Abstract
Single ray amputation after hand trauma or infection can result in good aesthetic and functional outcomes. The role of this procedure in the management of aggressive benign or malignant hand tumors has been described only in case reports and small case series. We retrospectively reviewed the records of all 25 patients who underwent single ray amputations at our center during a 10-year period; there were seven index, five middle, six ring, and seven small ray amputations performed. The minimum followup was 2 months (mean, 36 months; range, 2–120 months), with four patients having a followup of 1 year or less. No patients had local recurrences, although two patients had positive resection margins. One underwent repeat resection followed by radiotherapy. The other was treated with radiotherapy alone, as local tumor control would have required a hand amputation. Functional assessment based on the Musculoskeletal Tumor Society staging system showed an average of 27.5 (range, 21–30). Patients who underwent perioperative radiotherapy experienced a decrease in functional ability. Grip strength was an average of 66% (range, 38%–100%) of the contralateral side. Our study suggests single ray amputation for hand tumors has a low local recurrence rate and high functional scores. However, function can be compromised by radiotherapy and a decrease in grip strength by a mean of 34% is to be expected.
Level of Evidence: Level IV, case series. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Although the role of single ray amputation for treatment of traumatic hand injuries and hand infection is well established [7, 14, 17, 19, 20], its functional and oncologic outcomes for management of hand tumors are not as well defined. Several variations in the procedure, when used for tumors, may affect the eventual outcome of surgery. For example, the adjacent interosseous muscle frequently is excised to achieve complete tumor clearance. Occasionally, the digital neurovascular bundles and tendons of adjacent digits must be sacrificed as well, with secondary reconstruction to restore function. Finally, perioperative radiotherapy may be required for large or high-grade tumors or when surgical resection margins are close, which is frequently the case in the hand [1, 11, 12, 21]. This will cause more fibrosis and stiffness of the hand, further affecting the range of motion, strength, and consequently, functional outcome. Ray amputation is an ablative and a limb-sparing surgery. Loss of the digit allows preservation of the hand and provides a good source of soft tissue cover from a fillet flap [13], and spare parts such as nerve, tendon, and even vessel grafts for secondary reconstruction, eliminating the need to use other donor sites. However, adequate resection is paramount, and at times, free tissue transfer from distant sites will be required instead.
Some authors report reasonable functional scores, grip and pinch strength, and high rates of return to work when performing single ray amputations as primary or secondary reconstructive procedures after hand trauma [7, 14, 17, 19, 20]. Many others focused on local recurrence and survival rates from treatments of aggressive benign and primary malignant hand tumors, and metastases to the hand [1–3, 5, 6, 8, 10–12, 15, 16, 18, 21]. None of these reports have looked specifically at local recurrence rates or the functional outcome after single ray resection for tumors.
We therefore asked the following questions: (1) Could we achieve negative margins with low local recurrence rates when performing single ray amputations for aggressive benign and malignant lesions of the hand? (2) Would more extensive surgery as compared with a standard posttraumatic ray amputation substantially compromise the functional outcome of the hand assessed using the Musculoskeletal Tumor Society (MSTS) score and grip strength? (3) Were there any factors (for example, patient age, affected digit, or use of perioperative radiation) that resulted in a lower MSTS score? We presumed we could achieve negative margins with low local recurrence rates while preserving good aesthetic appearance and functional use of the hand.
Materials and Methods
We retrospectively reviewed the records of all 25 patients who had a single ray amputation of the hand during the 10 years from 1997 to 2007 at our center by one surgeon (EAA). We excluded patients with lesions affecting the thumb, as the thumb contributes more to overall hand function and, therefore, its loss would be far more disabling than that of any other ray. Furthermore, no isolated first ray amputations were performed without some reconstruction to restore function. We focused on patients who were at least 2 years postsurgery to allow time for hand function to stabilize and to enable assessment for local recurrence. The minimum followup for all the patients was 2 months (mean, 36 months; range, 2–120 months). Six patients were lost to followup at a mean of 11 months (range, 2–24 months), whereas another five died during this period and had a mean followup of 10 months (range, 4–12 months).
