Abstract
Background
Osteoarthritis arising from cartilage degeneration is the most common cause of joint pain. However, the relationship between joint pain and cartilage degeneration is not well understood.
Questions/Purposes
We asked whether the inflammatory mediators participate in the joint pain in the presence of cartilage degeneration.
Methods
We observed electromyographic responses of hindlimb flexors to four inflammatory mediators (bradykinin, ATP, acetylcholine, and serotonin) injected in normal rat knees and in those with monosodium iodoacetate (MIA)-induced arthritis.
Results
Joint cartilage of all the rats with MIA-induced arthritis histologically showed severe degeneration. We observed greater magnitude and longer duration responses in the MIA-induced arthritis than normal joints with all four mediators.
Conclusions
The data suggested nociceptors in osteoarthritic joints are more sensitive to inflammatory mediators than in normal joints. Such nociceptive sensitization to inflammatory mediators may participate in the joint pain in osteoarthritis.
Introduction
Osteoarthritis is the most frequent and painful musculoskeletal disease. In general, osteoarthritis is characterized by progressive cartilage degeneration. However, the relationship between joint pain and cartilage degeneration is not well understood and the cartilage is not innervated by sensory nerve endings. However, the synovial fluid contains various inflammatory mediators such as bradykinin (BK), adenosine triphosphate (ATP), acetylcholine (Ach), and serotonin [21], and these are believed to directly stimulate nociceptors of sensory nerve terminals and participate in the process of pain generation [20, 24].
The relationship between chemical mediators and joint pain has been suggested in previous studies. BK has been identified in synovial effusions of human joint disease [16]. Local application of BK sensitizes afferent fibers innervating a cat knee [12]. Several reports suggest Ach is also a mediator [5, 11, 24] and the application of Ach increased excitability of C-fiber axons [15]. ATP is also reportedly an inflammatory mediator [4, 8, 19] and the concentration of products from extracellular ATP catabolism correlates with the degree of cartilage degeneration [19]. Serotonin activates nociceptive fine articular afferents [9, 21] and nociceptive receptors in arthritic joints are affected by serotonin [1, 2].
Pain evaluation in animals has been performed mostly by observation of behavioral changes induced by heat [10] and mechanical stimulation [12]. However, behavioral changes are subject to some interpretation. Yamashita et al. [25] reported intraarticular chemical nociceptive receptors are stimulated by the injection of BK. Other investigators also have used nociceptive flexion reflex as a tool to assess pain [6, 22, 23], because nociceptive joint receptor afferents increase the excitability of flexion reflex pathways [6]. We believe EMG responses may provide a more objective way to assess pain response than do behavioral changes.
We therefore determined whether (1) injection of mediators in a joint would elicit flexor reflex EMG patterns, (2) injection of mediators in a degenerated joint would cause a greater EMG signal magnitude than in a normal joint, and (3) injection of mediators in a degenerated joint would cause a greater EMG signal duration than in a normal joint.
Materials and Methods
We used 48 adult male Wistar rats (200–250 g) (Fig. 1). To generate a cartilage degeneration model in rat knees, we used a monosodium iodoacetate (MIA)-induced arthritis model as this model manifests human osteoarthritis-like cartilage degeneration by histologic analysis [3, 13]. Twenty-five microliters of MIA solution at a concentration of 60 mg/mL was injected in the right knee of 24 animals (MIA group). The other 24 animals were injected with 25 μL of physiologic saline (control group). After the injection, the animals were maintained in separate cages and allowed free movement and free access to food and water. Fourteen days later, we conducted the following experiment. To evaluate the degree of induced joint pain in animals, EMG responses of hindlimb flexors through the flexion withdrawal reflex were analyzed [22, 23]. All animal procedures were reviewed and approved by the Committee of Asahikawa Medical College for Laboratory Animal Care and Use and complied with its guidelines.
Fig. 1.
A flow chart shows the numbers of rats given each chemical in the control and the monosodium iodoacetate (MIA) groups.
