Abstract
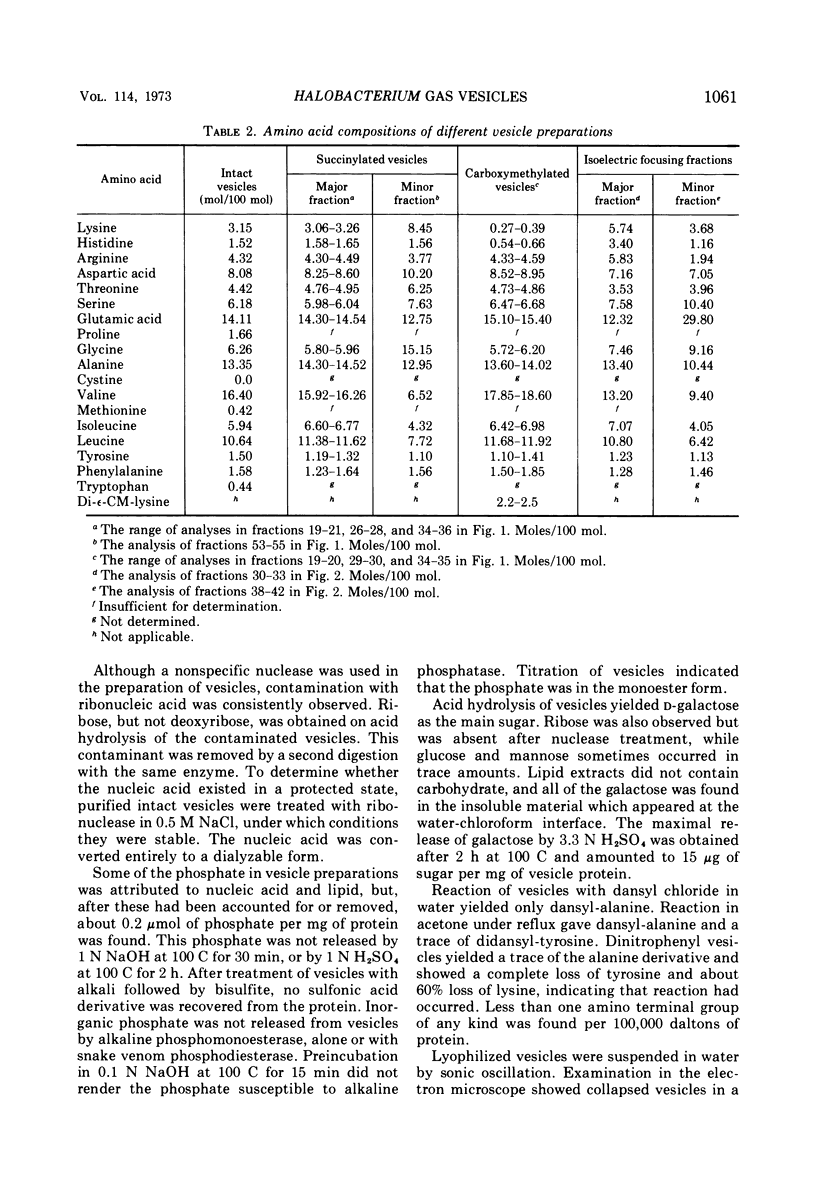
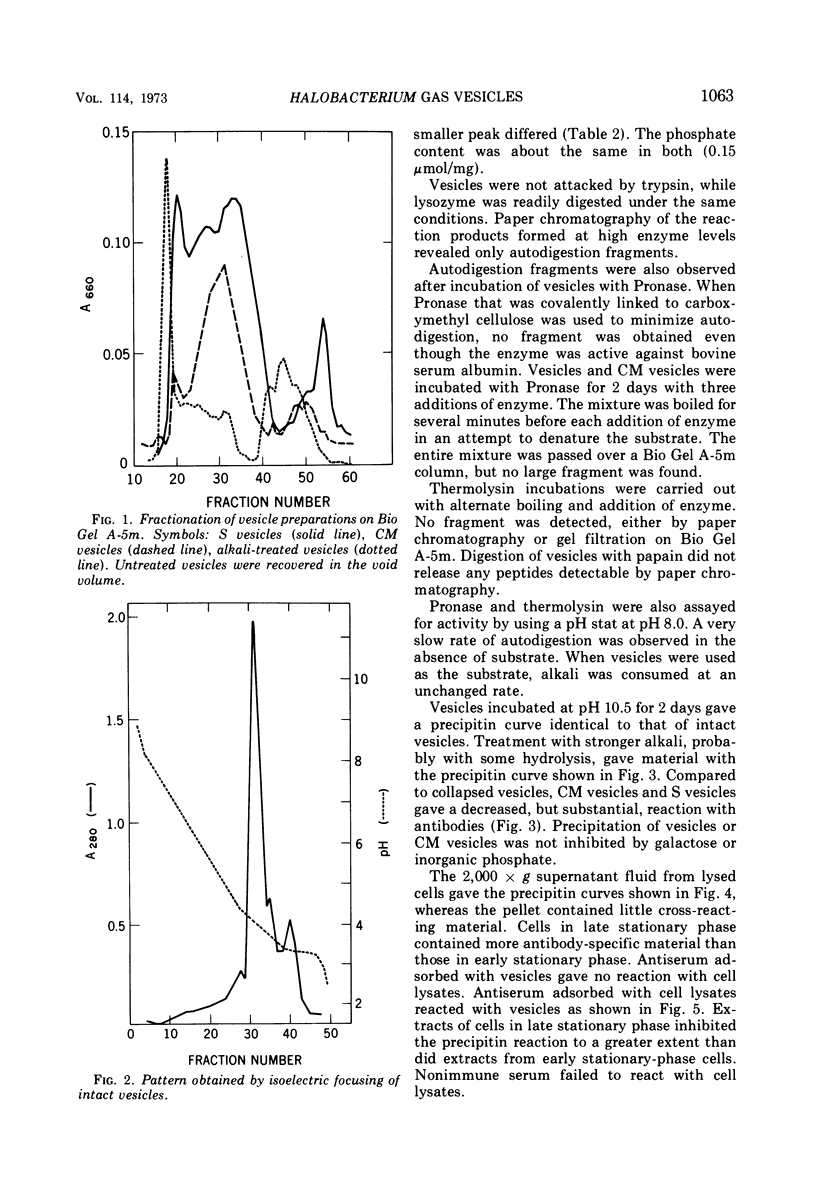
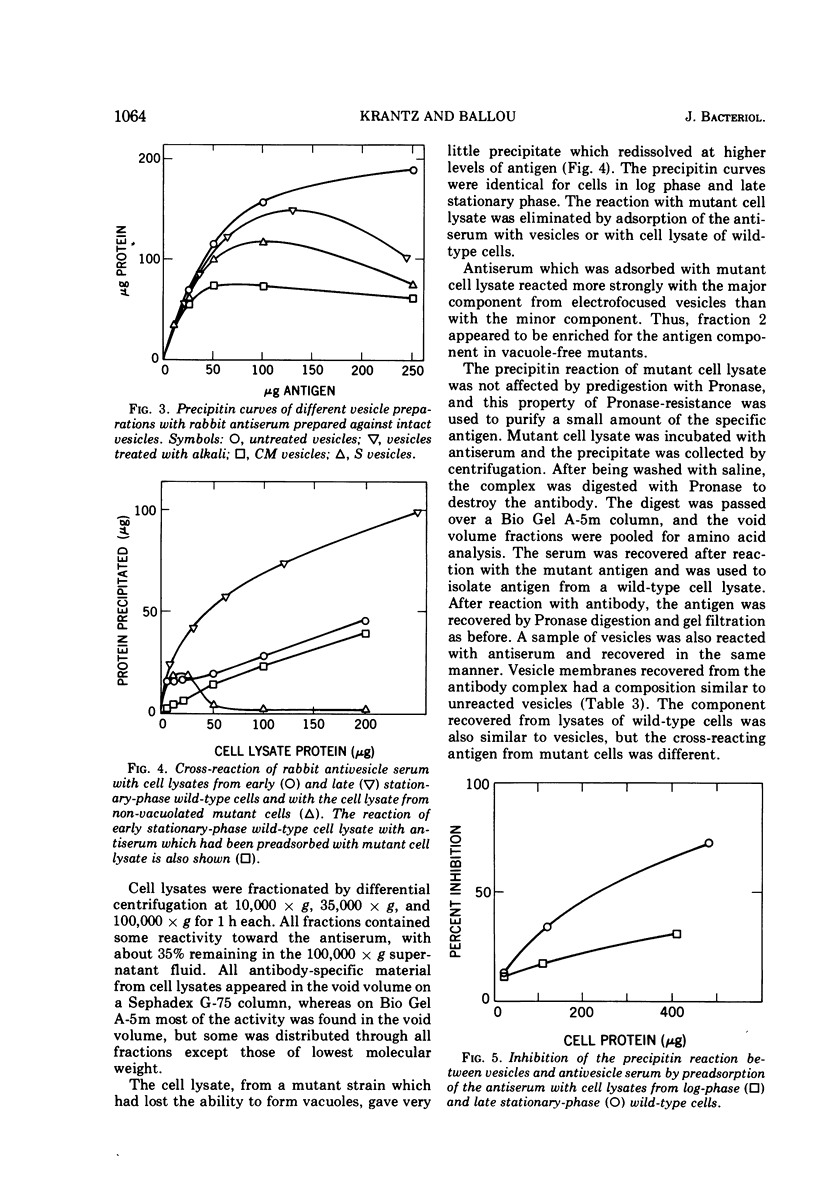
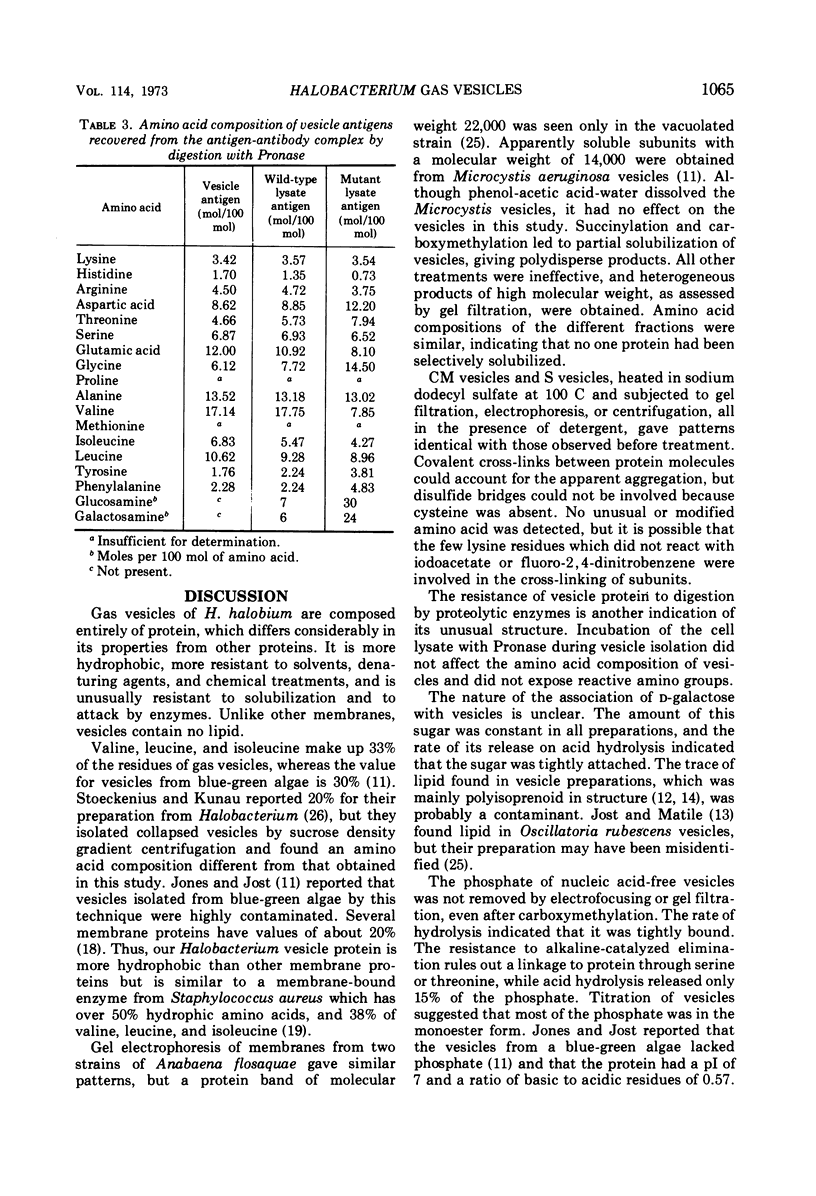
Gas vesicles, isolated from lysed Halobacterium halobium cells, gave an amino acid analysis which accounted for 78% of the weight, and the balance was mainly salt and water. One percent of tightly bound d-galactose was found, as well as 2% of phosphate that was not released by treatment which promotes β-elimination, by hydrolytic release of the galactose, by carboxymethylation of lysine, or by alkaline phosphatase digestion. Only a trace of lipid was detected, and it appeared to have a polyisoprenoid structure. The vesicles were not solubilized by extremes of pH, by agents such as urea, guanidine hydrochloride, formic acid, and detergents, or by organic solvents. Succinylation and carboxymethylation gave partial dispersion, but the products were heterogeneous and of high molecular weight. The amino acid composition of vesicles was independent of fragment size. No band was obtained by polyacrylamide gel electrophoresis, with neutral, acidic, and alkaline systems, with or without sodium dodecyl sulfate and urea, before or after chemical modification. No amino terminus was detected. Electrofocusing of a vesicle dispersion showed a major component with a pI of 4.0 and an amino acid composition of