Abstract
Osteolysis secondary to polyethylene wear is one of the major factors limiting long-term performance of TKA. Oxidized zirconium is a new material that combines the strength of a metal with the wear properties of a ceramic. It remains unknown whether implants with a zirconium femoral component can be used safely in TKA. To answer that question, we reviewed, at a minimum of 5 years, the clinical outcome and survivorship of a ceramic-surfaced oxidized zirconium femoral component implanted during 98 primary TKAs between April 2001 and December 2003. Survivorship was 98.7% at 7 years postoperatively. No revision was necessary and only one component failed because of aseptic loosening. Mean Knee Society score improved from 36 to 89. No adverse events were observed clinically or radiologically. These results justify pursuing the use of oxidized zirconium as an alternative bearing surface for a femoral component in TKA.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
One of the major factors limiting long-term performance of TKA is the osteolysis secondary to polyethylene (PE) wear. Therefore, reduction in the amount of wear debris released to periprosthetic tissues can be one of the most effective ways to improve longevity of total joint arthroplasties and to minimize bone loss. During the past 2 decades, there have been numerous attempts to decrease the wear of PE in knee arthroplasty prostheses, but efforts have focused primarily on improving implant design and quality of ultrahigh-molecular-weight PE [10, 12, 20, 25, 28].
However, as is the case for THA, a new approach to decrease PE wear could be to change the countersurface that articulates with PE. Because it is more difficult to adapt “hard on hard” technology like ceramics for TKA than for THA, a new metal alloy, oxidized zirconium (Zr), has been developed for the femoral component of TKA. This oxidized Zr femoral component is expected to result in less PE wear of the tibial component than femoral components composed of cobalt-chromium (Co-Cr) alloy [27]. Thermally driven oxygen diffusion transforms the metallic Zr alloy surface into a durable low-friction oxide [9]. The oxide layer is not a coating, but rather the surface zone of the metal alloy [24], conferring bearing properties of ceramic without the fracture risk [11]. Several in vitro studies have indicated oxidized Zr hip and knee implants have excellent wear resistance, which is significantly greater than that of Co-Cr [2, 9, 26, 28]. A study by the manufacturer under abrasive conditions showed oxidized Zr was less affected by the roughening procedure used than Co-Cr and that the wear of PE by oxidized Zr was considerably less than by Co-Cr [22]. An independent laboratory study showed oxidized Zr, when used for the femoral component of a prosthesis, reduced wear of tibial PE inserts by approximately 42% compared with identical femoral components made of a Co-Cr alloy [6]. Furthermore, the friction between cartilage articulating this material and a metallic femoral trochlear surface, a situation that occurs when the patella is not resurfaced during TKA, has been studied. The coefficient of friction of the cartilage against oxidized Zr was 30% less than that of the cast Co-Cr-Mo [22].
An additional advantage of an oxidized Zr femoral component is that it has been shown to be safe in patients with nickel sensitivity because there is no traceable nickel in this material [13].
However, it is well known that preclinical laboratory testing of materials is not entirely reliable, sometimes leading to catastrophic failure, and that proof for all new technologies must come from clinical trials. The first aim of this study was to evaluate the 5-year clinical outcome, as defined by the Knee Society score, and the patellofemoral function of these TKAs. The secondary purpose was to assess the 7-year survivorship as gleaned from the radiographic appearance of a series of primary TKAs with a femoral component made of oxidized Zr.
Materials and Methods
After approval by the Institutional Review Board, we reviewed the results of a selected series of 98 TKAs performed in 94 patients with an oxidized Zr femoral component (Genesis II®, OxiniumTM; Smith & Nephew, Memphis, TN) at the authors’ institution from April 2001 to December 2003. This study was performed in accordance with the Declaration of Helsinki. All patients gave informed consent before inclusion in the study.
