Abstract
We report the case of a 13-year-old boy with bilateral distal femoral unicameral bone cysts (UBCs) associated with acquired generalized lipodystrophy. As opposed to congenital generalized lipodystrophy, cystic bone lesions in acquired generalized lipodystrophy are rare. After radiographic and histologic confirmation of the UBCs, we performed percutaneous intramedullary decompression, curettage, and grafting. UBCs can be an important manifestation of acquired generalized lipodystrophy. Cystic bone lesions appear to be less common in acquired generalized lipodystrophy than in congenital generalized lipodystrophy, and intramedullary adipose tissue loss may be a predisposing factor for the development of bone lesions in patients with acquired generalized lipodystrophy. When evaluating a patient with lipodystrophy, doctors should recognize the clinical course may include the development of UBCs.
Introduction
Lipodystrophic syndromes are characterized by complete or partial absence of adipose tissue [10]. They can be classified as localized or generalized and congenital or acquired. Differentiation of lipodystrophies is complex because clinical presentations usually overlap. In general, localized lipodystrophies are primarily cosmetic and limited to focal atrophy of subcutaneous adipose tissue. Generalized lipodystrophies may include severe systemic metabolic abnormalities involving various organ systems. Subclassification of generalized lipodystrophies is based primarily on age at onset: congenital generalized lipodystrophy (CGL) is present at birth, and acquired generalized lipodystrophy (AGL) develops during childhood [10].
CGL is the most common lipodystrophy associated with bone lesions. Numerous authors have described the development of lytic lesions of the appendicular skeleton in these patients [1, 4, 10, 12, 18]. In contrast, bony lesions only rarely have been mentioned in AGL [6, 13, 19] and apparently were symptomatic in only one of the reported cases [19]. In none of these few reports of AGL are the characteristics, diagnosis, or treatment of these bony lesions reported.
We report the case of a boy with autoimmune-type AGL with symptomatic bilateral distal femoral UBCs. We show that UBCs can be an important clinical manifestation of AGL. We also speculate why the prevalence of bone cysts appears to be much greater in CGL than in AGL.
Case Report
A 13-year-old boy presented to the emergency department with a 1-week history of poorly localized, burning, left lower extremity pain. There was no history of trauma or other precipitating event. His medical history was remarkable for AGL, insulin-resistant diabetes mellitus secondary to leptin deficiency, autoimmune hepatitis, and autoimmune thrombocytopenia.
At initial physical examination, the patient appeared thin and nontoxemic and had a slightly antalgic gait. He had full active and passive ROM of the hips, knees, and ankles bilaterally. No knee erythema or effusion was noted. He had no focal tenderness over the thighs, calves, or knees. At the 1-week followup, the patient reported improvement of his pain. He stood with level shoulders on a level pelvis, but exaggerated thoracic kyphosis was noted. He was able to perform a full heel walk, toe walk, and deep knee bend without difficulty. Bilateral hamstring tightness was present, with a popliteal angle of 30°. The patient also had decreased girth of his right calf and thigh compared with the left.
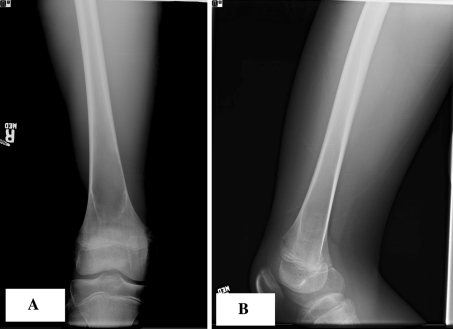
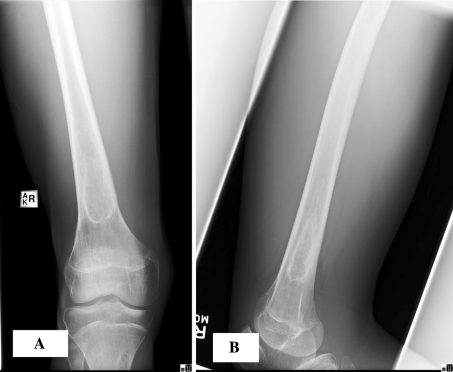
Initial radiographs showed a well-defined, central, lytic lesion with cortical thinning on the metadiaphysis region of the left femur (Fig. 1). There was no periosteal reaction, cortical disruption, scalloping, or other signs of aggressiveness. Contralateral radiographs were taken at followup and showed a similar well-defined lucent lesion of the right femoral metadiaphyseal region (Fig. 2). Both lesions were Grade IA using the classification of Lodwick et al. [15] based on their radiographic characteristics. In this classification, Grade 1 reflects better delineated lesions with a geographic distribution, Grade II is moth-eaten lesions, and Grade III is permeative lesions; Grades IA, IB, and IC are differentiated by their presumed aggressiveness.
