Abstract
Axis inhibition proteins 1 and 2 (Axin1 and Axin2) are scaffolding proteins that modulate at least two signaling pathways that are crucial in skeletogenesis: the Wnt/β-catenin and TGF-β signaling pathways. To determine whether Axin2 is important in skeletogenesis, we examined the skeletal phenotype of Axin2-null mice in a wild-type or Axin1+/− background. Animals with disrupted Axin2 expression displayed a runt phenotype when compared to heterozygous littermates. Whole-mount and tissue β-galactosidase staining of Axin2LacZ/LacZ mice revealed that Axin2 is expressed in cartilage tissue, and histological sections from knockout animals showed shorter hypertrophic zones in the growth plate. Primary chondrocytes were isolated from Axin2-null and wild-type mice, cultured, and assayed for type X collagen gene expression. While type II collagen levels were depressed in cells from Axin2-deficient animals, type X collagen gene expression was enhanced. There was no difference in BrdU incorporation between null and heterozygous mice, suggesting that loss of Axin2 does not alter chondrocyte proliferation. Taken together, these findings reveal that disruption of Axin2 expression results in accelerated chondrocyte maturation. In the presence of a heterozygous deficiency of Axin1, Axin2 was also shown to play a critical role in craniofacial and axial skeleton development.
Keywords: Axin, endochondral, chondrocyte, β-catenin, TGF-β
During embryonic development, skeletal elements are formed via two distinct processes: intramembranous and endochondral ossification. Several molecular signals coordinating endochondral bone formation have been discovered, including Wnt/β-catenin signaling and TGF-β signaling. Recent studies have demonstrated that Wnt/β-catenin signaling is required to drive proper osteoblast and chondrocyte differentiation during endochondral ossification.1–3 Additionally, TGF-β has been shown to stimulate early stages of chondrogenesis and chondrocyte proliferation while inhibiting the terminal differentiation of chondrocytes.4–6 Thus, Wnt and TGF-β signaling appear to oppose one another during select periods of endochondral bone development.
Axins regulate both the Wnt/β-catenin and TGF-β signaling pathways. In the absence of Wnt signal, Axins bind GSK-3β and β-catenin, facilitating GSK-3β-mediated phosphorylation of β-catenin, marking the protein for ubiquitination and proteasomal degradation.7 Axins are stabilized when phosphorylated by GSK-3β.8 In the presence of Wnt signal, Axins are recruited to the Wnt co-receptor LRP5 or 6, and are dephosphorylated. 9,10 The protein complex formed by Axin is therefore destabilized, allowing β-catenin to accumulate and translocate to the nucleus where it regulates gene transcription through TCF and LEF transcription factors.11,12 In a negative feedback loop, Wnt/β-catenin/TCF signaling induces Axin2 expression, which in turn inhibits canonical Wnt signaling.13,14 While Axins inhibit Wnt signaling, they enhance TGF-β signaling in two ways. First, Axins facilitate the phosphorylation and activation of Smad3.15 Activated Smad3 associates with Smad4, translocates to the nucleus, and initiates gene transcription. Second, Axins facilitate phosphorylation of the inhibitory Smad7, which competitively inhibits Smad3 activity. Phosphorylation of Smad7 marks it for ubiquitination and proteasomal degradation.16
Axin1 and Axin2 are master scaffolding proteins originally identified as negative regulators of canonical Wnt/β-catenin signaling (see review17). Although these proteins are similar in function, Axin1 appears to be ubiquitously expressed, while Axin2 has a more restricted expression pattern.18 Further, Axin2 expression is directly induced by canonical Wnt signaling and therefore acts in a negative feedback loop.8,13 In addition to interactions with several canonical Wnt signaling proteins,9,19 the Axin proteins have been shown to interact with MEKK1 and Smad3 establishing their importance in other key signaling pathways.15,20
Given the role of Axins in at least two pathways relevant to endochondral ossification and embryogenesis in general, it is likely that Axins specifically contribute to normal skeletogenesis. Therefore, we examined Axin2 expression in the cartilage of Axin2LacZ/LacZ mice and examined the skeletal phenotype of Axin2-deficient mice to ascertain the effects of disrupted Wnt/β-catenin signaling on endochondral ossification. Intramembranous bone formation has already been shown to involve Axin2 as Axin2-deficient mice are characterized by craniofacial defects.21 Interestingly, mice deficient in the functional homolog Axin1 die in utero and are characterized by the presence of axis determination defects22–24; however, mice heterozygous for a mutation in Axin1 survive with no abnormalities. Hence, we also explored the skeletal phenotype of Axin2-deficient mice on a genetic background of Axin1 heterozygosity to determine if the presence of Axin2 in Axin1 heterozygotes is compensates for the absence of Axin1. We hypothesized that Axin2 would be expressed in cartilage cells, that Axin2-deficient mice would have significant changes in endochondral skeletal development when compared to heterozygous or wild-type littermate controls, and that these alterations would be even more profound in the Axin1+/−; Axin2−/− animals.