We considered patients candidates for oxidized Zr based primarily on age (younger than 65 years), relatively high activity levels, and overall health status as judged by the surgeon, but older patients also were considered if they were sensitive to nickel. Exclusion criteria were patients with low functional demands and patients with knees with severe global instability.
Three patients (three knees) were lost to followup at a minimum of 2 years (mean, 2.5 years; range, 2–4 years) after the operation. These patients had no symptoms or radiologic evidence of loosening at the time of the last followup. Overall, 95 knees (91 patients) were available for inclusion in the current study with a minimum followup of 5 years (mean, 6.2 years; range, 5–7 years).
The mean age of the patients was 58.8 years (range, 36–78 years). Thirty-two knees were from males and 63 were from females. The preoperative diagnoses were osteoarthritis in 78 patients (82 knees [86.3%]), hemophilic arthropathy in five patients (five knees [5.2%]), posttraumatic arthritis in three patients (three knees [3.2%]), osteonecrosis in three patients (three knees [3.2%]), and rheumatoid arthritis in two patients (two knees [2.1%]).
The Genesis II® is a modular implant whose tibial component is modular with a ram-extruded, ethylene oxide sterilized PE insert in a titanium-aluminum-vanadium alloy baseplate. The baseplate is asymmetric so as to match the cut surface of the tibia. In five patients (5.2%) who were nickel-sensitive, we used an all-PE nonmodular tibial component of the same design. The femoral component geometry is such that flexion space filling is obtainable without having to externally rotate the component but specifically designed with a thicker posterolateral femoral condyle than a posteromedial femoral one. The femoral component has a deeper and more lateralized trochlear groove to improve patellar contact and tracking. The other characteristics of the implant were described previously [18].
Oxidized Zr is composed of Zr (97.5%) and niobium (2.5%). It is produced by submitting the alloy to heat in air to greater than 500°C. Thermal oxidation occurs, and as the oxygen diffuses through the alloy, the immediate surface oxidizes into a Zr ceramic approximately 5 μm thick. The alloy immediately underlying the ceramic surface has a high oxygen concentration and this gradually decreases until the alloy is just composed of the two base materials. This does not result in a coated surface treatment, but rather in a gradual transition of the material and its properties; the finished product is a stable monolithic crystalline structure [17].
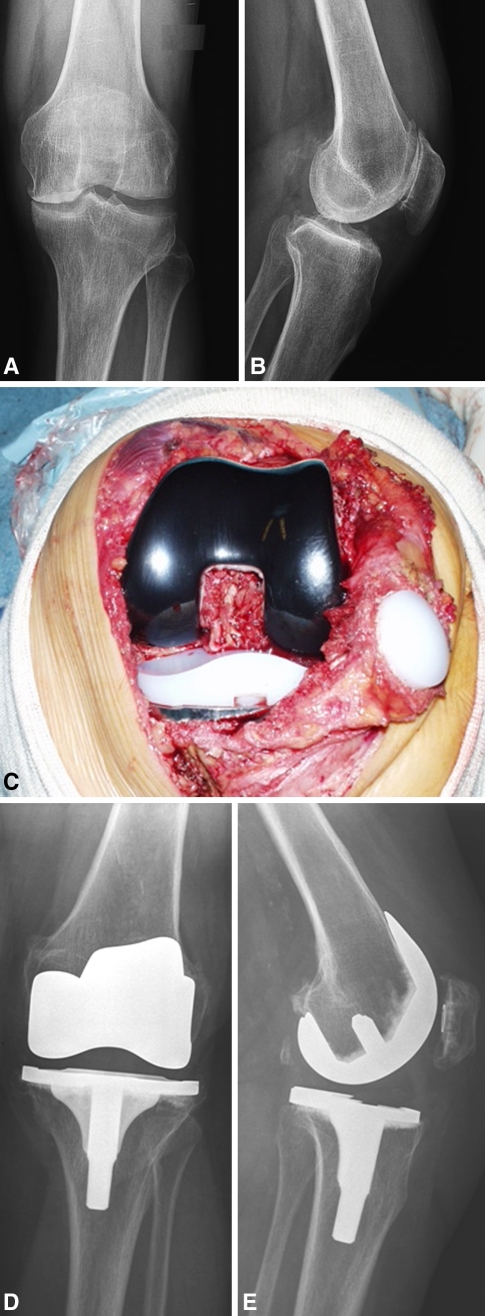
All procedures were performed by the first author (MI). A pneumatic tourniquet was applied about the upper thigh and inflated to approximately 250 mmHg in all cases. The tourniquet was released after the cement had set to allow hemostasis before wound closure. All the operations were performed through a standard medial parapatellar approach with patellar eversion. We used intramedullary femoral and extramedullary tibial instruments and they were set to obtain 95° alignment in the coronal plane, 180° alignment in the sagittal plane of the femoral component, 90° alignment in the coronal plane, and 87° alignment in the