Fig. 1A–B.
(A) AP and (B) lateral radiographs of the left femur show a large, fairly well-defined, lytic lesion of the distal diaphyseal-metaphyseal region. There is no periosteal reaction or any signs of aggressiveness.
Fig. 2A–B.
(A) AP and (B) lateral radiographs of the right femur show a similar large, well-defined, lytic lesion in the distal diaphyseal-metaphyseal region.
MRI of both femurs better defined the lesions. There was a homogeneous low T1 signal and hyperintense T2 signal. These findings were suggestive of a simple bone cyst. The left lesion measured 13 cm in length, and the right measured 10 cm at its greatest dimension.
Owing to the other anomalies, size, and location of the femoral lesions, we recommended surgery for diagnostic confirmation and management. The patient underwent bilateral cyst aspiration and cystogram, followed by incisional biopsy and intraoperative frozen section. Histologic confirmation of simple bone cyst was followed by intralesional curettage, intramedullary decompression, and grafting with medical-grade calcium sulfate (MGCS) pellets (Osteoset®; Wright Medical Technology, Inc, Memphis, TN) following a previously described technique [9].
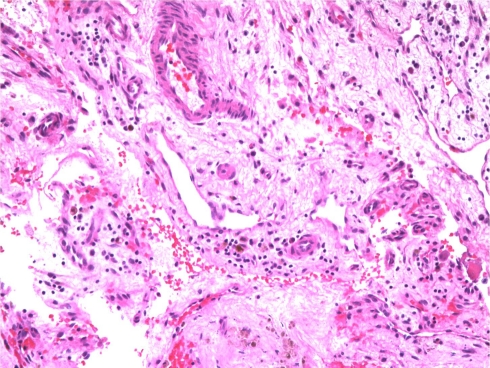
The histopathology of the cyst linings revealed fragments of trabecular bone and marrow elements with adjacent fibromyxoid tissue containing chronic inflammation, granulation tissue, hemosiderin-laden macrophages, rare foamy histiocytes, and multinucleated giant cells. No definite cyst lining was identified. These features are consistent with a benign bone cyst or UBC (Fig. 3).
Fig. 3.
Initial intraoperative frozen section from the right distal femur shows fragments of trabecular bone with marrow elements, adjacent fibromyxoid tissue containing chronic inflammation, granulation tissue, hemosiderin-laden macrophages, rare foamy histiocytes, and multinucleated giant cells (Stain, hematoxylin and eosin; original magnification, ×10). These features are consistent with benign bone cysts.
Postoperatively, the patient was allowed to weightbear as tolerated with a four-point gait. He was discharged from the hospital 2 days after surgery and was doing well until 2 weeks postoperatively, when he started complaining of right thigh pain. Although radiographs were unremarkable, the patient wore a long-leg cast for a suspected stress fracture (Fig. 4), perhaps from the surgical procedure. The cast was removed in 4 weeks with complete resolution of the symptoms.
Fig. 4A–B.
Two weeks postoperatively, (A) AP and (B) lateral radiographs of the right femur show no evidence of fracture.