We performed seven index, five middle, six ring, and seven small single ray amputations (Table 1). Surgery was performed on the dominant hands of nine patients and the nondominant hands of the other 16 patients. The mean age of these patients at the time of surgery was 48.8 years (range, 8–86 years). Eighteen patients had malignant soft tissue lesions, four had metastasis to the hand (three lung, one renal), two had giant cell tumors of the bone, and one had a malignant primary bone sarcoma. One patient with a primary malignant soft tissue tumor had metastatic disease on presentation. Ten patients underwent postoperative radiotherapy, two for positive margins and eight for close margins. Eight patients required the use of flaps to close the wound, two had transposition flaps, five had fillet flaps, and one had a free vascularized skin flap. Complete reconstruction was performed in many patients after tumor resection, when deemed appropriate, with flaps, tendon, and nerve grafts using spare parts from the amputated digit wherever possible. Only rarely were grafts taken from distant sites. This minimized donor site morbidity.
Table 1.
Summary of patients
| Patient | Age (years) | Gender | Ray | Histology | Tumor | Radiation | AJCC stage | Margins | Followup (months) | MSTS score | Grip (%) | Current status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | Female | IF | STS | Primary | No | 2 | Negative | 96 | 30 | 40 | NED |
| 2 | 51 | Male | IF | NSCLC | Metastasis | Yes | 4 | Negative | 118 | 27 | NA | DOD |
| 3 | 68 | Female | IF | STS | Primary | No | 2 | Negative | 24 | 27 | 80 | NED |
| 4 | 75 | Male | IF | STS | Recurrent | No | 2 | Negative | 12 | 29 | NA | NED |
| 5 | 79 | Female | IF | STS | Recurrent | Yes | 2 | Positive | 12 | 25 | NA | DOC |
| 6 | 80 | Male | IF | STS | Primary | No | 2 | Negative | 24 | 24 | NA | NED |
| 7 | 73 | Male | IF | NSCLC | Metastasis | No | 4 | Negative | 2 | NA | NA | DOD |
| 8 | 27 | Female | MF | GCT bone | Primary | Yes | NA | Negative | 60 | 29 | NA | NED |
| 9 | 37 | Male | MF | Sweat gland carcinoma | Recurrent | No | 4 | Negative | 12 | 27 | NA | DOD |
| 10 | 39 | Male | MF | STS | Recurrent | Yes | 2 | Negative | 36 | 28 | NA | NED |
| 11 | 51 | Female | MF | STS | Primary | Yes | 1 | Negative | 4 | 30 | 94 | NED |
| 12 | 72 | Male | MF | NSCLC | Metastasis | No | 4 | Negative | 12 | 29 | NA | DOD |
| 13 | 27 | Female | RF | STS | Primary | No | 2 | Negative | 24 | 29 | 100 | NED |
| 14 | 29 | Female | RF | GCT bone | Primary | No | NA | Negative | 33 | 29 | 71 | NED |
| 15 | 40 | Male | RF | Chondrosarcoma | Primary | Yes | 1 | Negative | 120 | 30 | 96 | NED |
| 16 | 41 | Female | RF | STS | Primary | No | 2 | Negative | 6 | 28 | NA | NED |
| 17 | 49 | Female | RF | STS | Primary | No | 2 | Negative | 24 | 26 | 38 | NED |
| 18 | 51 | Female | RF | STS | Primary | No | 2 | Negative | 24 | 28 | 93 | NED |
| 19 | 12 | Female | LF | STS | Recurrent | No | 2 | Negative | 66 | 28 | 67 | NED |
| 20 | 27 | Female | LF | STS | Primary | Yes | 2 | Negative | 29 | 21 | NA | NED |
| 21 | 33 | Female | LF | STS | Primary | No | 2 | Positive | 66 | 25 | NA | NED |
| 22 | 38 | Male | LF | STS | Primary | Yes | 2 | Negative | 68 | 28 | 70 | NED |
| 23 | 61 | Female | LF | STS | Primary | Yes | 1 | Negative | 14 | 30 | NA | NED |
| 24 | 66 | Female | LF | STS | Primary | Yes | 1 | Negative | 6 | 27 | NA | NED |
| 25 | 86 | Female | LF | RCC | Metastasis | No | 4 | Negative | 12 | 27 | NA | DOD |
AJCC = American Joint Committee on Cancer; MSTS = Musculoskeletal Tumor Society; IF = index finger; MF = middle finger; RF = ring finger; LF = little finger; STS = soft tissue sarcoma; GCT bone = giant cell tumor of the bone; NSCLC = nonsmall-cell lung cancer; RCC = renal cell carcinoma; NA = not available; NED = no evidence of disease; DOD = dead from disease; DOC = dead from other causes.