The animals were anesthetized with 3% halothane. After tracheal cannulation and decerebration by suction ablation at the level of the pons, the anesthesia was discontinued and animals were respirated with room air [18]. A pair of stainless wire electrodes (50 μm in diameter, 5 mm apart) as a lead for EMG was inserted into the belly of the knee flexor muscle (biceps femoris). Under direct vision of the central part of the muscle belly from a small skin incision, the tips of the electrodes were placed at a depth of 3 mm from the fasciae. The hindlimb was fixed at a slightly knee-flexed position. The tip of a thin flexible needle was inserted in the knee cavity for mediator injection. After waiting 3 minutes for the EMG signal to return to baseline after insertion, 25 μL of a mediator was injected using a microsyringe. For chemical application, a 27-gauge needle connected to an injection syringe with an extension tube was used, and the tip of the needle was inserted in the patellofemoral joint space through a patellar tendon. The following mediators were tested: (1) BK: 1.25 μg; (2) ATP: 12.5 μg; (3) Ach: 25 μg; and (4) serotonin: 12.5 μg. The dose of each mediator was the minimal dose for which EMG activities consistently appeared in the normal knee [17]. In the normal and MIA groups, six animals were given one of the four mediators. One injection and observation was performed per joint.
EMG signals elicited by each mediator were recorded using an electrodiagnostic system (Counterpoint; Dantec Co, Skuvlunde, Denmark). Raw EMG signal was full-wave rectified and integrated with a 3 Hz low-pass filter, finally processed as a linear envelope waveform in which the height reflected the degree of muscle activity. We observed EMG signals and their integrated wave shapes using an oscilloscope and recorded all signals on paper simultaneously.
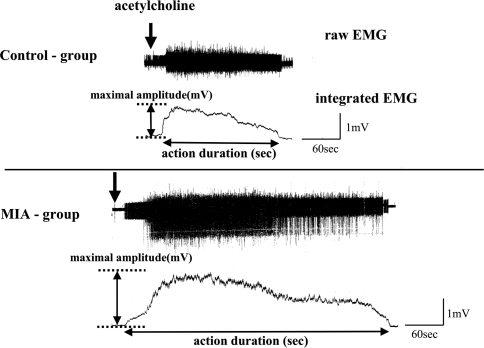
Using the integrated EMG waveforms, we recorded the maximal amplitude (mV) and action duration (seconds) for all responses to each mediator. The maximal amplitude (mV) was the difference measured between the maximal height during the response and the preinjection level. Action duration (seconds) was the time of the response between the onset and the point returning to the preinjection level (Fig. 2).
Fig. 2.
Representative responses to acetylcholine in the control and the monosodium iodoacetate (MIA) groups are shown. Using an integrated EMG waveform, the maximal amplitude (mV) and action duration (seconds) were measured for all the responses to the mediators.
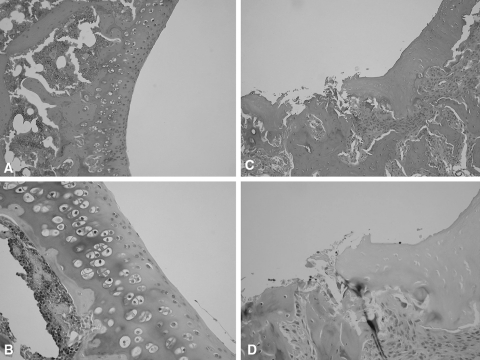
After the measurements, the rats were euthanized with an excess dosage of sodium pentobarbital through an intraperitoneal injection. We dissected all knees and placed them in 10% neutral formalin. We then prepared 5-μ thin sections in a sagittal plane at 1-mm intervals. The sections were stained with hematoxylin-eosin and Safranin-O. And we examined the sections for cartilage degeneration using light microscopy. All the sections obtained from the MIA group showed irregular surfaces and ulceration on the femoral and tibial sides. In contrast, none of the sections obtained from the control group showed such changes (Fig. 3).
