BP and cardiovascular disease rates.
Acknowledgements
We would like to thank Xinqun Hu, MS, Arjun Rash, MS, Raelene Listhrop, MS, and Nikhil Sharma, MS, for technical assistance with animal studies and Western blotting.
This work was supported by NHLBI grants HL074142 and HL073193 (to C.M.E.) and the American Heart Association Established Investigator Award (to C.M.E.).
References
- 1.Barbry P, Hofman P. Molecular biology of Na+ absorption. Am J Physiol. 1997;273:G571–G585. doi: 10.1152/ajpgi.1997.273.3.G571. [DOI] [PubMed] [Google Scholar]
- 2.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 3.Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol. 1998;274:C1373–C1379. doi: 10.1152/ajpcell.1998.274.5.C1373. [DOI] [PubMed] [Google Scholar]
- 4.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 5.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 6.Riazi S, Madala Halagappa VK, Hu X, Ecelbarger CA. Sex and body-type interactions in the regulation of renal sodium transporter abundance, urinary excretion, and activity in lean and obese Zucker rats. Gend Med. 2006;3:309–327. doi: 10.1016/s1550-8579(06)80219-6. [DOI] [PubMed] [Google Scholar]
- 7.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masilamani S, Wang X, Kim GH, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol. 2002;283:F648–F657. doi: 10.1152/ajprenal.00016.2002. [DOI] [PubMed] [Google Scholar]
- 9.Song J, Hu X, Khan O, Tian Y, Verbalis JG, Ecelbarger CA. Increased blood pressure, aldosterone activity, and regional differences in renal ENaC protein during vasopressin escape. Am J Physiol Renal Physiol. 2004;287:F1076–F1083. doi: 10.1152/ajprenal.00075.2004. [DOI] [PubMed] [Google Scholar]
- 10.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol. 2006;290:F1055–F1064. doi: 10.1152/ajprenal.00108.2005. [DOI] [PubMed] [Google Scholar]
- 11.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol. 2000;279:F46–F53. doi: 10.1152/ajprenal.2000.279.1.F46. [DOI] [PubMed] [Google Scholar]
- 12.Ecelbarger CA, Knepper MA, Verbalis JG. Increased abundance of distal sodium transporters in rat kidney during vasopressin escape. J Am Soc Nephrol. 2001;12:207–217. doi: 10.1681/ASN.V122207. [DOI] [PubMed] [Google Scholar]
- 13.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol. 1999;276:F96–F103. doi: 10.1152/ajprenal.1999.276.1.F96. [DOI] [PubMed] [Google Scholar]
- 14.Kim GH, Martin SW, Fernandez-Llama P, Masilamani S, Packer RK, Knepper MA. Long-term regulation of renal Na-dependent cotransporters and ENaC: response to altered acid-base intake. Am J Physiol Renal Physiol. 2000;279:F459–F467. doi: 10.1152/ajprenal.2000.279.3.F459. [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J, Hu X, Shi M, Knepper MA, Ecelbarger CA. Effects of dietary fat, NaCl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am J Physiol Renal Physiol. 2004;287:F1204–F1212. doi: 10.1152/ajprenal.00063.2004. [DOI] [PubMed] [Google Scholar]
- 17.Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–453. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–846. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- 20.Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC. Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest. 1998;101:1661–1669. doi: 10.1172/JCI601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R794–R799. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 22.Oestreicher EM, Guo C, Seely EW, Kikuchi T, Martinez-Vasquez D, Jonasson L, Yao T, Burr D, Mayoral S, Roubsanthisuk W, Ricchiuti V, Adler GK. Estradiol increases proteinuria and angiotensin II type 1 receptor in kidneys of rats receiving L-NAME and angiotensin II. Kidney Int. 2006;70:1759–1768. doi: 10.1038/sj.ki.5001897. [DOI] [PubMed] [Google Scholar]
- 23.Nicco C, Wittner M, DiStefano A, Jounier S, Bankir L, Bouby N. Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension. 2001;38:1143–1149. doi: 10.1161/hy1001.092641. [DOI] [PubMed] [Google Scholar]
- 24.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006;291:F683–F693. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- 25.Guipponi M, Vuagniaux G, Wattenhofer M, Shibuya K, Vazquez M, Dougherty L, Scamuffa N, Guida E, Okui M, Rossier C, Hancock M, Buchet K, Reymond A, Hummler E, Marzella PL, Kudoh J, Shimizu N, Scott HS, Antonarakis SE, Rossier BC. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet. 2002;11:2829–2836. doi: 10.1093/hmg/11.23.2829. [DOI] [PubMed] [Google Scholar]
- 26.Shimkets RA, Lifton R, Canessa CM. In vivo phosphorylation of the epithelial sodium channel. Proc Natl Acad Sci USA. 1998;95:3301–3305. doi: 10.1073/pnas.95.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]