Abstract
Background
Osteonecrosis is a complication of corticosteroid therapy with limited treatment options in young, active patients. These options include debridement, core decompression, osteotomy, allografting, and partial or total knee replacement. Few studies exist regarding the use of osteochondral allografts for treatment of steroid-associated osteonecrosis.
Questions/purposes
We asked if fresh osteochondral allografts would (1) heal to host bone in the presence of osteonecrosis, (2) provide a clinically meaningful decrease in pain and improvement in function, and (3) prevent or postpone the need for prosthetic arthroplasty.
Patients and Methods
Twenty-two patients (28 knees) who underwent osteochondral allografting for high-grade, corticosteroid-associated osteonecrosis were evaluated. Their average age was 24.3 years (range, 16–44 years). The mean graft surface area was 10.8 cm2 (range, 5.0–19.0 cm2). Evaluation included a modified (for the knee) D’Aubigné and Postel (18-point) score, International Knee Documentation Committee (IKDC), and Knee Society function scores. The minimum followup was 25 months (mean, 67 months; range, 25–235 months).
Results
Five knees failed. The graft survival rate was 89% (25 of 28). The mean D’Aubigné and Postel score improved from 11.3 to 15.8; 19 of 25 (76%) had a score greater than 15. The mean IKDC pain score improved from 7.1 to 2.0, mean IKDC function score from 3.5 to 8.3, and mean Knee Society function score from 60.0 to 85.7.
Conclusions
Our data suggest osteochondral allografting is a reasonable salvage option for osteonecrosis of the femoral condyles. TKA was avoided in 27 of the 28 of knees at last followup.
Level of Evidence
Level IV, case series. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Aseptic osteonecrosis of the distal femur is a relatively rare but potentially serious side effect of acute or chronic systemic corticosteroid administration. After the femoral head, the femoral condyles are the second most common anatomic structure to be affected [12]. Juxtaarticular, epiphyseal lesions frequently lead to subchondral fracture and overlying chondrosis, progressing to joint collapse and, ultimately, to secondary arthritis (Fig. 1).
Fig. 1.
A posteroanterior, 45° flexion (Rosenberg) view radiograph demonstrating steroid-associated osteonecrosis of the left lateral femoral condyle in a 44-year-old woman.
Chronic, high-dose therapy is implicated most often in the etiology of steroid-associated osteonecrosis, with cumulative threshold doses as low as 480 mg having been reported as potentially causative [4]. However, the risk for osteonecrosis in patients with prednisone use less than 15 to 20 mg per day is considered very low [1]. Furthermore, systemic lupus erythematosus on its own is a recognized risk factor for osteonecrosis, but patients with systemic lupus erythematosus treated with high-dose corticosteroids have greater risk for development of osteonecrosis than with the presence of either risk factor alone [13].
The management of osteonecrosis of the knee remains challenging and controversial. There is little consensus regarding treatment options for osteonecrosis and many different therapeutic approaches have been proposed. These options range from activity modification to surgical intervention including arthroscopic debridement [10], core decompression [11], osteotomy [9], osteochondral grafting [2], and partial or total knee arthroplasty [14, 15]. Nonoperative treatment is largely ineffective in patients with symptomatic osteonecrotic lesions of the knee, leading to high rates of failure [12]. Retrograde drilling with small- [11] or large-diameter trephines, with or without bone grafting, arthroscopic debridement [10], and osteotomy [9] have been postulated for early stages (modified Ficat/Arlet Stages I–II) of osteonecrosis, with reported clinical success rates between 72% in medium-sized lesions and 92% in small lesions at short-term followup. None of these modalities has proven reproducibly effective in treating symptomatic, high-grade (modified Ficat/Arlet Stages III–IV) osteonecrotic lesions of the distal femur, often necessitating total joint arthroplasty.
Although generally successful, TKA is a reluctant choice in young, active patients owing to concerns regarding implant durability. Fresh osteochondral allografting may be considered a biologic alternative to arthroplasty in young (younger than 50 years) individuals with osteonecrosis of the knee. Osteochondral allografting is particularly attractive for this indication, as the allograft can address the osseous and chondral components of juxtaarticular necrotic lesions by replacing them with orthotopic tissue.
In this study, we asked if fresh osteochondral allografts would (1) heal to host bone in the presence of osteonecrosis, (2) provide a clinically meaningful decrease in pain and improvement in function, and (3) prevent or postpone the need for prosthetic arthroplasty.