Fig. 3A–D.
(A) No abnormality is present in this normal rat knee (Stain, hematoxylin and eosin; original magnification, ×10), (B) or this normal rat knee (Stain, Safranin-O; original magnification, ×20). (C) The irregular surface of the joint cartilage and exposure of subchondral bone are evident in this monosodium iodoacetate (MIA) rat knee (Stain, hematoxylin and eosin; original magnification, ×10). (D) Ulceration and fibrillation of the cartilage with inflammatory cell infiltration are present in this MIA rat knee (Stain, Safranin-O; original magnification, ×20).
Using an unpaired t-test, we compared the maximal amplitudes and action durations for each chemical mediator between the control and the MIA groups to assess whether there is a difference in the values resulting from the presence of cartilage degeneration. Statistical analysis was performed using StatView Version 5.0 (SAS Institute, Cary, NC). The level for statistical significance was set at a value of p < 0.05.
Results
Changes in EMG activities were elicited in all the animals after application of each mediator. The common reaction pattern was observed in which the EMG activity increased within a few seconds after injection, reached the maximum quickly, and then gradually decreased, returning to the preinjection level (Fig. 2).
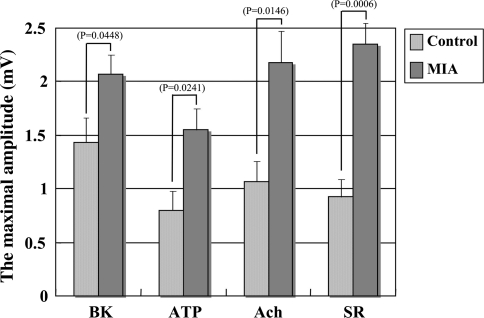
All maximal amplitudes were greater after injection of the mediators in the MIA group compared with those in the control group (Fig. 4). The maximal amplitude by BK was greater (p = 0.0448) in the MIA group (mean value: 2.07 mV) than in the control group (mean value: 1.43 mV). The maximal amplitude by ATP also was greater (p = 0.0241) in the MIA group (mean value: 1.55 mV) than in the control group (mean value: 0.80 mV). That by Ach was greater (p = 0.0146) in the MIA group (mean value: 2.18 mV) than in the control group (mean value: 1.07 mV) and that by serotonin also was greater (p < 0.001) in the MIA group (mean value: 2.35 mV) than in the control group (mean value: 0.93 mV).
Fig. 4.
A comparison of the maximal amplitudes (mV) for each mediator between the control and the monosodium iodoacetate (MIA) groups is shown. BK = bradykinin; ATP = adenosine triphosphate; Ach = acetylcholine; SR = serotonin.
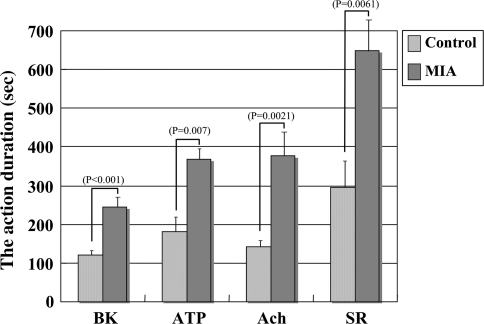
All action durations were longer after injection of the mediators in the MIA group compared with those in the control group (Fig. 5). The action duration by BK was longer (p < 0.001) in the MIA group (mean value: 245.0 seconds) than in the control group (mean value: 121.7 seconds). That by ATP also was longer (p = 0.007) in the MIA group (mean value: 366.7 seconds) than in the control group (mean value: 180.0 seconds). The action duration by Ach was longer (p = 0.0021) in the MIA group (mean value: 376.7 seconds) than in the control group (mean value: 143.3 seconds), and that by serotonin was longer (p = 0.0061) in the MIA group (mean value: 648.3 seconds) than in the control group (mean value: 296.7 seconds).