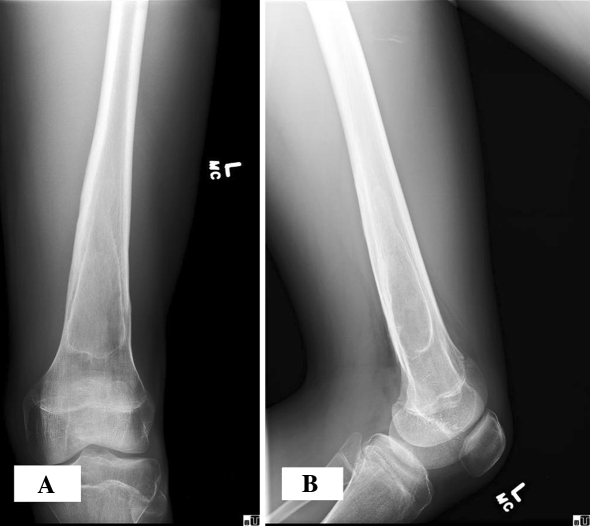
Although initial radiographs showed progressive healing, the patient had worsening bilateral leg pain develop. Radiographs at 20 months showed persistence or recurrence of both cysts, again with features consistent with Grade IA lesions (Lodwick et al. [15]) (Figs. 5, 6). MRI of both femurs again suggested findings consistent with a simple bone cyst. The patient underwent repeat bilateral cyst aspiration and cystogram followed by incisional biopsy and intraoperative frozen section, as described above. Histopathology again confirmed the diagnosis as UBC, and intralesional curettage, intramedullary decompression, and grafting with MGCS pellets were performed.
Fig. 5A–B.
At the 20-month followup, (A) AP and (B) lateral radiographs of the left femur show persistence of the cystic area.
Fig. 6A–B.
At the 20-month followup, (A) AP and (B) lateral radiographs of the right femur show persistence of the cystic area.
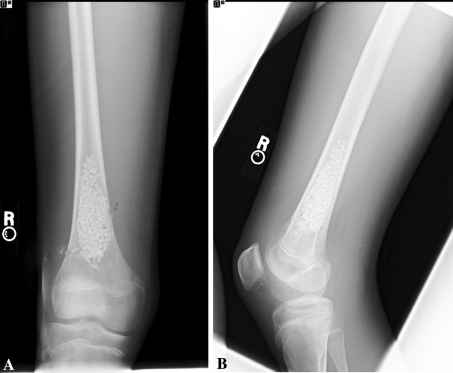
Initial radiographs at the 2-month followup after the second procedures showed interval bony ingrowth into the right UBC (Fig. 7). At this time, the patient had a superficial wound irritation of the left distal femur develop, which resolved with antibiotics. At the 4-month followup, the patient complained of some mild greater trochanteric pain of the left hip that was suggestive of bursitis. He was otherwise asymptomatic, fully active, and doing very well. The initially larger left UBC showed improvement and continued bony ingrowth (Fig. 8). At the most recent 7-month followup, he remains asymptomatic and fully active. We planned additional followup to determine if the lesions healed or whether they recur.
Fig. 7A–B.
Two months after reoperation, (A) AP and (B) lateral radiographs of the right femur show the presence of MGCS pellets and bony ingrowth into the cystic area.
Fig. 8A–B.
Four months after reoperation, (A) AP and (B) lateral radiographs of the left femur show continued bony ingrowth into the cystic area.
Discussion
Lipodystrophies are a group of disorders characterized by the loss of adipose tissue [11]. They are classified based on the pattern and timing of adipose tissue loss. The extent of adipose tissue loss corresponds to the severity of metabolic manifestations. Patients with localized lipodystrophies can experience only cosmetic problems, whereas those with generalized lipodystrophies can experience systemic problems such as insulin resistance, early diabetes mellitus, or autoimmune hepatitis or thrombocytopenia. Musculoskeletal manifestations can include advanced bone age, development of lytic bone lesions, and intramedullary adipose tissue loss.
Lipodystrophies are rare. CGL has been estimated to have a prevalence of 1 in 12 million [11]. AGL is even rarer. Only approximately 63 cases of AGL have been reported since the condition was first described in 1928 [17]. CGL has a well-characterized relationship to the development of nonspecific lytic bone lesions [4, 10, 18]. In AGL, lytic bone lesions have been reported only three times [6, 13, 19] and have never been diagnosed or treated. The descriptions of these lesions are nonspecific and therefore of limited clinical value. Our report documents the presence of UBCs in this disorder.