METHODS
Axin2-Deficient Mice
Axin2LacZ/LacZ mice were a generous gift from Dr. Wei Hsu and have been described by his group.21 In this article, the term “Axin2−/−” connotes “Axin2LacZ/LacZ.” Care and use of experimental animals complied with the guidelines and policies of the University Committee on Animal Resources at the University of Rochester.
Histology and β-Galactosidase Staining
Whole-mount embryo or frozen tissue section β-galactosidase staining was performed as previously described.21,25 Stained tissue sections were then washed in PBS, counterstained with nuclear fast red, dehydrated, and coverslipped using standard mounting media.
Bromodeoxyuridine (BrdU) labeling was achieved in 1-week-old mice by administering the labeling reagent (1 mL/100g body weight; Zymed, San Francisco, CA) 3 h before sacrifice via i.p. injection. BrdU incorporation was examined using immunohistochemistry on paraffin-embedded sections with a primary mouse monoclonal antibody against BrdU (Lab Vision, Fremont, CA).
RNA Extraction and Quantitative Reverse-Transcriptase PCR from Chondrocytes
Chondrocytes were isolated from the sterna and ribs of 3-day-old mice as previously described.26 Cells were plated in 12-well plates at 5 × 104 cells per well for RNA isolation. Total RNA was extracted from primary chondrocytes using the Trizol (Invitrogen, Carlsbad, CA) protocol following the manufacturer’s recommendations. One microgram of total RNA was reverse-transcribed using the i-Script cDNA synthesis kit (Biorad, Hercules, CA) following the manufacturer’s recommended protocol. Two microliters of reverse-transcribed cDNA was used for quantitative PCR. cDNA levels were measured in real-time using the fluorescent dye SYBR Green I (SYBR Green PCR Master Mix, Applied Biosystems, Foster City, CA) and specific primers designed for mouse type X collagen (Forward: 5′-ACC CCA AGG ACC TAA AGG AA-3′; Reverse: 5′-CCC CAG GAT ACC CTG TTT TT-3′), type II collagen (Forward: 5′-ACT GGT AAG TGG GGC AAG AC-3′; Reverse: 5′-CCA CAC CAA ATT CCT GTT CA-3′), and β-actin (Forward: 5′-TGT TAC CAA CTG GGA CGA CA-3′; Reverse: 5′-CTG GGT CAT CTT TTC ACG GT-3′). The PCR reaction used the RotorGene real-time DNA amplification system (Corbett Research, Sydney, Australia) and the following protocol: 95°C denaturation step for 10 min followed by 45 cycles with denaturation for 30 s at 95°, annealing for 30 s at 55°C, and extension for 30 s at 72°C. Detection of the fluorescent product occurred after each extension period. PCR products were subjected to melting curve analysis, and the data were analyzed and quantified with the RotorGene analysis software. Gene expression was normalized to β-actin expression levels.
Micro-CT and Skeletal Staining
Embryos were fixed overnight in 10% neutral buffered formalin following evisceration and skinning, then dehydrated in a graded series of ethanol. Fixed embryos were scanned at a resolution of 12.5 µm using a ScanCo Medical VivaCT40 (Basserdorf, Switzerland) with x-ray settings of 55 kVp and 145 µA, and an integration time of 300 ms. Three-dimensional composite images were created with a threshold value of 150. Skeletal staining of whole embryos using alcian blue and alizarin red was performed as previously described,27 immediately following micro-CT analysis.