Fig. 5.
A comparison of the action duration (seconds) for each mediator between the control and the monosodium iodoacetate (MIA) groups is shown. BK = bradykinin; ATP = adenosine triphosphate; Ach = acetylcholine; SR = serotonin.
Discussion
Joint pain in osteoarthritis is derived mainly from cartilage degeneration. However, the relationship between joint pain and cartilage degeneration is not well understood. We therefore determined whether (1) injection of mediators in a joint would elicit flexor reflex EMG patterns, (2) injection of mediators in a degenerated joint would cause a greater EMG signal magnitude than in a normal joint, and (3) injection of mediators in a degenerated joint would cause a greater EMG signal duration than in a normal joint.
We acknowledge several limitations of our study design and experiment. First, we could not distinguish the effects of cartilage degeneration and the inflammation on the EMG responses in our experimental arthritic model. However, the two typically occur together clinically. Second, we did not examine the degree of joint degeneration in histologic examinations, nor did we attempt to correlate the degree of the degeneration with the EMG responses; rather, with the small numbers of animals in each subgroup, we considered this a pilot study to simply determine whether the responses were greater in the MIA animals with the various mediators.
Two reports describe neurobiologic mechanisms of arthralgia [7, 20]. Numerous sensory nerves are distributed in the synovial lining, joint capsules, and ligaments [21]. They include polymodal receptors in the free nerve endings, which are sensitive to mechanical stimuli and chemical mediators [14]. In the final stage of the pain generation process, electrical responses are evoked as afferent action potentials, which finally cause pain. In our control group, we observed EMG activities after application of all four mediators, which confirms receptors for these mediators exist on the free nerve endings in the joint cavity. Using our experimental methods, we evaluated changes in sensitivity of intraarticular polymodal receptors to exogenously applied chemical mediators.
Two previous experimental studies of joint pain were performed with an acute inflammatory arthritis [2, 18] and thus did not explore the relationship of pain and cartilage degeneration. We used a MIA-induced arthritis model to evaluate the influence of cartilage degeneration. MIA is an inhibitor of glycolysis, disrupts chondrocyte metabolism, and induces cartilage degeneration. MIA-induced arthritis has similar histopathologic characteristics as human osteoarthritis [3, 13]. The EMG responses to all the mediators used in our study were augmented when the mediators were applied to the MIA group. The data suggest the sensitivity of polymodal receptors in a knee is increased in the presence of cartilage degeneration. Such hypersensitivity of polymodal receptors may be based on existence of other mediators in osteoarthritis such as prostaglandins [21].
We observed pain-generating properties of four chemical mediators by analyzing EMG responses using rat knees. MIA-induced arthritis had major effects on the mediator sensitivities of joint polymodal receptors. Such changes may participate in the generation of joint pain in osteoarthritis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alstergren P, Kopp S. Pain and synovial fluid concentration of serotonin in arthritic temporomandibular joints. Pain. 1997;72:137–143. doi: 10.1016/S0304-3959(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 2.Birrell GJ, McQueen DS, Iggo A, Grubb BD. The effects of 5-HT on articular sensory receptors in normal and arthritic rats. Br J Pharmacol. 1990;101:715–721. doi: 10.1111/j.1476-5381.1990.tb14146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004;370:236–240. doi: 10.1016/j.neulet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Dowd E, McQueen DS, Chessell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan CG, Lockhart JC, Ferrell WR. Pathophysiology of vascular dysfunction in a rat model of chronic joint inflammation. J Physiol. 