sagittal plane for the tibial component. In 59 cases (62.1%), a cruciate-retaining implant with a deep-dished, more conforming PE was used, and in 36 cases (47.9%), a posterior-stabilized design was used. All tibial and femoral components were placed with cement fixation on the nonstemmed portion of the implant in the metaphysis and with cementless fixation of the titanium stems. We used high-viscosity Palacos® cement (Biomet, Warsaw, IN) for fixation. Selective resurfacing of the patella was used. Criteria not to resurface were good quality of the remaining articular cartilage, absence of eburneated bone, normal patellar shape, absence of inflammatory synovial tissue, and congruent intraoperative patellar tracking. According to these criteria, 37 patellae (38.9%) were resurfaced and 58 patellae (61.1%) were left unresurfaced (Fig. 1).
Fig. 1A–E.
These (A) anteroposterior and (B) lateral radiographs show the varus osteoarthritis in the left knee of a 64-year-old man with a body mass index 22.4 kg/m2. (C) An intraoperative photograph shows the implant with the oxidized Zr femoral component. (D) Anteroposterior and (E) lateral radiographs show the cruciate-retaining Genesis II implant with an Oxinium femoral component and patellar resurfacing at the 6-year followup.
We performed clinical and radiographic assessments before surgery and at the latest followup. The Knee Society rating system was used for the clinical evaluation. Two separate scores were assigned: one for walking, stairclimbing, and use of walking aids (functional score) and another for pain, ROM, and stability (knee score). Knee scores greater than 90 points were considered excellent; 80 to 89 points were considered good; 70 to 79 points were considered fair; and less than 69 points were considered poor [14]. We evaluated patellofemoral symptoms preoperatively and postoperatively with a specific patellar score assigning 0 to 15 points for anterior knee pain, 1 to 5 points for quadriceps strength, 0 to 5 points for ability to rise from a chair, and 2 to 5 points for stairclimbing [7]. Radiographs were taken preoperatively, 4 weeks postoperatively, and then yearly after surgery. The images included an AP view (weightbearing), a lateral view, and a patellar skyline view. The radiolucency results were recorded according to the method recommended by the Knee Society [5]. Radiolucent lines were measured in millimeters in each designated zone for the femoral and tibial prostheses in the coronal and sagittal planes [5]. Aseptic loosening was defined according to this radiographic scoring system of the Knee Society. The system assesses the quality of fixation by measuring the width of the radiolucent lines in millimeters for each of the zones of the three components.
This produces a numerical score that was rated as possible or impending failure when the summed total of widths of radiolucent lines from all zones for each component was 10 mm or greater [5]. PE wear was assessed by measuring the medial and lateral joint spaces to the nearest millimeter fraction on the weightbearing AP radiograph. Although there may be limitations with this method of assessment, it provides an estimate of gross in vivo PE wear and was used in a similar study [3]. Evaluation was assessed by all the authors (MI, RC, MV, CC, FM).