RESULTS
Axin2 Knockout Mice Display a Runt Phenotype
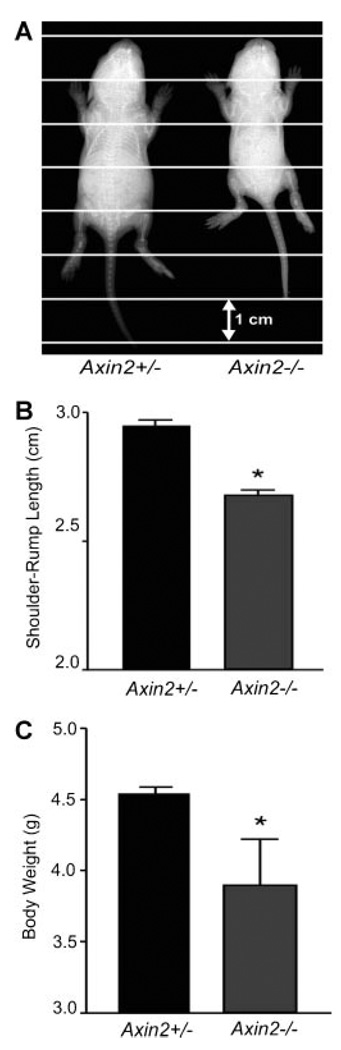
Yu and colleagues have demonstrated that Axin2 plays a critical role in intramembranous bone formation such that disruption of Axin2 in mice results in skeletal abnormalities, particularly a craniosynostosis-like phenotype.21 Measurement of Axin2−/− and Axin2+/− littermates reveals an overall runt phenotype in the null mice (Fig. 1A). One-week-old Axin2−/− mice (n = 11) had an approximate 12.5% decrease in shoulder-to-rump length when compared to heterozygous littermates (n = 12) (Fig. 1B). Accordingly, the Axin2−/− mice weighed less, averaging 3.8 g at 1 week, compared to Axin2+/− littermates, which averaged 4.5 g at the same time point (Fig. 1C). This decrease in body size suggests that Axin2 plays a critical role not only in intramembranous bone formation of the skull, but also in endochondral bone formation, which is critical to development of the axial and appendicular skeleton. No difference in body size or weight was observed between heterozygous and homozygous wild-type animals.
Figure 1.
One-week-old Axin2-null mice (n = 11) display a runt phenotype compared to heterozygous littermates (n = 12). (A) Plain x-ray; (B) body length in centimeters; (C) mass in grams. (B) and (C) are shown as the mean ± SD. *p<0.05 using unpaired t-test.
Axin2 Is Expressed in Cartilage
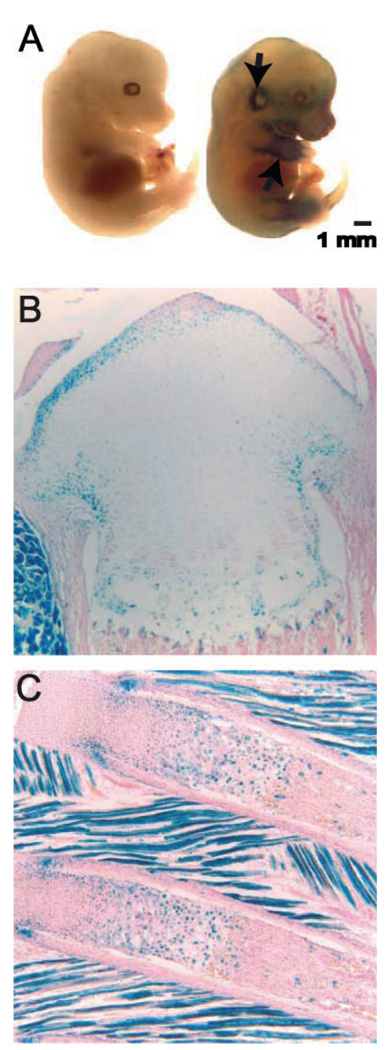
It has previously been established that Axin2 is specifically expressed in neural crest-derived skeletal elements during postnatal development.21 Whole-mount β-galactosidase staining of E13.5 Axin2LacZ/LacZ embryos reveals Axin2 expression in cartilaginous areas of the axial and appendicular skeleton during embryonic development (Fig. 2A). Thus, positively stained regions at this stage reveal that Axin2 is expressed in tissues derived from paraxial and lateral-plate mesoderm, as well as in neural crest derivatives.
Figure 2.
Axin2 is expressed in cartilage during development. (A) β-galactosidase staining of whole wild-type or Axin2-null embryos at day E13.5. Arrowhead indicates β-galactosidase activity. Representative photomicrographs (original magnification, × 100) of β-galactosidase stained frozen sections: tibia (B) and ribs (C).
