Abstract
Background
Subchondral insufficiency fracture of the femoral head occurs mainly in elderly patients with osteoporosis. Spontaneous resolution is observed after nonoperative treatment in some patients whereas other show progressive joint destruction requiring THA. Several studies report the occurrence of subchondral insufficiency fracture of the femoral head in dysplastic hips.
Questions/purposes
We asked whether the extent of hip dysplasia or osteoporosis was greater in patients with subchondral insufficiency fracture of the femoral head than in normal control subjects.
Patients and Methods
We compared the clinical and imaging findings of 13 patients with subchondral insufficiency fractures of the femoral head and 12 patients scheduled for TKA with asymptomatic hips. Age, gender, and body mass index were comparable in the two groups.
Results
Higher mean Sharp angles, lower acetabular head indices, lower center-edge angles, and higher acetabular roof angles in patients with subchondral insufficiency fracture of the femoral head than in those with asymptomatic hips suggested a greater degree of hip dysplasia. Bone mineral density and serum levels of Type I collagen cross-linked N-telopeptide and bone alkaline phosphatase were similar in the two groups.
Conclusions
We speculate an excessive amount of stress on the acetabular edge from dysplasia may be associated with the occurrence of subchondral insufficiency fracture of the femoral head.
Introduction
A subchondral insufficiency fracture of the femoral head (SIF) often manifests as acute hip pain without obvious antecedent trauma, primarily occurs in elderly patients with osteoporosis, and is more common with obesity [1, 5, 17, 20]. Initial radiographs may not show any obvious abnormalities. MRI is useful in the diagnosis of SIF, especially during the early stage, which is characterized by a bone marrow edema pattern (diffuse low signal intensity on the T1-weighted images and high signal intensity on the T2-weighted images) in the femoral head to the neck and by the presence of a low signal intensity band in the subchondral region [22]. Yamamoto and Bullough [20] reported histologic findings of 10 patients with SIF in which thin disconnected bone trabeculae indicative of osteopenia were observed throughout the femoral head in all cases. Several reports of SIF include patients with dysplasia of the acetabulum [5, 13, 14].
We therefore asked (1) whether the extent of hip dysplasia or osteoporosis was greater in patients with SIF than in control subjects and (2) whether any of various clinical parameters (Sharp angle, acetabular head index [AHI], center-edge [CE] angle, acetabular roof angle, pelvic tilt, bone mineral density [BMD], serum Type I collagen cross-linked N-telopeptide [NTx], and serum bone alkaline phosphatase [BAP]) occurred more commonly in patients with SIF than in control subjects.
Patients and Methods
We retrospectively reviewed all 27 patients who had SIF (diagnostic criteria below) at our hospital between March 2001 and May 2008. Fourteen of these patients had associated degenerative changes and were excluded, leaving 13 for analysis. There was one man and 12 women, ranging from 61 to 81 years of age (mean age, 71.2 years). Six patients were affected on the left side, and seven were affected on the right side. All of the 13 patients with SIF initially were treated nonoperatively with protected weightbearing and/or mild analgesics (Fig. 1). The diagnosis of SIF was made by following previously published criteria [20, 22]: (1) hip pain that begins with no apparent history of trauma; (2) initial radiographs that are normal or may show collapse of the femoral head; (3) a bone marrow edema pattern in the femoral head and/or neck observed on MRI; and (4) a subchondral low signal intensity band on the T1-weighted MR image that is serpiginous or is parallel to the articular surface. We performed 26 TKAs between July 2008 and December 2008 at our hospital. Of these, 12 patients (12 women) served as a control group (asymptomatic hip control). None of these patients had any hip symptoms. The AP hip radiographs were normal (no signs of joint space narrowing, collapse of the femoral head, and osteophyte formation). Patients with hip dysplasia were not excluded. The mean age of control subjects at the time of surgery was 74.9 years (range, 65–82 years). The study was approved by the local ethics committee, and the patients provided informed consent for participation in this study.