We analyzed the clinical difference between the resurfaced and nonresurfaced patella using an independent two-sample Student’s t test. Kaplan-Meier analysis was performed using as end point revision or indication for revision for aseptic loosening as assessed by radiography. Survival tables were constructed using 12-month intervals. For each interval, the total number of TKAs entering the interval, the number of failures and withdrawals, the number at risk, the annual rates of failure and success, and the cumulative success rate were calculated. Second, patients who were lost to followup or had died were considered to have failed results, and a new curve was constructed and termed as the worst-case scenario.
Confidence intervals (95%) were calculated using the Rothman formula as described by Murray et al. [19].
Results
The mean femorotibial angle in the coronal plane averaged 6° varus preoperatively (range, 0°–10°) in 58 patients and 12.6° valgus (range, 10°–14°) in 33 patients. The mean position of the femoral component was 4.4° valgus (range, 1.9°–7.7°) relative to the anatomic axis of the femur on the weightbearing AP radiograph and 0.8° flexion (range, 0.8° extension to 3.8° flexion) on the lateral radiograph. The mean alignment of the tibial component was 90.8° (range, 87.8°–92.8°) relative to the mechanical axis of the tibia on the weightbearing AP radiograph and 4.8° posterior slope (range, 1.6°–8.8°) on the lateral radiograph. Nonprogressive radiolucent lines less than 1 mm in width were seen in 10 tibial components (10.5%) and in one (1.05%) femoral component. Of the tibial lucencies, seven were in Zone 11, one was in Zone 14, and two were in Zone 8. Their presence was not associated with clinical symptoms. There was no detectable asymmetry (less than 1 mm) of the PE space between the lateral and medial compartments on the weightbearing AP radiograph.
The mean Knee Society roentgenographic evaluation and scoring system scores were 2.1 (range: 0–14) for the femoral component and 1.3 (range: 0–7) for the tibial component.
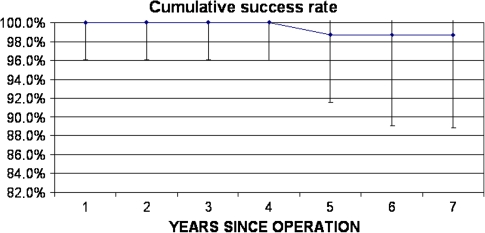
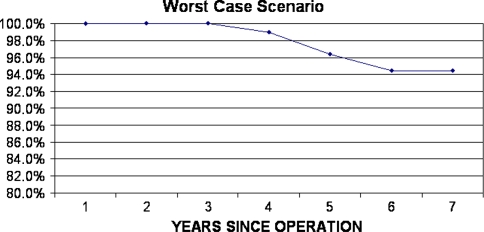
No knee was revised. There was no episode of deep joint infection. Radiographic analysis revealed one (1.05%) femoral component with definite loosening and it was scheduled for revision. No other knee implants had signs of possible loosening or change in component position. Therefore, according to the Knee Society score that we have described the mean score was for the entire cohort of 98 knees, Kaplan-Meier survival analysis, with failure defined as radiographic evidence of aseptic loosening, indicated a 98.7% survival rate at 7 years (95% confidence interval, 90.3%–99.8%) (Fig. 2); the worst case scenario curve showed a 94.5% success rate at 7 years (Fig. 3).
Fig. 2.
A 7-year Kaplan-Meier survival curve is shown.
Fig. 3.
A 7-year Kaplan-Meier survival curve for the worst case scenario assuming all patients lost to followup or died as having failed results in the year of their withdrawal is shown.
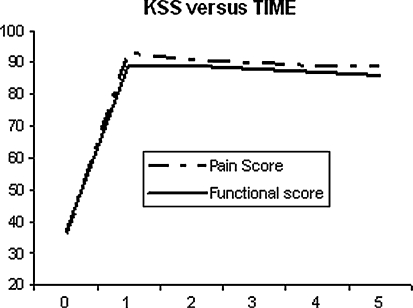
At the final followup, the knee score improved from an average of 36 points (range, 13–57) preoperatively to 89 points (range, 64–100) at the latest evaluation. The functional score improved from 37 points (range, 0–55) to 86 points (range, 55–100) (Fig. 4). The mean total flexion arc improved from 92° (range, 23°–140°) preoperatively to 118° (range, 80°–145°) at the time of the latest followup.