2004;557:635–643. doi: 10.1113/jphysiol.2004.062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrero AT, Verri WA, Jr, Cuna TM, Silva TA, Rocha FA, Ferreira SH, Cunha FQ, Parada CA. Hypernociception elicited by tibio-tarsal joint flexion in mice: a novel experimental arthritis model for pharmacological screening. Pharmacol Biochem Behav. 2006;84:244–251. doi: 10.1016/j.pbb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Gwilym SE, Pollard TC, Carr AJ. Understanding pain in osteoarthritis. J Bone Joint Surg Br. 2008;90:280–287. doi: 10.1302/0301-620X.90B3.20167. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton SG, McMahon SB. ATP as peripheral mediator of pain. J Auton Nerv Syst. 2000;81:187–194. doi: 10.1016/S0165-1838(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 9.Herbert MK, Schmidt RF. Activation of normal and inflamed fine articular afferent units by serotonin. Pain. 1992;50:79–88. doi: 10.1016/0304-3959(92)90115-R. [DOI] [PubMed] [Google Scholar]
- 10.Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9:463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- 11.Juan H, Lembeck F. Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn Schmiedebergs Arch Pharmacol. 1974;283:151–164. doi: 10.1007/BF00501142. [DOI] [PubMed] [Google Scholar]
- 12.Kanaka R, Schaible HG, Schmidt RF. Activation of fine articular afferent units by bradykinin. Brain Res. 1985;327:81–90. doi: 10.1016/0006-8993(85)91501-X. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, Komatsu H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003;65:1195–1199. doi: 10.1292/jvms.65.1195. [DOI] [PubMed] [Google Scholar]
- 14.Kumazawa T. The polymodal receptor: bio-warning and defense system. Prog Brain Res. 1996;113:3–18. doi: 10.1016/S0079-6123(08)61078-X. [DOI] [PubMed] [Google Scholar]
- 15.Lang PM, Burgstahler R, Sippel W, Irnich D, Schlotter-Weigel B, Grafe P. Characterization of neuronal nicotinic acetylcholine receptors in the membrane of unmyelinated human c-fiber axons by in vitro studies. J Neurophysiol. 2003;90:3295–3303. doi: 10.1152/jn.00512.2003. [DOI] [PubMed] [Google Scholar]
- 16.Melmon KL, Webster ME, Goldfinger SE, Seegmiller JE. The presence of a kinin in inflammatory synovial effusion from arthritides of varying etologies. Arthritis Rheum. 1967;10:13–20. doi: 10.1002/art.1780100103. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto M, Atsuta Y, Machida K. Pain-generating properties of chemical mediators in normal and osteoarthritic knee joints of rats. Transactions of the 52nd Annual Meeting of the Orthopaedic Research Society. Paper No 1533. 2006:113. Available at: http://www.ors.org/web/Transactions/52/1533.PDF. Accessed November 30, 2009.
- 18.Okamoto T, Atsuta Y, Shimazaki S. Sensory afferent properties of immobilised or inflamed rat knees during continuous passive movement. J Bone Joint Surg Br. 1999;81:171–177. doi: 10.1302/0301-620X.81B1.8566. [DOI] [PubMed] [Google Scholar]
- 19.Park W, Masuda I, Cardenal-Escarcena A, Palmer DL, McCarty DJ. Inorganic pyrophosphate generation from adenosine triphosphate by cell-free human synovial fluid. J Rheumatol. 1996;23:665–671. [PubMed] [Google Scholar]
- 20.Schaible HG, Ebersberger A, Banchet GS. Mechanisms of pain in arthritis. Ann NY Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 21.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- 22.Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans: review article. Pain. 2002;96:3–8. doi: 10.1016/S0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 23.Willer JC. Nociceptive flexion reflexes as a tool for pain research in man. Adv Neurol. 1983;39:809–827. [PubMed] [Google Scholar]
- 24.Wood JN, Docherty R. Mediator activators of sensory neurons. Annu Rev Physiol. 1997;59:457–482. doi: 10.1146/annurev.physiol.59.1.457. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita I, Atsuta Y, Shimazaki S, Miyatsu M. [Effects of prostaglandin E2 and sodium hyaluronate on bradykinin induced knee joint pain in rat] [in Japanese] Nippon Seikeigeka Gakkai Zasshi. 1995;69:735–743. [PubMed] [Google Scholar]