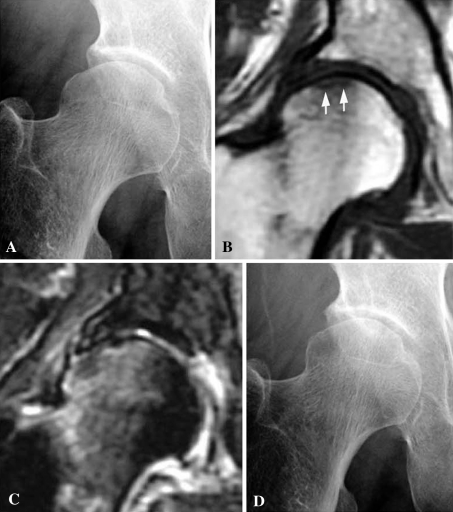
Fig. 1A–D.
A 76-year-old woman was treated nonoperatively for SIF. (A) Her initial radiograph shows a slight collapse of the lateral portion of the right femoral head. (B) A coronal T1-weighted fast spin echo MR image shows a low signal intensity band in the subchondral area (white arrows) associated with diffuse low signal intensity in the femoral head and neck. (C) A coronal fat-suppressed T2-weighted fast spin echo MR image shows a linear pattern of low signal intensity in the subchondral area with diffuse high signal intensity in the femoral head and neck. (D) A radiograph taken 10 months after the onset of hip pain shows no obvious change. The patient has only slight hip pain when walking.
We compared body mass index (BMI), BMD shown by the percentage of the young adult mean [16], five radiographic indices (below), and two hematologic factors (below) between the two groups. The BMI, calculated as the body weight in kilograms divided by the height in meters squared, was used to determine whether the patient was obese.
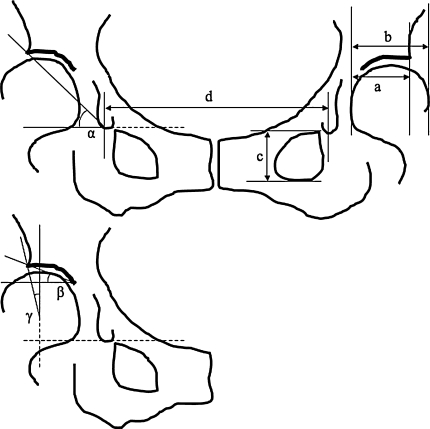
We made the following radiographic measurements: Sharp angle [18], AHI [6], CE angle, acetabular roof angle [11], and pelvic tilt [8] on a supine AP radiograph (Fig. 2). We determined pelvic tilt using the following published formula: (angle of the pelvic tilt, in degrees) = (height of the obturator foramen)/([teardrop distance] × A−B), where A = 207.0 and B = 32.0 in the female subjects and A = 137.4 and B = 23.1 in the male subjects [8] (Fig. 2). The calculated angle indicates how much the pelvis tilts backward from the neutral position. All markings and reference points, including lateral acetabular margin and a distal point of the teardrop, were decided and marked on a radiograph on a consensus by two of the investigators (KI, KM). After this process, one of the investigators (KI) made the measurements. To test the reproducibility of the radiographic measurements, two authors (KI, KM) and another orthopaedic resident (ES) measured the five radiographic indices in five randomly selected hips; KI was an orthopaedic resident and not the treating surgeon. KM was one of the treating surgeons. Each observer measured each hip three times, with an interval of 1 week between measurements. The values were averaged. The data were analyzed for intraobserver and interobserver variances. The coefficients of variation were calculated and ranged from 0.6% to 4.9% which we considered reasonable [24].
Fig. 2.
The radiographic indices used for evaluation of the hip are shown. α = Sharp angle; β = acetabular roof angle; γ = CE angle. The AHI was calculated with the formula: a/b × 100. Pelvic tilt was calculated with the formula: c/(d × A−B), where A = 207.0 and B = 32.0 in the female subjects and A = 137.4 and B = 23.1 in the male subjects.
Fasting blood samples in the morning were obtained on admission to examine the biochemical markers of bone turnover related to osteoporosis, including the levels of the serum Type I collagen cross-linked NTx and serum BAP [15].
We used Shapiro-Wilk tests to examine if the continuous data (age, BMI, Sharp angle, AHI, CE angle, acetabular roof angle, pelvic tilt, BMD, NTx, and BAP) were normally distributed. Shapiro-Wilk tests showed the clinical data examined (Table 1) were normally distributed with W values ranging from 0.9561 to 0.9856. We determined differences in age, BMI, Sharp angle, AHI, CE angle, acetabular roof angle, pelvic tilt, BMD, NTx, and BAP between the SIF and asymptomatic hip control groups using unpaired Student’s t test. Fisher’s exact test was used for the gender proportion in the SIF and control groups. These analyses were performed using the StatView® software program, Version 5.0.1 (SAS Institute Inc, Cary, NC).