Fig. 4.
A plot of the Knee Society score (KSS) as a function of length of followup is shown.
The mean patellar scores were similar when comparing the nonresurfaced patella group (26.8) with the resurfaced patella group (25.8). Anterior knee pain was present in two patients (5.4%) of the resurfaced patella group (one severe, one moderate) and in one patient (1.7%) of the nonresurfaced patella group.
Discussion
We reviewed the medium-term, 5- to 7-year results of TKA with a femoral component made of oxidized Zr, which was designed specifically to reduce PE wear of the tibial component. To our knowledge, this is the first study in which the results of TKA with a femoral component made of oxidized Zr were analyzed with a minimum followup of 5 years by a team not involved with the design or invention of the implant.
Some limitations were identified and need to be considered when interpreting these data. First, it was a nonrandomized study of selected rather than consecutive cases with a potential for patient selection bias. Second, a control group was not available for direct comparison, so we could not show that a Co-Cr femoral component would have the same results. We therefore relied on comparisons to published reports to judge the relative adequacy of this implant. The third limitation is the lack of statistical analysis for interobserver and intraobserver reliability of the radiographic evaluation of TKAs; assessment of radiographs was done by the authors on the basis of their knowledge and experience. Finally, a limitation could be the relative short duration of followup. However, it is known that the clinical introduction of a new material always brings a risk of early, rather than late, unexpected adverse events.
In the 98 patients in our series, the survival rate at 7 years was 98.7% and the rate of aseptic loosening was low (1.05%). No adverse events or complications related to the new material were seen. The observed rates of radiologic loosening and wear were similar to those in other studies of primary TKAs that had a similar length of followup [4, 8, 15, 18]. Furthermore, we noted a slightly better patellar score in the nonresurfaced patella group; this difference just approached but did not achieve statistical significance. However, it could be related to a positive effect of the oxidized Zr femoral component when articulating with the native patella, because the lower coefficient of friction against cartilage and the greater lubricity could allow better coupling of the nonresurfaced patella with the oxidized Zr femoral component.
Previous attempts to improve PE wear immediately underscored limitations that exist in establishing efficacy through preclinical tests. For example, carbon fiber reinforcement and heat pressing of PE performed poorly when introduced in TKA despite having gone through considerable laboratory testing [29]. More recently, a highly crystalline PE (Hylamer®; DePuy-DuPont Orthopaedics, Wilmington, DE) was introduced for use in acetabular, tibial, patellar, and glenoid components. The clinical performance was poor, including that in TKA, showing high wear rates and premature failure [1, 23]. Therefore, even the medium-term results of a new material become crucial to understand whether it can be safely pursued or if it has to be abandoned.
Medium- to long-term followup results for ceramic on PE knee arthroplasties have been reported. Oonishi et al. [21] reported a clinical and in vitro study of an all-ceramic (alumina) implant with a mean followup of 6.6 years in 67 patients with preoperative diagnoses of rheumatoid arthritis (RA) and osteoarthritis; 107 TKAs were performed with nine aseptic loosenings at final followup. Only two implants in patients with RA failed in the femoral and tibial components, whereas loosening of single components was observed in the remaining cases. Koshino et al. [16] reported their results of approximately 90 alumina implants in 64 patients affected by RA; survivorship was 99.1% at 8 years followup.
However, because clinical introduction of the material was relatively recent, there are few published reviews analyzing the clinical results of oxidized Zr.