Patients and Methods
We retrospectively reviewed 34 patients (43 knees) who underwent osteochondral allografting for osteonecrosis of the knee between 1984 and 2006. These patients declined prosthetic arthroplasty and were referred for consideration of allografting as an alternative to arthroplasty owing to their young age (younger than 50 years) and symptoms that did not respond to other treatment modalities and were enrolled in an Institutional Review Board-approved study to evaluate the effectiveness of osteochondral allografting for various knee diseases. All 34 patients were at least 2 years postoperative at the time they were identified from the database. Of these, 23 patients (29 knees) had received osteochondral allografts for osteoarticular lesions sustained secondary to steroid-associated osteonecrosis of the femoral condyles. One patient (one knee) was deceased attributable to his underlying condition and the status of his knee was not ascertained. The remaining 22 patients (28 knees) comprise the current study population, including 16 females and six males with an average age of 24.3 years (range, 16–44 years) and a mean body mass index of 21.5 (range, 17.1–28.1). All patients were referred for consideration of osteochondral allografting as an alternative to arthroplasty and presented with radiographic evidence of advanced, Stages III–IV (modified Ficat/Arlet stage) [12] lesions at the time of surgery. All had a history of a medical diagnosis requiring prednisone use exceeding doses of 20 mg per day, but only two of 22 were actively receiving corticosteroid therapy at the time of surgery (Table 1). All patients were evaluated preoperatively for limb malalignment with 54-inch standing radiographs and realignment osteotomy was ruled out as a treatment option before consideration of osteochondral allografting. No patients were lost to followup. All data were entered prospectively in an Institutional Review Board-approved clinical database.
Table 1.
Patient data
| Patient number | Knee | Gender | Age (years) | Underlying diagnosis | Steroid use at time of OCA | Number of grafts | Condyle | Total graft area (cm2) | Graft technique | Bone grafting | Followup (months) | 18-point D’Aubigné and Postel score/outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 01 | Female | 19 | Sickle cell anemia | No | 1 | Medial | Shell | No | 48 | 18 | |
| 2 | 02 | Male | 17 | Leukemia | No | 1 | Lateral | 9.9 | Shell | Yes | 27 | 17 |
| 03 | Male | 17 | Leukemia | No | 1 | Lateral | 11.6 | Shell | Yes | 27 | 17 | |
| 3 | 04 | Female | 16 | Leukemia | No | 2 | Medial and lateral | 11.8 | Shell | No | 45* | Revision allograft |
| 05 | Female | 17 | Leukemia | No | 1 | Lateral | 11.0 | Shell | Yes | 34 | 12 | |
| 4 | 06 | Female | 25 | SLE | No | 2 | Medial and lateral | 9.25 | Shell | No | 34 | 14 |
| 5 | 07 | Female | 39 | Crohn’s disease | No | 1 | Lateral | 17.2 | Shell | No | 122 | 11 |
| 08 | Female | 40 | Crohn’s disease | No | 2 | Medial and lateral | 9.2 | Shell | No | 115 | 11 | |
| 6 | 09 | Male | 25 | SLE | No | 1 | Medial | 17.5 | Shell | Yes | 46 | 18 |
| 7 | 10 | Male | 29 | Leukemia | No | 2 | Lateral | 6.3 | Plug | Yes | 25 | 18 |
| 8 | 11 | Female | 25 | SLE | Yes (unknown dosage) | 1 | Lateral | 5.25 | Shell | Yes | 30 | 17 |
| 9 | 12 | Male | 35 | Ulcerative colitis | No | 1 | Medial | 9.0 | Plug | Yes | 93 | 11 |
| 13 | Male | 32 | Ulcerative colitis | No | 2 | Medial and lateral | 7.4 | Shell | Yes | 127 | 11 | |
| 10 | 14 | Female | 21 | Leukemia | No | 1 | Medial | 10.0 | Shell | No | 96 | 16 |
| 11 | 15 | Female | 36 | Closed head injury | No | 1 | Lateral | 7.8 | Shell | No | 235 | 18 |
| 12 | 16 | Female | 17 | Hodgkin’s lymphoma | No | 3 | Medial | 11.2 | Plug | Yes | 33 | 16 |
| 17 | Female | 17 | Hodgkin’s lymphoma | No | 3 | Medial | 12.0 | Plug | Yes | 33 | 16 | |
| 13 | 18 | Female | 21 | Leukemia | No | 3 | Medial and lateral | 19.0 | Plug | Yes | 64 | 16 |
| 14 | 19 | Female | 18 | Renal transplant | No | 1 | Lateral | 11.0 | Shell | Yes | 98 | 17 |
| 15 | 20 | Female | 30 | Transient allergies | No | 2 | Medial and lateral | 13.75 | Shell | Yes | 78* | TKA |
| 16 | 21 | Female | 23 | SLE | No | 2 | Lateral | 10.5 | Shell | Yes | 159 | 16 |
| 17 | 22 | Female | 18 | Leukemia | No | 1 | Lateral | 5.0 | Plug | No | 35 | 18 |
| 23 | Female | 18 | Leukemia | No | 1 | Medial | 9.7 | Shell | Yes | 35 | 18 | |
| 18 | 24 | Female | 20 | Ulcerative colitis | No | 2 | Medial | 6.3 | Plug | Yes | 44 | 18 |
| 19 | 25 | Female | 18 | SLE | No | 3 | Medial and lateral | 19.0 | Plug | Yes | 40* | Revision allograft |
| 20 | 26 | Female | 44 | Renal infection | No | 1 | Lateral | 10 | Shell | No | 26 | 17 |
| 21 | 27 | Male | 28 | Ulcerative colitis | No | 2 | Medial | 8.5 | Plug | Yes | 46 | 17 |
| 22 | 28 | Male | 16 | Myositis | Yes (40 mg/day) | 1 | Lateral | 12.6 | Shell | No | 48 | 16 |
* Time to revision/conversion; OCA = osteochondral allografting; SLE = systemic lupus erythematosus.