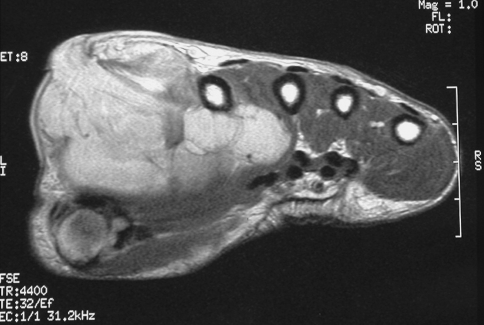
Preoperative resection was planned with the help of high-resolution MRI scans (Fig. 1). Patients selected for single ray amputations were (1) those with lesions that could be seen on imaging to affect the bone (metacarpal or proximal phalanx) of only one ray and the soft tissue around it or (2) those with soft tissue lesions that required a wide resection that would result in substantially impaired function of the digit. Once a tumor had invaded the bone of the adjacent ray or involved both neurovascular bundles of the adjacent digit, a more radical resection (with a double ray amputation or more) would be required. This was performed for four patients during the same period.
Fig. 1.
MRI shows a soft tissue sarcoma (liposarcoma) in the first web space and extending close to the third metacarpal.
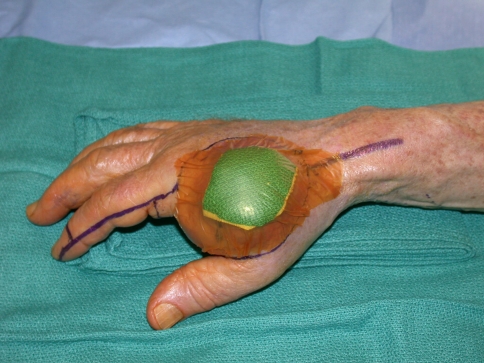
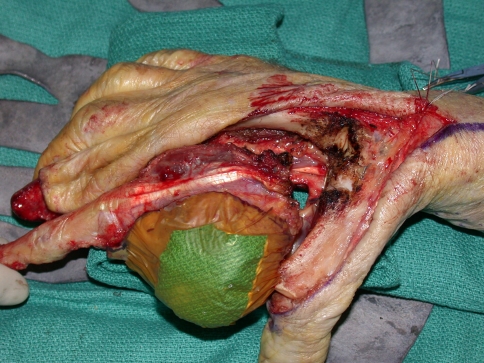
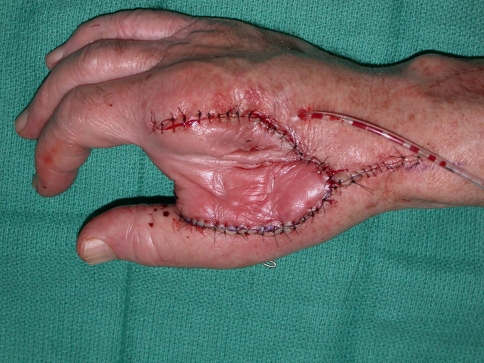
We performed tumor excision through a volar Z-Brunner type incision and dorsal longitudinal extension along the digit, incorporating the previous biopsy incision (Fig. 2). Whenever required, a fillet flap was raised from the amputated ray based on the volar digital arteries. The extensor mechanism then was incised proximally, the adjacent interosseous muscles incised, and dissection performed around the tumor with the interosseous muscles taken as a margin. Often, subperiosteal dissection on the adjacent metacarpal was required. We made the volar incisions and identified the neurovascular bundles. Where necessary, the common digital neurovascular bundles were sacrificed to completely excise the tumor. The intermetacarpal ligaments then were transected and the flexor tendons transected proximally. We made bone cuts through the base of the metacarpal or disarticulated the ray through the carpometacarpal joint. Circumferential dissection then was performed to ensure complete en bloc removal of the tumor (Fig. 3). For central ray amputations, we then repaired or reconstructed the remnants of the adjacent intermetacarpal ligaments, ensuring appropriate rotation of the digits. The skin flaps were fashioned and closed over a drain either primarily or with the fillet flap (Fig. 4), which was performed in five patients. Ray transposition was not performed for any of the patients.