Laskin [17], one of the developers of the prosthesis, was the first to report the 5-year results of 76 patients treated with TKAs with a femoral component made of oxidized Zr. At the 5-year followup, no aseptic loosening and adverse events had been observed clinically or radiographically in the cemented oxidized Zr femoral component. In a second study in which patients were randomized to receive either a Co-Cr-Mo or an oxidized Zr femoral component, there was a statistically significant increase in the rapidity of regaining flexion by patients with oxidized Zr implants [18]. The results of the current study are comparable at midterm followup with these studies in terms of implant failure and early minor complications.
Our 5-year followup study shows that, unlike for other new bearing materials, no adverse events have been observed clinically or radiologically with oxidized Zr. It supports ongoing study of the use of an oxidized Zr femoral component in TKA.
Acknowledgments
We thank Jacques Bruhwyler from Squarepoint-Pointcarre for help in preparing the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Ahn NU, Nallamshetty L, Ahn UM, Buchowski JM, Rose PS, Lemma MA, Wenz JF. Early failure associated with the use of Hylamer-M spacers in three primary AMK total knee arthroplasties. J Arthroplasty. 2001;16:136–139. doi: 10.1054/arth.2001.9052. [DOI] [PubMed] [Google Scholar]
- 2.Bourne RB, Barrack R, Rorabeck CH, Salehi A, Good V. Arthroplasty options for the young patient: Oxinium on cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:159–167. doi: 10.1097/01.blo.0000193813.08458.e2. [DOI] [PubMed] [Google Scholar]
- 3.Colizza WA, Insall JN, Scuderi GR. The posterior stabilized total knee prosthesis: assessment of polyethylene damage and osteolysis after a ten-year-minimum follow-up. J Bone Joint Surg Am. 1995;77:1713–1720. doi: 10.2106/00004623-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Dalury DF, Barrett WP, Mason JB, Goldstein WM, Murphy JA, Roche MW. Midterm survival of a contemporary modular total knee replacement: a multicentre study of 1970 knees. J Bone Joint Surg Br. 2008;90:1594–1596. doi: 10.1302/0301-620X.90B12.21064. [DOI] [PubMed] [Google Scholar]
- 5.Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 6.Ezzet KA, Hermida JC, Colwell CW, Jr, D’Lima DD. Oxidized zirconium femoral components reduce polyethylene wear in a knee wear simulator. Clin Orthop Relat Res. 2004;428:120–124. doi: 10.1097/01.blo.0000148576.70780.13. [DOI] [PubMed] [Google Scholar]
- 7.Feller JA, Bartlett RJ, Lang DM. Patellar resurfacing versus retention in total knee arthroplasty. J Bone Joint Surg Br. 1996;78:226–228. [PubMed] [Google Scholar]
- 8.Fuchs R, Mills EL, Clarke HD, Scuderi GR, Scott WN, Insall JN. A third-generation, posterior-stabilized knee prosthesis: early results after follow-up of 2 to 6 years. J Arthroplasty. 2006;21:821–825. doi: 10.1016/j.arth.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Good V, Ries M, Barrack RL, Widding K, Hunter G, Heuer D. Reduced wear with oxidized zirconium femoral heads. J Bone Joint Surg Am. 2003;85(suppl 4):105–110. doi: 10.2106/00004623-200300004-00013. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SK, Chu A, Ranawat AS, Slamin J, Ranawat CS. Osteolysis after total knee arthroplasty. J Arthroplasty. 2007;22:787–799. doi: 10.1016/j.arth.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Hernigou P, Nogier A, Manicom O, Poignard A, Abreu L, Filippini P. Alternative femoral bearing surface options for knee replacement in young patients. Knee. 2004;11:169–172. doi: 10.1016/j.knee.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Hodrick JT, Severson EP, McAlister DS, Dahl B, Hofmann AA. Highly crosslinked polyethylene is safe for use in total knee arthroplasty. Clin Orthop Relat Res. 2008;466:2806–2812. doi: 10.1007/s11999-008-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter G, Jones WM, Spector M. Oxidized zirconium. In: Bellemans J, Ries MD, Victor J, editors. Total Knee Arthroplasty. Heidelberg, Germany: Springer-Verlag; 2005. pp. 370–377. [Google Scholar]