Axin2 continues to be expressed in cartilaginous elements postnatally. At 1 week of age, β-galactosidase staining of frozen tissue sections from Axin2−/− mice reveals Axin2 expression in chondrocytes of the ribs, vertebra, and long bone growth regions, specifically in peripheral epiphyseal chondrocytes and prehypertrophic/hypertrophic chondrocytes (Fig. 2). These findings are consistent with the idea that Axin2 functions during endochondral bone formation, and likely accounts for the runt phenotype observed in Axin2-null mice.
Axin2 Regulates Chondrocyte Maturation
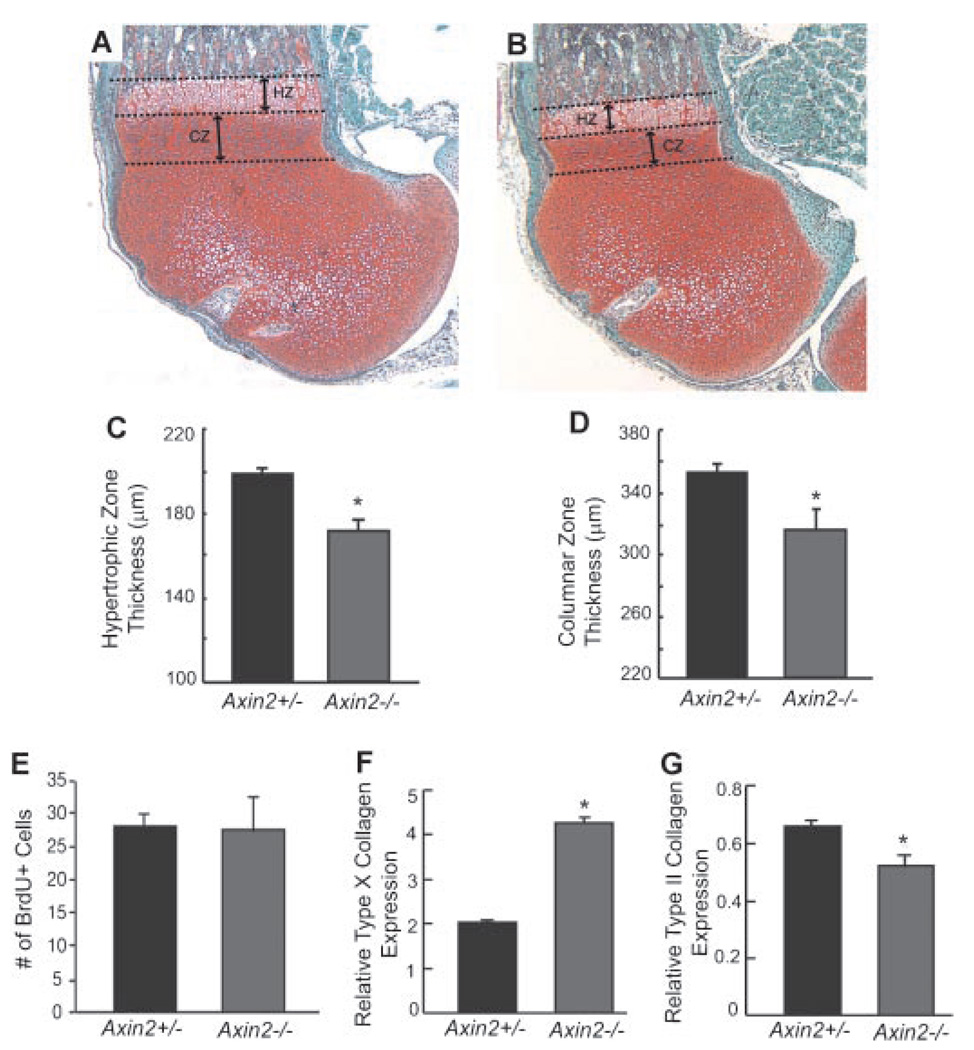
While defects in intramembranous bone formation leading to craniosynostosis in Axin2−/− mice have been attributed to abnormal osteoblast proliferation and differentiation,28 the defects observed during endochondral bone formation appear to result exclusively from abnormal chondrocyte maturation. Histological sections of distal femurs from 1-week-old Axin2−/− mice (n = 7) reveal thinner hypertrophic and columnar zones when compared to Axin2+/− littermates (n = 13) (Fig. 3A–D). This finding is consistent with an overall acceleration in both the initiation of hypertrophy and terminal differentiation processes resulting in shorter limb length, reduced rib cage size, and a shorter axial skeleton. To examine whether loss of Axin2 disrupts chondrocyte proliferation, BrdU staining was performed on growth region chondrocytes of 1-week-old Axin2+/− (n = 13) and Axin2−/− (n = 7) hindlimb sections. No difference was observed in BrdU labeling between these two groups (Fig. 3E), suggesting that Axin2 does not regulate chondrocyte proliferation. To determine the effects of Axin2 on chondrocyte maturation, mRNA was extracted from primary sternal and rib chondrocytes of 3-day-old Axin2−/− and Axin2+/− mice, and the expression of chondrocyte maturation marker genes was examined. Real-time RT-PCR analyses revealed a twofold increase in gene expression of the hypertrophic chondrocyte marker, type X collagen, in Axin2−/− cells (Fig. 3F). Accordingly, there is an approximate 20% decrease in type II collagen gene expression, a marker of immature chondrocytes (Fig. 3G). Together, these data indicate that loss of Axin2 leads to accelerated chondrocyte maturation without any obvious change in cell proliferation, demonstrating a specific regulatory role for Axin2 in differentiating chondrocytes.