Fig. 2.
The incision was planned incorporating the previous biopsy track.
Fig. 3.
An extended second ray amputation was performed, with circumferential dissection to ensure complete en bloc removal of the tumor.
Fig. 4.
After the extended second ray amputation, the wound was closed with a fillet flap.
Some considerations were specific to the ray being amputated. During small finger ray amputation, special attention must be paid to the ulnar artery and nerve as it winds around the hook of the hamate. Similarly, the radial artery must be protected as it crosses the base of the second metacarpal. For the central digits, the deep motor branch of the ulnar nerve and deep arch should be protected during the osteotomy at the base of the metacarpal or disarticulation through the carpometacarpal joint.
Patients stayed overnight in the hospital and were discharged the day after surgery after range of motion therapy was initiated by the surgeon. They were reviewed on the tenth postoperative day when stitches were removed, and hand rehabilitation continued under the supervision of a hand occupational therapist.
Patients were reviewed at 6 weeks, 3 months, and then every 3 months during the first year, every 4 months for the next 2 years, and every 6 months during the fourth and fifth years. Hand function was assessed when we believed it had stabilized, on average 6 months after surgery, using the MSTS score [4]. Grip strength also was measured at this time using a Baseline® Hydraulic Hand Dynamometer (Fabrication Enterprises Inc, Irvington, NY), although these data were recorded in only 10 of the 25 patients. Chest radiographs were performed at these visits and CT scans of the chest were taken yearly.
Results
Of the 25 patients, five died from disease (including the four patients with metastatic lesions to the hand and one with a primary lesion in the hand who had distant metastasis at the time of surgery), one died from other causes, and 19 remained completely disease-free (Table 1). Two patients had positive resection margins. One underwent repeat resection to negative margins followed by radiotherapy and is currently disease-free 4 years after surgery. The other was treated with radiotherapy alone, as local surgical control would have required a hand amputation, which was refused by the patient; she died from nondisease-related causes 18 months after ray amputation. None of the patients had local recurrence.
We observed no differences in function between (1) amputation performed as a primary tumor excision versus excision of a locally recurrent tumor, (2) flap versus primary wound closure, (3) amputations of different rays, or (4) surgery on the dominant versus nondominant hand. The average MSTS score was 27.5 (range, 21–30). We measured grip strength in 10 patients, with an average of 66% (range, 38%–100%) of the contralateral side.
Slightly lower MSTS scores were seen in patients who had received radiotherapy than in those who did not (average 26.4 versus 28.5, respectively). The age of the patient at the time of surgery had no impact on the MSTS score.
Discussion
We conducted this retrospective review of patient records for those who had single ray amputation for treatment of aggressive local and malignant tumors to answer the following questions: (1) Could we achieve negative margins with low local recurrence rates when performing single ray amputations for aggressive benign and malignant lesions of the hand? (2) Would more extensive surgery as compared with a standard posttraumatic ray amputation substantially compromise the functional outcome of the hand assessed using the MSTS score and grip strength? (3) Did any factors (for example, patient age, affected digit, or use of perioperative radiation) result in a lower MSTS score? We presumed we could achieve negative margins with low local recurrence rates while preserving good aesthetic appearance and functional use of the hand.