UBCs are a common benign bone tumor seen in the growing child [2]. They are often asymptomatic and the diagnosis frequently is made at the time of a pathologic fracture [2]. The pathophysiology behind the development of AGL remains speculative, although the destruction of adipocytes has been hypothesized to result from either cell-mediated or antibody-mediated autoimmune processes [17]. Additionally, despite intensive research, the pathogenesis of UBCs remains elusive. The specific metabolic abnormalities predisposing patients with AGL to the development of cystic bone lesions is unknown.
We speculate the loss of intramedullary adipose tissue may contribute to the development of UBCs in patients with lipodystrophy. It has been hypothesized a reduction in bone marrow fat or local hypervascularity may induce an osteoclastic response contributing to the development of osseous cysts [8]. In CGL, where the development of UBCs is well characterized, destruction of bone marrow fat is commonly seen [11]. In AGL, destruction of bone marrow fat is less common. In a relatively large case series of 16 patients with AGL, all had well-preserved bone marrow fat [17]. Cystic bone lesions were not seen in any of these patients. It is unknown whether intramedullary adipose destruction is present in the rare patients with AGL in which cystic bone lesions have been identified [6, 13, 19]. We hypothesize the lower incidence of development of cystic bone lesions in patients with AGL could reflect the general lack of intramedullary fat involvement in this disorder. In the rare patients with AGL who do have bone cysts develop, perhaps unidentified or subclinical bone marrow fat destruction is a contributing factor.
Traditional methods of treatment for UBCs have included observation, open curettage and bone grafting, and steroid injections. Some techniques have integrated cyst decompression as a treatment, reflecting the hypothesis that obstructed venous outflow may be a cause of UBCs [5, 7]. Additionally, some techniques have begun to use bone graft substitutes such as demineralized bone matrix or MGCS to fill benign bone defects [3, 14]. Treatment of UBCs is difficult, and recurrence is frequent, regardless of the method used [2]. Given this high recurrence rate, indications for surgical intervention generally are limited to critical weightbearing areas or to prevent an impending pathologic fracture. In our patient, although the right UBC was asymptomatic, it appeared very similar, radiographically, to the symptomatic, and therefore likely functionally unstable, left side. For this reason, we believed bilateral surgery was appropriate. We used a minimally invasive percutaneous technique as previously described [9]. This technique combines the healing benefits of intramedullary decompression, curettage, and grafting with the lower morbidity of a percutaneous procedure and the decreased donor site morbidity from the use of MGCS pellets [9]. However, regardless of surgical technique, recurrence of UBCs is common [2, 9, 16].
Development of lytic bone lesions in AGL is rare, and these lesions have not been described or diagnosed. We reported a case of bilateral cystic lesions in a patient with AGL and, for the first time, diagnosed them as UBCs. We used histology and advanced imaging techniques to confirm our diagnosis and described the use of a newer technique of UBC treatment in this patient. We identified cystic bone lesions can be an important manifestation of AGL and observed these lesions may be less common in AGL than in CGL. We suggest intramedullary adipose tissue loss in patients with AGL may be a predisposing factor for the development of bone lesions. We encourage additional case collection to determine the coexistence between UBCs and AGL.