Figure 3.
Accelerated chondrocyte maturation in Axin2−/− mice. Representative photomicrographs (original magnification, ×40) of distal femurs in 1-week-old Axin2+/− [n = 13 (A)] and Axin2−/− [n = 7 (B)]. Histomorphometric measurements of hypertrophic zone (HZ) thickness (C) and columnar zone (CZ) thickness (D). (E) Quantification of BrdU-positive cells in the epiphyseal region. Real-time RT-PCR analysis of type X collagen (F) and type II collagen (G) gene expression in primary chondrocytes isolated from Axin2+/− and Axin2−/− mice. Results are shown as mean ± SD. *p<0.05 using unpaired t-test.
Axial Skeletal Defects in Absence of Axin2 and Axin1
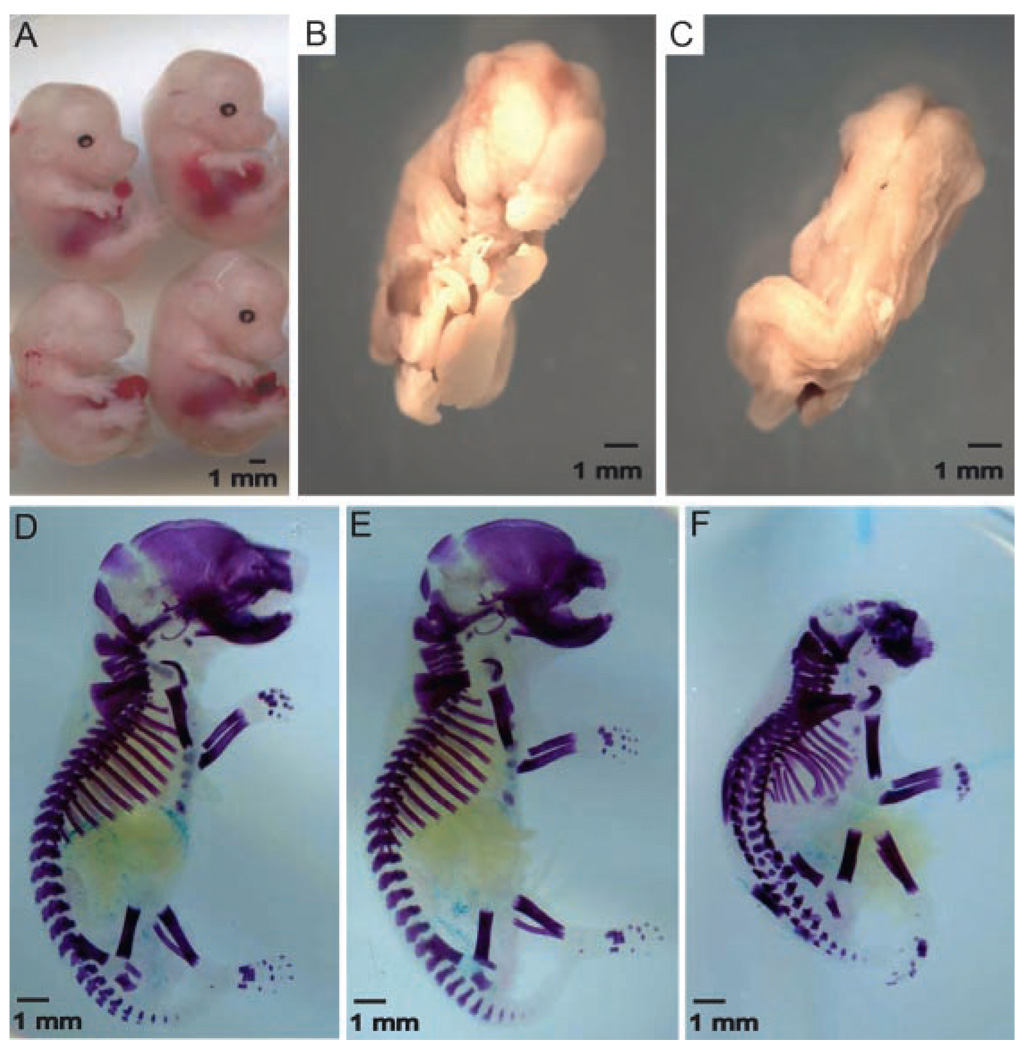
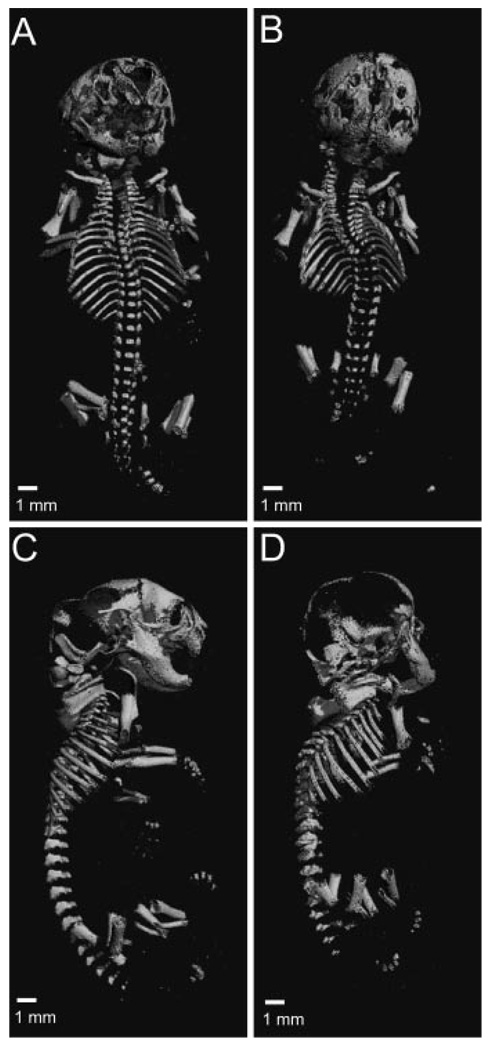
To determine if deletion of Axin2, the functional homolog of Axin1, produces defects in axial development as found in the embryonic lethal Axin1 deficiency, we crossed the Axin2−/− mice onto an Axin1+/− background. Compared to the Axin2−/− and Axin1+/−; Axin2+/− mice, the Axin1+/−; Axin2−/− mice (hereafter referred to as Axin 3/4 knockout) were significantly smaller at day E14.5 and also lacked bilateral eye formation (Fig. 4A). Profound abnormalities persisted at day E16.5 where the Axin 3/4 knockout embryos demonstrated incomplete midline fusion of the cranium and marked scoliosis (Fig. 4B, C). Staining of the complete embryonic skeleton at day E18.5 shows several defects of axial skeleton development, including incomplete calvarial formation, and deformities of the vertebrae and ribs (Fig. 4D–F). The appendicular skeleton of the Axin 3/4 knockout embryos appeared similar to that of the Axin2−/− animals at day E18.5. E18.5 Axin 3/4 knockout embryos were also examined using micro-CT scanning, where the small size and scoliosis were very apparent compared to the double heterozygous littermates (Fig. 5A, B). When seen in profile, the E18.5 Axin 3/4 knockout embryo demonstrates much reduced mineralization of the calvaria, especially in the parietal and occipital regions (Fig. 5D). In addition, fusions of the lumbar vertebrae are also apparent. Since loss of Axin2 function in the background of Axin1 heterozygosity results in marked defects in embryo size and axial skeletal formation, these findings suggest that Axin2 regulates endochondral bone formation, as well as axial skeleton patterning and development.