Table 1.
Clinical information for patients with SIF and for control subjects
| Variable | SIF (n = 13) | Control subjects | p Value |
|---|---|---|---|
| (n = 12) | |||
| Age (years) | 71.2 ± 7.0 | 74.9 ± 5.6 | 0.1522 |
| Gender (male:female) | 1:12 | 0:12 | 0.9999 |
| Body mass index (kg/m2) | 23.9 ± 4.7 | 25.3 ± 4.0 | 0.4145 |
| Sharp angle (degrees) | 42.8 ± 4.0 | 37.8 ± 2.5 | 0.0012 |
| Acetabular head index | 72.6 ± 6.5 | 87.4 ± 7.2 | < 0.0001 |
| Center-edge angle (degrees) | 15.3 ± 8.5 | 29.5 ± 7.7 | 0.0002 |
| Acetabular roof angle (degrees) | 17.9 ± 4.1 | 7.2 ± 4.0 | < 0.0001 |
| Pelvic tilt (degrees) | 17.6 ± 10.5 | 19.8 ± 7.3 | 0.5539 |
| BMD (% of the young adult mean) | 85.1 ± 22.0* | 83.4 ± 14.9 | 0.8427 |
| NTx (nmol BCE/L) | 17.7 ± 2.4* | 17.1 ± 6.0 | 0.8503 |
| BAP (U/L) | 29.7 ± 9.6* | 25.2 ± 7.9 | 0.4121 |
Values are expressed as mean ± SD; *data concerning BMD, NTx, and BAP in the SIF group were available in seven, four, and three patients, respectively; SIF = subchondral insufficiency fracture of the femoral head; BMD = bone mineral density; BCE = bone collagen equivalents; NTx = Type I collagen cross-linked N-telopeptide; BAP = bone alkaline phosphatase.
Results
The Sharp angle was greater (p = 0.0012) in the SIF group than in the control group (Table 1). The AHI was lower (p < 0.0001) in the SIF group than in the control group. The CE angle was lower (p = 0.0002) in the SIF group than in the control group. The acetabular roof angle was greater (p < 0.0001) in the SIF group than in the control group. Age, gender proportion, BMI, pelvic tilt, BMD, and serum levels of NTx and BAP were similar between the two groups.
Discussion
The pathogenesis of SIF remains unclear. Histologic analysis of SIF cases has shown thin disconnected bone trabeculae in the femoral head, indicating its osteoporotic nature [20]. The fact that SIF occurs in most cases without any antecedent trauma suggests there may be underlying subchondral bone insufficiency or excessive mechanical stress on the subchondral bone. Several reports note the presence of hip dysplasia in patients with SIF [5, 13, 14]. This raises a question regarding whether dysplasia could contribute to SIF. We therefore asked whether (1) the extent of hip dysplasia or osteoporosis was greater in patients with SIF than in control subjects and (2) whether any of various clinical parameters (Sharp angle, AHI, CE angle, acetabular roof angle, pelvic tilt, BMD, NTx, and BAP) occurred more commonly in patients with SIF compared with control subjects.
We note limitations to our study. First, the number of patients with SIF was small. Some patients with SIF have disappearance of the joint space and/or collapse of the femoral head during the natural course [21], indicating SIF may be diagnosed as osteoarthritis as it progresses. To eliminate the effect of osteoarthritis, we excluded patients with possible SIF who had osteoarthritic changes (joint space narrowing and osteophyte formation), which limited the number of patients with SIF in this study. Second, our control patients had TKAs for osteoarthritis of the knee and we cannot ensure they had no early osteoarthritis. However, they had no hip pain and their AP hip radiographs showed no joint space narrowing or osteophytes. We thus considered them control subjects with asymptomatic hips.