Acknowledgments
We thank The Children’s Hospital of Philadelphia and the clinical research coordinators in the Department of Orthopaedics for their hard work.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case, that all the investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at The Children’s Hospital of Philadelphia.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11999-010-1371-z
References
- 1.Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O’Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NH, Bindl L, Herbst K, Kennel K, Patel SB, Al-Gazali L, Garg A. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88:4840–4847. doi: 10.1210/jc.2003-030855. [DOI] [PubMed] [Google Scholar]
- 2.Baig R, Eady JL. Unicameral (simple) bone cysts. South Med J. 2006;99:966–976. doi: 10.1097/01.smj.0000235498.40200.36. [DOI] [PubMed] [Google Scholar]
- 3.Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986;204:25–36. [PubMed] [Google Scholar]
- 4.Chandalia M, Garg A, Vuitch F, Nizzi F. Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1995;80:3077–3081. doi: 10.1210/jc.80.10.3077. [DOI] [PubMed] [Google Scholar]
- 5.Chigira M, Maehara S, Arita S, Udagawa E. The aetiology and treatment of simple bone cysts. J Bone Joint Surg Br. 1983;65:633–637. doi: 10.1302/0301-620X.65B5.6643570. [DOI] [PubMed] [Google Scholar]
- 6.Coelho PC, Jordao A, Andre O, Queiroz MV, Eurico-Lisboa P. Rheumatological manifestations of lipoatrophic diabetes. Clin Rheumatol. 1995;14:229–230. doi: 10.1007/BF02214953. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Simple bone cysts: studies of cyst fluid in six cases with a theory of pathogenesis. J Bone Joint Surg Am. 1960;42:609–616. [PubMed] [Google Scholar]
- 8.Wazieres B, Wendling D, Test T, Morin G, Dupond JL. [Bone and visceral manifestations of lipoatrophic diabetes: apropos of a case] [in French] Rev Rheum Mal Osteoartic. 1992;59:761–764. [PubMed] [Google Scholar]
- 9.Dormans JP, Sankar WN, Moroz L, Erol B. Percutaneous intramedullary decompression, curettage, and grafting with medical-grade calcium sulfate pellets for unicameral bone cysts in children: a new minimally invasive technique. J Pediatr Orthop. 2005;25:804–811. doi: 10.1097/01.bpo.0000184647.03981.a5. [DOI] [PubMed] [Google Scholar]
- 10.Fleckenstein JL, Garg A, Bonte FJ, Vuitch MF, Peshock RM. The skeleton in congenital generalized lipodystrophy: evaluation using whole-body radiographic surveys, magnetic resonance imaging and technetium-99 m bone scintigraphy. Skeletal Radiol. 1992;21:381–386. doi: 10.1007/BF00241817. [DOI] [PubMed] [Google Scholar]
- 11.Garg A. Lipodystrophies. Am J Med. 2000;108:143–152. doi: 10.1016/S0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 12.Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety) J Clin Endocrinol Metab. 1999;84:170–174. doi: 10.1210/jc.84.1.170. [DOI] [PubMed] [Google Scholar]
- 13.Goebel FD, Dorfler H, Goebel HH, Zollner N. Muscle capillary basement membrane thickness in lipoatrophic diabetes. Res Exp Med (Berl) 1977;171:271–276. doi: 10.1007/BF01851511. [DOI] [PubMed] [Google Scholar]
- 14.Inoue O, Ibaraki K, Shimabukuro H, Shingaki Y. Packing with high-porosity hydroxyapatite cubes alone for the treatment of simple bone cyst. Clin Orthop Relat Res. 1993;293:287–292. [PubMed] [Google Scholar]
- 15.Lodwick GS, Wilson AJ, Farrell C, Virtama P, Smeltzer FM, Dittrich F. Determining growth rates of focal lesions of bone from radiographs. Radiology. 1980;134:577–583. doi: 10.1148/radiology.134.3.6928321. [DOI] [PubMed] [Google Scholar]
- 16.Mik G, Arkader A, Manteghi A, Dormans JP. Results of a minimally invasive technique for treatment of unicameral bone cysts. Clin Orthop Relat Res. 2009;467:2949–2954. doi: 10.1007/s11999-009-1008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003;82:129–146. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Westvik J. Radiological features in generalized lipodystrophy. Acta Paediatr Suppl. 1996;413:44–51. doi: 10.1111/j.1651-2227.1996.tb14265.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson DE, Chan IF, Stevenson KB, Horton SC, Schipke C. Eucaloric substitution of medium chain triglycerides for dietary long chain fatty acids in acquired total lipodystrophy: effects on hyperlipoproteinemia and endogenous insulin resistance. J Clin Endocrinol Metab. 1983;57:517–523. doi: 10.1210/jcem-57-3-517. [DOI] [PubMed] [Google Scholar]