Figure 4.
Profound abnormalities in Axin 3/4 knockout embryos. (A) Whole mounts of representative E14.5 embryos lacking one or more copies of the Axin1 or Axin2 genes. Clockwise from the upper left, the genotypes are: Axin2+/−, Axin2−/−, Axin1+/−; Axin2+/−, and Axin 3/4 knockout. Ventral (B) and dorsal (C) views of E16.5 Axin 3/4 knockout embryos. Skeletal staining of E18.5 Axin1+/− Axin2+/− (D), Axin2−/− (E), and Axin 3/4 knockout (F) embryos.
Figure 5.
Defects of the mineralized skeleton in E18.5 Axin 3/4 knockout embryos. Ventral (A, B) or profile view (C, D) of double-heterozygous skeleton (A, C) or Axin 3/4 knockout skeleton (B, D) as rendered by micro-CT analysis.
DISCUSSION
The findings presented above establish that Axin2 is expressed in both axial and appendicular cartilage during skeletal development, and is an inhibitor of chondrocyte maturation. Axin2 is expressed in lateral plate and paraxial mesoderm-derived tissue, namely in the cartilage of limbs, spine, and ribs. Interestingly, we show that Axin2 expression is primarily restricted to hypertrophic chondrocytes and a subset of the most peripheral epiphyseal chondrocytes. Loss of Axin2 function accelerates hypertrophic differentiation, resulting in reduced endochondral bone growth and a runt phenotype in mutant mice. Therefore, these data emphasize the important role Axin2 plays during endochondral bone formation.
Previous work has shown that Axin2 regulates both proliferation and differentiation in osteoblasts, primarily through its actions in Wnt/β-catenin signaling. 28 In this article, we show that Axin2 also affects chondrocytes. It is probable that the lack of a chondrocyte proliferation phenotype in Axin2−/− mice is due to the fact that Axin2 expression is primarily restricted to differentiating chondrocytes in the prehypertrophic and hypertrophic zones, but not proliferating chondrocytes. The accelerated cartilage differentiation phenotype is likely caused by an increase in localized Wnt signaling in those differentiating cells, which is similar to cartilage phenotypes of animal models with Wnt gain-of-function modifications.
Although the role of Axin2 in inhibiting Wnt signaling has been well established, recently Axins have been shown to play active roles in the TGF-β and JNK signaling pathways as well. Axins enhance TGF-β signaling by facilitating Smad3 phosphorylation and activation, as well as by enhancing Smad7 phosphorylation and degradation.15,16 In mitogen-activated protein kinase (MAPK) signaling, Axins facilitate the activation of c-Jun N-terminal Kinase (JNK), a MAPK regulator, through interactions with the protein kinases MEKK1, 4, and 7.20 Recently, we have shown that Axins are negatively regulated by TGF-β and mediate crosstalk between the TGF-β and Wnt signaling pathways in chondrocytes.29 The overall effect of this crosstalk is an enhancement of β-catenin signaling and an inhibition of Smad3 signaling that results in chondrocyte maturation. This is consistent with the in vivo findings presented here, such that disruption of Axin2 signaling results in accelerated chondrocyte maturation and shortening of endochondral bones. β-Catenin gain-of-function in vivo produces a similar phenotype (shorter limbs and craniofacial abnormalities), which provides further evidence for the role of Axin proteins in the regulation of this signaling cascade.30 A mechanism through which this occurs may lie in the removal of Axin2 as a mediator that balances TGF-β and Wnt/β-catenin signaling, which are thought to oppose one another during chondrocyte proliferation and maturation.
Interestingly, Axin1 protein is ubiquitously expressed, but does not apparently function to preserve normal endochondral bone formation in the absence of Axin2. Axin2, in turn, cannot compensate for the lack of Axin1 as Axin1−/− animals do not survive. This is likely due to the limited expression pattern of Axin2.18 The results presented here also indicate that both Axin1 and Axin2 are involved in axial and appendicular skeletogenesis involving both intramembranous and endochondral ossification. In particular, midline fusion appears to be tightly linked to Wnt/β-catenin signaling, as GSK-3β-null mice also exhibit incomplete midline fusion and cleft palate.31 Regarding the role of Axins in intramembranous bone formation, several groups have found that manipulation of the Wnt/β-catenin or TGF-β signaling pathways results in craniofacial deformities, including cleft palate and calvarial agenesis.32–34 Axin proteins may also play other roles during embryogenesis, as suggested by the lack of eye development in the Axin 3/4 knockout embryos. Indeed, van de Water et al. described an eyeless phenotype in zebrafish with mutations in the Axin1 gene.35
In summary, our work identifies differentiating chondrocytes as a specific target for Axin2 activity; Axin2 normally prevents early chondrocyte maturation. Combined with work we have previously reported, the runt phenotype of Axin2−/− mice described here can be accounted for, at least in part, by the involvement of Axin2 in mediating TGF-β and Wnt/β-catenin crosstalk, where the loss of Axin2 tips the balance between these two signaling pathways in favor of chondrocyte maturation. Examination of the Axin 3/4 knockout mice also indicates that both Axin proteins are involved in intramembranous bone formation and participate in early midline events during skeletogenesis.