Our data suggest hips with SIF had more characteristics of dysplasia compared with those of the control subjects. Although we suspected anterior uncovering of the acetabulum attributable to posterior pelvic tilt might be associated with SIF, the pelvic tilt was similar in the two groups. Previous biomechanical studies showed the joint contact pressure of dysplastic hips is concentrated on the lateral edge of the acetabulum [2, 12, 19]. Increased stress on the acetabular rim also reportedly is associated with acetabular lesions such as labral tears and ganglion cysts [9, 10]. Resultant labrum tears in dysplastic hips are associated with the development of osteoarthritis [3, 4]. Therefore, we presumed the increased joint contact pressure on the anterolateral edge of the dysplastic acetabulum could produce a subchondral fracture in SIF. This presumption is consistent with case reports in which initial collapse occurred in the superolateral portion of the femoral head corresponding to the acetabular rim [13, 14, 23]. Furthermore, Motomura et al. [14] presented a SIF case in which a patient with developmental dysplasia of the acetabulum had subchondral fractures in the femoral head and the acetabulum, suggesting an impact on the acetabular edge led to the fractures in both sides.
The Sharp angle (42.8°), AHI (72.6), CE angle (15.3°), and acetabular roof angle (17.9°) indicated the extent of dysplasia was slight in our patients with SIF despite the difference compared with the control subjects. Severe deformity of the femoral head or acetabulum rarely was observed and the joint congruity was relatively stable. These observations suggest the pathogenesis of SIF may be multifactorial and no one factor may be able to adequately explain its etiology. Other possible etiologic factors may include labrum tear and joint laxity. Underlying subchondral bone insufficiency may be a possible reason for the occurrence of a fracture on the femoral side in SIF. Additional investigations are needed to clarify this issue conclusively.
We found BMD and blood markers of the osteoporosis-related bone turnover (NTx and BAP) were similar in the two groups. However, we still consider treatment for osteoporosis important for patients with SIF because osteoporosis is a major cause of the fractures in elderly patients [7], and numerous reports strongly support the occurrence of SIF in patients with osteoporosis [1, 5, 13, 14, 17, 20–23].
Our data suggest the hips in our patients with SIF were more likely associated with imaging findings of dysplasia compared with those of control subjects. We interpret these findings as suggesting an excessive amount of stress on the acetabular edge may be associated with the occurrence of SIF.
Acknowledgments
We thank Dr. Eiji Suenaga for help during the data collection.
Footnotes
One or more of the authors (KM) have received funding to promote the hospital functions of Japan Labour Health and Welfare Organization and a grant from Japan Orthopaedics and Traumatology Foundation, Inc (Number 0165).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bangil M, Soubrier M, Dubost JJ, Rami S, Carcanagues Y, Ristori JM, Bussiere JL. Subchondral insufficiency fracture of the femoral head. Rev Rhum Engl Ed. 1996;63:859–861. [PubMed] [Google Scholar]
- 2.Genda E, Konishi N, Hasegawa Y, Miura T. A computer simulation study of normal and abnormal hip joint contact pressure. Arch Orthop Trauma Surg. 1995;114:202–206. doi: 10.1007/BF00444263. [DOI] [PubMed] [Google Scholar]
- 3.Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2:105–117. doi: 10.1007/s12178-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haene RA, Bradley M, Villar RN. Hip dysplasia and the torn acetabular labrum: an inexact relationship. J Bone Joint Surg Br. 2007;89:1289–1292. doi: 10.1302/0301-620X.89B10.17319. [DOI] [PubMed] [Google Scholar]
- 5.Hagino H, Okano T, Teshima R, Nishi T, Yamamoto K. Insufficiency fracture of the femoral head in patients with severe osteoporosis: report of 2 cases. Acta Orthop Scand. 1999;70:87–89. doi: 10.3109/17453679909000966. [DOI] [PubMed] [Google Scholar]
- 6.Heyman CH, Herndon CH. Legg-Perthes disease: a method for the measurement of the roentgenographic result. J Bone Joint Surg Am. 1950;32:767–778. [PubMed] [Google Scholar]