the whole vesicles, and a minor band with pI 3.4 which had an amino acid composition different from whole vesicles. Vesicle protein was resistant to digestion by Pronase, trypsin, thermolysin, and papain. The precipitin reaction with rabbit antivesicle serum was not inhibited by galactose or inorganic phosphate. Succinylated and carboxymethylated vesicles cross-reacted with antivesicle serum. Cell lysates contained material which reacted with antiserum, but it was heterogeneous and mainly larger than 5 × 106 daltons. Material from nonvacuolated mutants reacted weakly with antiserum, but the amino acid composition of the precipitated antigen was different from that of vesicles and of soluble cross-reacting material from vacuolated cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bowen C. C., Jensen T. E. Blue-Green Algae: Fine Structure of the Gas Vacuoles. Science. 1965 Mar 19;147(3664):1460–1462. doi: 10.1126/science.147.3664.1460. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature. 1954 Nov 13;174(4437):930–931. doi: 10.1038/174930b0. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- HOUWINK A. L. Flagella, gas vacuoles and cell-wall structure in Halobacterium halobium; an electron microscope study. J Gen Microbiol. 1956 Aug;15(1):146–150. doi: 10.1099/00221287-15-1-146. [DOI] [PubMed] [Google Scholar]
- Holmes P. K., Dundas I. E., Halvorson H. O. Halophilic enzymes in cell-free extracts of Halobacterium salinarium. J Bacteriol. 1965 Oct;90(4):1159–1160. doi: 10.1128/jb.90.4.1159-1160.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. D., Jost M. Isolation and chemical characterization of gas-vacuole membranes from Microcystis aeruginosa Kuetz. emend. Elenkin. Arch Mikrobiol. 1970;70(1):43–64. doi: 10.1007/BF00691059. [DOI] [PubMed] [Google Scholar]
- Joo C. N., Kates M. Synthesis of the naturally occurring phytanyl diether analogs of phosphatidyl glycerophosphate and phosphatidyl glycerol. Biochim Biophys Acta. 1969 Mar 4;176(2):278–297. doi: 10.1016/0005-2760(69)90186-6. [DOI] [PubMed] [Google Scholar]
- Jost M., Matile P. Zur Charakterisierung der Gasvacuolen der Blaualge Oscillatoria rubescens. Arch Mikrobiol. 1966 Feb 1;53(1):50–58. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Mazia D., Ruby A. Dissolution of erythrocyte membranes in water and comparison of the membrane protein with other structural proteins. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1005–1012. doi: 10.1073/pnas.61.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAM E., MOORE S., BIGWOOD E. J. Chromatographic determination of cystine as cysteic acid. Biochem J. 1954 May;57(1):33–37. doi: 10.1042/bj0570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. C 55 -isoprenoid alcohol phosphokinase: an extremely hydrophobic protein from the bacterial membrane. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2441–2443. doi: 10.1073/pnas.68.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968 Sep;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Siakotos A. N. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc. 1965 Nov;42(11):913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Peat A., Bailey C. J. The isolation and characterisation of gas-cylinder membranes and alpha-granules from Anabaena flos-aquae D 124. Arch Mikrobiol. 1969;65(2):87–97. doi: 10.1007/BF00693312. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Rowen R. A morphological study of Halobacterium halobium and its lysis in media of low salt concentration. J Cell Biol. 1967 Jul;34(1):365–393. doi: 10.1083/jcb.34.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., MacLennan D. H., Tzagoloff A., Stoner C. D. Studies on the electron transfer system. LXVII. Polyacrylamide gel electrophoresis of the mitochondrial electron transfer complexes. Arch Biochem Biophys. 1966 Apr;114(1):223–230. doi: 10.1016/0003-9861(66)90324-9. [DOI] [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaland J. R., Branton D. Gas vacuole development in a blue-green alga. Science. 1969 Mar 21;163(3873):1339–1341. doi: 10.1126/science.163.3873.1339. [DOI] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