Twelve of the surgeries involved only the left knee, four involved only the right, and six involved bilateral surgeries, for a total of 28 knees treated. Twenty-one knees had unicondylar lesions (12 lateral, nine medial), whereas seven knees had bicondylar involvement (medial and lateral femoral condyles in the same knee) and received allografts to both condyles. The mean total allograft surface area was 10.8 cm2 (range, 5.0–19.0 cm2). Thirteen of 28 (46%) knees had multiple grafts; these included grafts to both condyles in the case of bicondylar involvement, two grafts on the same condyle in the case of large lesions undergoing the plug technique, and additional nonstructural particulate bone grafting of necrotic areas beneath the osteochondral allografts in 18 of the 28 patients. Fourteen of 28 (50%) knees had an average of 1.5 previous surgeries (range, 1–5 surgeries), including arthroscopic debridement (seven), drilling (four), loose body removal (four), bone grafting (three), and distal femoral osteotomy (one). The remaining 14 had no prior surgery. The minimum followup in the 25 surviving grafts was 25 months (mean, 67 months; range, 25–235 months).
Preoperatively, donor and recipient were matched based on mediolateral dimension of the tibial plateau using a standard AP radiograph of the recipient corrected for magnification and direct measurement of the tibial width of the donor. No blood or tissue typing was performed and no immunosuppressive therapy was used. Fresh anatomically appropriate tissue was obtained from healthy donors aged 15 to 40 years who met the criteria of the American Association of Tissue Banks. Donor tissue was recovered within 24 hours of donor death and then processed and stored fresh at 4°C in tissue culture media (Modified Eagle’s Medium with 10% fetal bovine serum [Mediatech Inc, Herndon, VA]). Grafts were implanted between 5 and 21 days of donor death.
Surgery was performed with the patient in the supine position under tourniquet control using a full or mini-arthrotomy through a midline incision, as previously described [6]. The necrotic lesion(s) was debrided to identify the location, size, and shape. These characteristics determined whether a shell (Fig. 2) or plug type graft(s) was used. After initial debridement, graft beds were prepared down to healthy bleeding bone to a maximum depth of 12 mm (Fig. 3). Lesions extending deeper than 12 mm were curetted out manually and particulate bone graft was placed before seating the osteochondral allograft. Allograft thickness did not exceed 12 mm and we considered a 50% viable, bleeding bone in the prepared host bed acceptable for graft placement. The grafts were prepared to match the prepared lesion in size, shape, and depth (Fig. 4). Trial fittings were performed after the grafts were copiously pulse lavaged with saline to remove debris and marrow elements to decrease the immunogenicity of the graft. After grafts had been positioned and an acceptable fit and condylar reconstruction were established, fixation was supplemented using absorbable internal fixation devices or compression screws, if necessary, for graft stability (Fig. 5). The use of supplemental screw fixation was determined on a case-by-case basis and typically used when grafts extended to the medial, lateral, or posterior edge of the condyle and therefore were uncontained and did not have a stable press fit. The decision to use a shell or plug graft depended on the size and location of the lesion. For example, lesions of the posterior femoral condyles were inaccessible to the instruments used for plug grafts and thus a shell technique was used. The shell technique involves fashioning the graft and recipient site into complementary geometric shapes (trapezoidal) using burrs and hand tools. More anterior lesions less than 30 mm in diameter (the maximum dimension of the available instruments) were treated with a round plug graft. The plug technique involves preparation of the lesion site with a reaming tool placed over a guide wire and preparing a cylindrical graft using a coring device (Fig. 6). This requires placement of a guide wire and instruments perpendicular to the articular surface. Large, extensive lesions or long and narrow lesions were treated either with one trapezoidal shell graft or multiple overlapping round plugs, depending on which technique would result in the least amount of removal of healthy bone and cartilage. Seven of nine knees treated with the plug technique had multiple grafts. Regardless of the type of technique, no graft exceeded 12 mm in maximum thickness.