- 7.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 8.Konishi N, Mieno T. Determination of acetabular coverage of the femoral head with use of a single anteroposterior radiograph: a new computerized technique. J Bone Joint Surg Am. 1993;75:1318–1333. doi: 10.2106/00004623-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Leunig M, Podeszwa D, Beck M, Werlen S, Ganz R. Magnetic resonance arthrography of labral disorders in hips with dysplasia and impingement. Clin Orthop Relat Res. 2004;418:74–80. doi: 10.1097/00003086-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Lin YK, Tien YC, Lin SY. Hip ganglion cyst associated with developmental dysplasia of hip in a child: a case report. Acta Orthop Scand. 2002;73:109–110. doi: 10.1080/000164702317281512. [DOI] [PubMed] [Google Scholar]
- 11.Massie WK, Howorth MB. Congenital dislocation of the hip. Part I. Method of grading results. J Bone Joint Surg Am. 1950;32:519–531. [PubMed] [Google Scholar]
- 12.Mavcic B, Pompe B, Antolic V, Daniel M, Iglic A, Kralj-Iglic V. Mathematical estimation of stress distribution in normal and dysplastic human hips. J Orthop Res. 2002;20:1025–1030. doi: 10.1016/S0736-0266(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 13.Miyanishi K, Hara T, Hamada T, Maekawa M, Tsurusaki S, Moro-Oka TA, Kamo Y, Jingushi S, Torisu T. Co-occurrence of subchondral insufficiency fracture of the femoral head and contralateral femoral neck fracture in a rheumatic patient receiving steroid treatment. Mod Rheumatol. 2008;18:619–622. doi: 10.1007/s10165-008-0093-5. [DOI] [PubMed] [Google Scholar]
- 14.Motomura G, Yamamoto T, Miyanishi K, Shirasawa K, Noguchi Y, Iwamoto Y. Subchondral insufficiency fracture of the femoral head and acetabulum: a case report. J Bone Joint Surg Am. 2002;84:1205–1209. doi: 10.1302/0301-620X.84B8.13739. [DOI] [PubMed] [Google Scholar]
- 15.Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, Fukunaga M, Hosoi T, Miki T, Chaki O, Ichimura S, Nakatsuka K, Miura M. Committee on the Guidelines for the Use of Biochemical Markers of Bone Turnover in Osteoporosis. Japan Osteoporosis Society Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004) J Bone Miner Metab. 2005;23:97–104. doi: 10.1007/s00774-004-0547-6. [DOI] [PubMed] [Google Scholar]
- 16.Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H. Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 17.Rafii M, Mitnick H, Klug J, Firooznia H. Insufficiency fracture of the femoral head: MR imaging in three patients. AJR Am J Roentgenol. 1997;168:159–163. doi: 10.2214/ajr.168.1.8976940. [DOI] [PubMed] [Google Scholar]
- 18.Sharp IK. Acetabular dysplasia: the acetabular angle. J Bone Joint Surg Br. 1961;43:268–272. [Google Scholar]
- 19.Tsumura H, Kaku N, Ikeda S, Torisu T. A computer simulation of rotational acetabular osteotomy for dysplastic hip joint: does the optimal transposition of the acetabular fragment exist? J Orthop Sci. 2005;10:145–151. doi: 10.1007/s00776-004-0866-4. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Bullough PG. Subchondral insufficiency fracture of the femoral head: a differential diagnosis in acute onset of coxarthrosis in the elderly. Arthritis Rheum. 1999;42:2719–2723. doi: 10.1002/1529-0131(199912)42:12<2719::AID-ANR31>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Bullough PG. The role of subchondral insufficiency fracture in rapid destruction of the hip joint: a preliminary report. Arthritis Rheum. 2000;43:2423–2427. doi: 10.1002/1529-0131(200011)43:11<2423::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Schneider R, Bullough PG. Subchondral insufficiency fracture of the femoral head: histopathologic correlation with MRI. Skeletal Radiol. 2001;30:247–254. doi: 10.1007/s002560100348. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Takabatake K, Iwamoto Y. Subchondral insufficiency fracture of the femoral head resulting in rapid destruction of the hip joint: a sequential radiographic study. AJR Am J Roentgenol. 2002;178:435–437. doi: 10.2214/ajr.178.2.1780435. [DOI] [PubMed] [Google Scholar]
- 24.Yasunaga Y, Ochi M, Terayama H, Tanaka R, Yamasaki T, Ishii Y. Rotational acetabular osteotomy for advanced osteoarthritis secondary to dysplasia of the hip. J Bone Joint Surg Am. 2006;88:1915–1919. doi: 10.2106/JBJS.E.00715. [DOI] [PubMed] [Google Scholar]