This study is first limited by its small size. However, small size is inevitable given the rarity of tumors of the hand that require ray amputation. As the small number of patients limits our ability to perform meaningful survival analyses or subset analysis, we have described certain trends instead—for example, lower MSTS scores in patients who received perioperative radiation to the hand. Second, single ray amputations of patients with tumors vary more widely than do those performed in the trauma setting.
We managed to achieve negative resection margins in 23 of the 25 patients. None of our patients had local recurrence during the period of this study. We believe surgical planning was greatly helped by high-resolution MRI scans of the hand. Our standard approach to managing patients who had close surgical margins was to use postoperative radiotherapy, even though we anticipated this would compromise functional outcome. When resection margins were positive, repeat resection to clear margins followed by radiotherapy was performed in one patient and radiotherapy alone in the other. The former option offers the best chance for local control of the tumor, although it may not make a difference to overall survival in the setting of soft tissue sarcomas [9, 11].
Good functional results reflected in the high mean MSTS score of 27.5 were achieved for patients who underwent single ray amputation for excision of tumors of the hand. The results compare favorably to those of a recent study that also included ray transposition and in which a mean MSTS score of 60% (18) was reported [16]. However, that group also included a patient who underwent ray amputation of the thumb followed by index ray amputation. Loss of the thumb ray expectedly resulted in a lower MSTS score that brought the mean score lower as well. Grip strength compared with the nonoperated side was only slightly poorer than that found in patients who underwent ray amputation in the trauma setting (66% versus 72% and 73%) [14, 19], and was equivalent for those who had concurrent ray transposition [7]. This most likely was attributable to the more extensive resection, including the interosseous muscle of adjacent digits, that was required for adequate tumor clearance.
Patients who had perioperative radiotherapy to the hand had somewhat lower MSTS scores, although the difference in the scores was not large and likely would not be clinically meaningful. Furthermore, as this group of patients generally had larger tumors and required more extensive surgery, it is uncertain if the poorer functional outcome was attributable to radiotherapy alone or a combination of factors. We were unable to identify any other factors having an effect on the MSTS score.
Surgical treatment options for malignant hand tumors include en bloc excision, partial amputations (single or multiple ray), or complete hand amputations. The first option allows preservation of all digits but may require multiple donor tissues for reconstruction. The more donor tissue required, the higher is the risk for donor site morbidity. Hand amputation gives the best tumor clearance but should be performed only when negative margins and reasonable retention of function are not possible. Partial hand amputations, in the form of a single or double ray amputation, offer a good oncologic solution with function superior to more proximal level amputation. Single ray amputation is an ablative and reconstructive surgical technique that has good oncologic functional and aesthetic outcomes. The amputated digit also can serve as a donor site for a fillet skin flap and nerve, vessel, and tendon grafts. In light of the excellent tumor control and good functional results, this technique should be considered when managing patients with benign tumors with metastatic potential or malignant tumors of the hand.
Footnotes
One of more of the authors (MEP) have received funding from the Daniel P. Sullivan Fellowship in Musculoskeletal Oncology.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anakwenze OA, Parker WL, Wold LE, Amrami KK, Amadio PC. Ewing’s sarcoma of the hand. J Hand Surg Eur Vol. 2009;34:35–39. doi: 10.1177/1753193408094922. [DOI] [PubMed] [Google Scholar]