- 14.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 15.Kolisek FR, Barnes CL. Scorpio posterior-stabilized knee system: 5-year clinical and functional results. J Arthroplasty. 2006;21:1187–1192. doi: 10.1016/j.arth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Koshino T, Okamoto R, Takagi T, Yamamoto K, Saito T. Cemented ceramic YMCK total knee arthroplasty in patients with severe rheumatoid arthritis. J Arthroplasty. 2002;17:1009–1015. doi: 10.1054/arth.2002.35826. [DOI] [PubMed] [Google Scholar]
- 17.Laskin RS. An oxidized Zr ceramic surfaced femoral component for total knee arthroplasty. Clin Orthop Relat Res. 2003;416:191–196. doi: 10.1097/01.blo.0000093003.90435.1f. [DOI] [PubMed] [Google Scholar]
- 18.Laskin RS, Davis J. Total knee replacement using the Genesis II prosthesis: a 5-year follow up study of the first 100 consecutive cases. Knee. 2005;12:163–167. doi: 10.1016/j.knee.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Murray DW, Carr AJ, Bulstrode C. Survival analysis of joint replacements. J Bone Joint Surg Br. 1993;75:697–704. doi: 10.1302/0301-620X.75B5.8376423. [DOI] [PubMed] [Google Scholar]
- 20.Naudie DD, Ammeen DJ, Engh GA, Rorabeck CH. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg. 2007;15:53–64. doi: 10.5435/00124635-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Oonishi H, Aono M, Murata N, Kushitani S. Alumina versus polyethylene in total knee arthroplasty. Clin Orthop Relat Res. 1992;282:95–104. [PubMed] [Google Scholar]
- 22.Patel AM, Spector M. Tribological evaluation of oxidized zirconium using an articular cartilage counterface: a novel material for potential use in hemiarthroplasty. Biomaterials. 1997;18:441–447. doi: 10.1016/S0142-9612(96)00152-4. [DOI] [PubMed] [Google Scholar]
- 23.Ries MD, Bellare A, Livingston BJ, Cohen RE, Spector M. Early delamination of a Hylamer-M tibial insert. J Arthroplasty. 1996;11:974–976. doi: 10.1016/S0883-5403(96)80143-4. [DOI] [PubMed] [Google Scholar]
- 24.Ries MD, Salehi A, Widding K, Hunter G. Polyethylene wear performance of oxidized zirconium and cobalt-chromium knee components under abrasive conditions. J Bone Joint Surg Am. 2002;84(suppl 2):129–135. doi: 10.2106/00004623-200200002-00018. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Spector BM, Ries MD, Bourne RB, Sauer WS, Long M, Hunter G. Wear performance of ultra-high molecular weight polyethylene on oxidized zirconium total knee femoral components. J Bone Joint Surg Am. 2001;83(suppl 2):80–86. doi: 10.2106/00004623-200100022-00004. [DOI] [PubMed] [Google Scholar]
- 27.White SE, Whiteside LA, McCarthy DS, Anthony M, Poggie RA. Simulated knee wear with cobalt chromium and oxidized zirconium knee femoral components. Clin Orthop Relat Res. 1994;309:176–184. [PubMed] [Google Scholar]
- 28.Wright TM. Polyethylene in knee arthroplasty: what is the future? Clin Orthop Relat Res. 2005;440:141–148. doi: 10.1097/01.blo.0000187811.48717.9d. [DOI] [PubMed] [Google Scholar]
- 29.Wright TM, Rimnac CM, Faris PM, Bansal M. Analysis of surface damage in retrieved carbon fiber-reinforced and plain polyethylene tibial components from posterior stabilized total knee replacements. J Bone Joint Surg Am. 1988;70:1312–1319. [PubMed] [Google Scholar]