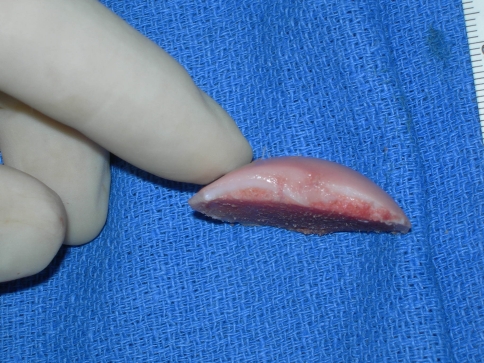
Fig. 2.
An intraoperative photograph shows the donor condyle with ink markings outlining dimensions of the planned shell allograft.
Fig. 3.
A lateral view of the prepared shell allograft shows the thickness of the compound graft aimed at restoring subchondral bone loss secondary to osteonecrosis.
Fig. 4.
A photograph shows a comparison view of the shell allograft (left) and removed pathologic recipient osteoarticular segment (right).
Fig. 5.
An intraoperative photograph of the osteochondral allograft in situ in anatomic position after fixation with five bioabsorbable chondral darts shows restoration of the weightbearing portion of the lateral femoral condyle.
Fig. 6.
An intraoperative photograph shows a reconstruction of the medial femoral condyle using two plug-type allografts.
Postoperative physical therapy included supervised ROM exercises and quadriceps strengthening. Closed chain exercises such as cycling were begun by 1 month after surgery. Weightbearing was limited to touch down for the first 6 weeks followed by gradual increases (single-crutch gait) until 3 months. Full weightbearing was allowed at 3 months if radiographs showed evidence of osseous integration of the graft (Fig. 7). Unrestricted activities were allowed at 6 months with counseling regarding potential risks of high-impact loading activities.
Fig. 7.
A Rosenberg view radiograph taken 3 months postoperatively, of the same patient as in Fig. 1, shows ongoing osseointegration of the osseous graft portion and restoration of the left lateral femoral condyle articular surface.
Clinical evaluation was performed preoperatively and at 6 weeks, 3 months, 6 months, and annually thereafter, using a modified D’Aubigné and Postel (18-point) scale [3], IKDC pain and function scores [8], and Knee Society function scores [7]. Patients unable to return for followup in person were contacted by telephone by one of us (SG), interviewed using a standardized questionnaire (Appendix 1), and asked to provide radiographs and clinical examination from their local physician. Sixteen patients (19 knees) were examined by the authors and six patients (nine knees) were interviewed by telephone only. We used a modified D’Aubigné and Postel scale [3] for assessing patients at each followup. Modification was made to the ROM component, with values relevant to the knee rather than the hip. This scale allots a maximum of six points each for absence of pain, knee ROM, and knee function, for a maximum total of 18 points. Any additional surgeries occurring on the operative joint were documented. Failure was defined as any subsequent operation on the allografted knee.
Radiographic evaluation for determination of allograft healing was performed by one unblinded observer (WDB) at 6 weeks, 3 months, 6 months, and 1 year. Three criteria were used to define healing: presence of bony trabeculae across the interface, disappearance of the initial radiolucent line between graft and host bone, and no change in position or fracture of the graft on serial radiographs. All patients completed the 1-year radiographic followup analysis. Fourteen knees (11 patients) had subsequent radiographic evaluation at a mean of 4.5 years (range, 2–7 years) to determine graft status. Failure was defined as resorption, collapse or fragmentation of the osseous portion of the graft, or loss of 50% of the initial joint space (representing the chondral portion of the allograft) in the involved compartment.
Preoperative and postoperative IKDC pain and function scores, Knee Society function scores, and the modified D’Aubigné and Postel scores were compared using nonparametric Wilcoxon signed-rank tests. Data were analyzed using SPSS® Version 13.0 (SPSS Inc, Chicago, IL).