- 2.Athanasian EA, Wold LE, Amadio PC. Giant cell tumors of the bones of the hand. J Hand Surg Am. 1997;22:91–98. doi: 10.1016/S0363-5023(05)80187-X. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RA. Fibro-osseous pseudotumour of the digit: amputation for a benign but aggressive lesion. J Hand Surg Br. 2005;30:504–506. doi: 10.1016/j.jhsb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 5.Gross E, Rao BN, Pappo AS, Michalkiewicz E, Hudson MM, Kaste SC, Greenwald CA, Pratt CB. Soft tissue sarcoma of the hand in children: clinical outcome and management. J Pediatr Surg. 1997;32:698–702. doi: 10.1016/S0022-3468(97)90008-7. [DOI] [PubMed] [Google Scholar]
- 6.Hamm JC, DeFranzo AJ, Argenta LC, White W. Keratoacanthoma necessitating metacarpal amputation. J Hand Surg Am. 1990;15:980–986. doi: 10.1016/0363-5023(90)90028-P. [DOI] [PubMed] [Google Scholar]
- 7.Hanel DP, Lederman ES. Index transposition after resection of the long finger ray. J Hand Surg Am. 1993;18:271–277. doi: 10.1016/0363-5023(93)90360-F. [DOI] [PubMed] [Google Scholar]
- 8.Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases: a study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68:743–746. [PubMed] [Google Scholar]
- 9.Heslin MJ, Woodruff J, Brennan MF. Prognostic significance of a positive microscopic margin in high-risk extremity soft tissue sarcoma: implications for management. J Clin Oncol. 1996;14:473–478. doi: 10.1200/JCO.1996.14.2.473. [DOI] [PubMed] [Google Scholar]
- 10.Jayakumar S, Jatavalabulla S, Miller IM. Peripheral primitive neuroectodermal tumour of the hand in an adult. J Hand Surg Eur Vol. 2007;32:460–461. doi: 10.1016/j.jhse.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone PA, Wexler LH, Venzon DJ, Jacobson J, Yang JC, Horowitz ME, DeLaney TF. Sarcomas of the hand and foot: analysis of local control and functional result with combined modality therapy in extremity preservation. Int J Radiat Oncol Biol Phys. 1994;29:735–745. doi: 10.1016/0360-3016(94)90561-4. [DOI] [PubMed] [Google Scholar]
- 12.Kobus RJ, Leinberry C, Kirkpatrick WH. Metastatic renal carcinoma in the hand: treatment with preoperative irradiation and ray resection. Orthop Rev. 1992;21:983–984. [PubMed] [Google Scholar]
- 13.Küntscher MV, Erdmann D, Homann HH, Steinau HU, Levin SL, Germann G. The concept of fillet flaps: classification, indications, and analysis of their clinical value. Plast Reconstr Surg. 2001;108:885–896. doi: 10.1097/00006534-200109150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Melikyan EY, Beg MS, Woodbridge S, Burke FD. The functional results of ray amputation. Hand Surg. 2003;8:47–51. doi: 10.1142/S0218810403001388. [DOI] [PubMed] [Google Scholar]
- 15.Mowlavi A, Quinn BM, Zook EG, Milner S. Ray amputation as a treatment for recurrent myxohyaline tumor of the distal extremity. Plast Reconstr Surg. 2003;111:1573–1574. doi: 10.1097/00006534-200304010-00046. [DOI] [PubMed] [Google Scholar]
- 16.Muramatsu K, Ihara K, Doi K, Hashimoto T, Seto S, Taguchi T. Primary reconstruction with digital ray transposition after resection of malignant tumor. Arch Orthop Trauma Surg. 2008;128:1017–1021. doi: 10.1007/s00402-007-0453-1. [DOI] [PubMed] [Google Scholar]
- 17.Nuzumlali E, Orhun E, Oztürk K, Cepel S, Polatkan S. Results of ray resection and amputation for ring avulsion injuries at the proximal interphalangeal joint. J Hand Surg Br. 2003;28:578–581. doi: 10.1016/S0266-7681(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 18.Patil S, Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28:602–608. doi: 10.1016/S0266-7681(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 19.Peimer CA, Wheeler DR, Barrett A, Goldschmidt PG. Hand function following single ray amputation. J Hand Surg Am. 1999;24:1245–1248. doi: 10.1053/jhsu.1999.1245. [DOI] [PubMed] [Google Scholar]
- 20.Slocum DB. Amputations of the fingers and the hand. Clin Orthop. 1959;15:35–59. [PubMed] [Google Scholar]
- 21.Talbert ML, Zagars GK, Sherman NE, Romsdahl MM. Conservative surgery and radiation therapy for soft tissue sarcoma of the wrist, hand, ankle, and foot. Cancer. 1990;66:2482–2491. doi: 10.1002/1097-0142(19901215)66:12<2482::AID-CNCR2820661207>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]