ACKNOWLEDGMENTS
This work was supported by National Health Service Awards AR048681 (R. J. O) and AR053717 (R. J. O).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Day TF, Guo X, Garrett-Beal L, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 3.Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Drissi H, Chen M, et al. Wnt-mediated regulation of chondrocyte maturation: modulation by TGF-beta. J Cell Biochem. 2005;95:1057–1068. doi: 10.1002/jcb.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato Y, Iwamoto M, Koike T, et al. Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor beta and serum factors. Proc Natl Acad Sci USA. 1988;85:9552–9556. doi: 10.1073/pnas.85.24.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Chen L, Xu X, et al. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Noort M, Meeldijk J, van der Zee R, et al. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda S, Kishida S, Yamamoto H, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3-beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao J, Wang J, Liu B, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 10.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of Axin releases beta-catenin from the Axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 12.Molenaar M, van de Wetering M, Oosterwegel M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 13.Jho EH, Zhang T, Domon C, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung JY, Kolligs FT, Wu R, et al. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 15.Furuhashi M, Yagi K, Yamamoto H, et al. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol. 2001;21:5132–5141. doi: 10.1128/MCB.21.15.5132-5141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Rui H, Wang J, et al. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikuchi A. Roles of Axin in the Wnt signalling pathway. Cell Signal. 1999;11:777–788. doi: 10.1016/s0898-6568(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 18.Chia IV, Costantini F. Mouse Axin and Axin2/conducting proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo W, Lin SC. Axin: a master scaffold for multiple signaling pathways. Neurosignals. 2004;13:99–113. doi: 10.1159/000076563. [DOI] [PubMed] [Google Scholar]
- 20.Zou H, Li Q, Lin SC, et al. Differential requirement of MKK4 and MKK7 in JNK activation by distinct scaffold proteins. FEBS Lett. 2007;581:196–202. doi: 10.1016/j.febslet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Yu HM, Jerchow B, Sheu TJ, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluecksohn-Schoenheimer S. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J Exp Zool. 1949;110:47–76. doi: 10.1002/jez.1401100105. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan RJ, O’Brien MC. Genetic analysis of mutations at the fused locus in the mouse. Proc Natl Acad Sci USA. 1986;83:4413–4417. doi: 10.1073/pnas.83.12.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng L, Fagotto F, Zhang T, et al. The mouse fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 25.Whiting J, Marshall H, Cook M, et al. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991;5:2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- 26.Li TF, Darowish M, Zuscik MJ, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Yu HM, Hsu W. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of beta-catenin in proliferation and differentiation. Dev Biol. 2007;301:298–308. doi: 10.1016/j.physletb.2003.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dao DY, Yang X, Chen D, et al. Axin1 and Axin2 are regulated by TGF- and mediate cross-talk between TGF- and Wnt signaling pathways. Ann NY Acad Sci. 2007;1116:82–99. doi: 10.1196/annals.1402.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama H, Lyons JP, Mori-Akiyama Y, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu KJ, Arron JR, Stankunas K, et al. Chemical rescue of cleft palate and midline defects in conditional GSK-3beta mice. Nature. 2007;446:79–82. doi: 10.1038/nature05557. [DOI] [PubMed] [Google Scholar]
- 32.Brault V, Moore R, Kutsch S, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 33.Chang J, Sonoyama W, Wang Z, et al. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- 34.Ito Y, Yeo JY, Chytil A, et al. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–5280. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- 35.van de Water S, van de Wetering M, Joore J, et al. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development. 2001;128:3877–3888. doi: 10.1242/dev.128.20.3877. [DOI] [PubMed] [Google Scholar]