Results
All knees showed radiographic evidence of graft healing within 1 year. Bony trabeculae crossing the graft-host interface were present in all grafts by 6 months, and disappearance of the radiolucent line between graft and host occurred in all knees by 1 year. No graft showed a change in position or fracture. Of the 14 knees with longer term radiographic followup, two showed signs of failure including resorption, fragmentation, and collapse of the allograft. No knees had loss of joint space of 50% or greater develop in the allografted compartment.
Eighteen of the 19 surviving patients (22 knees) reported reduced pain and improved function. The mean IKDC pain score improved (p < 0.001) from 7.1 to 2.0, and the mean IKDC function score increased (p = 0.002) from 3.5 to 8.3. Nineteen of the 25 (76%) knees with retained allografts scored 15 or greater on the 18-point D’Aubigné-Postel scale. The mean 18-point score improved (p < 0.001) from 11.3 preoperatively to 15.8 at the latest followup. The mean Knee Society function score improved (p = 0.005) from 60.0 to 85.7.
Twenty-seven of 28 (96%) avoided arthroplasty. Graft survival was 89% (25 of 28). Overall, five of the 28 knees (18%) had additional surgery and therefore were defined as having failed results. Allografts were revised or removed in three knees (11%). One patient had a TKA 78 months after the index (allograft) surgery owing to recurrent pain. This patient had bicondylar necrosis and multiple grafts but did not have radiographic evidence of graft failure. Two patients underwent repeat allografting. One had bicondylar involvement and had multiple grafts revised at 40 months. The other underwent distal femoral valgus osteotomy to correct residual valgus deformity at 26 months and subsequently underwent revision allografting of the lateral femoral condyle at 45 months from the initial allograft procedure. Both patients had recurrent pain and radiographic evidence of resorption, collapse, and fragmentation of their allografts. Two knees underwent surgery without removal of the allograft. One patient underwent a partial meniscectomy and another patient underwent two arthroscopic debridements at 22 and 34 months postoperatively.
Discussion
Osteochondral allografts are used increasingly in the treatment of certain knee conditions such as chondral injuries, osteochondritis dissecans, and posttraumatic reconstruction [6]. A major purpose of the comprehensive osteochondral allograft transplant program at our institution is to determine the outcome of this procedure for various indications. To our knowledge, this study is the largest clinical series of patients undergoing osteochondral allografting for steroid-associated osteonecrosis of the knee. Flynn et al. [5] reported on 15 patients with distal femoral osteonecrosis, but only seven patients had received corticosteroids. In the study by Bayne et al. [2], only four patients had steroid-associated osteonecrosis. Therefore, this study provides valuable information regarding the treatment of steroid-associated osteonecrosis of the femoral condyle. Specifically, we asked the following questions: would allografts heal to host bone in the presence of osteonecrosis, provide a clinically meaningful decrease in pain and improvement in function, and prevent or postpone the need for prosthetic arthroplasty.
This study has several limitations. The scoring instrument (modified D’Aubigné and Postel 18-point scale) has not been validated in the knee. This 18-point scale was chosen for historical comparison as this was the only outcome measure available for cases performed early during the study period, before the introduction of other validated knee outcome instruments. However, it provided a simple, standardized method of retrospectively evaluating patient outcomes on an objective basis, allowing comparison of patients over a relatively long followup. Using a telephone interview to obtain some of the outcome data in six of the 22 patients is not optimal. A telephone interview may not reveal some details of a patient’s status. Nonetheless, the use of a questionnaire with simple pain and function measures facilitated obtaining important outcome measures such as pain, function, reoperation, and revision surgery. Another limitation of this study is incomplete long-term radiographic followup. The interpretation of radiographs (or other imaging studies) could provide useful information regarding progression of disease in the treated joint, status of the allograft, and evidence of potential impending failure not observed by change in clinical performance.
Although many studies of osteochondral allografts do not report a significant rate of nonunion [6], an important question to ask is if steroid-associated osteonecrosis presents a uniquely different clinical situation for graft healing. Radiographic healing of fresh osteochondral allografts to host bone in the presence of osteonecrosis has not been shown comprehensively to date. Flynn et al. [5], using frozen allografts, reported that “all allografts demonstrated radiographic signs of union between 6 and 12 months,” but did not provide specific criteria. Our study showed that all allografts healed within 1 year, despite the presence of compromised host bone environment. We believe that surgical techniques that include stable graft fixation, removal and supplemental grafting of deep necrotic lesions, and protected weightbearing are important parts of our protocol that led to predictable healing of the allografts. Although not a specific aim of our study, the long-term radiograph performance of osteochondral allografts is also important. Two of 14 knees with long-term radiographs had graft collapse and were revised. The phenomenon of late graft failure also was reported by Bayne et al. [2]. They found that late collapse occurred in all of the patients who continued to take steroids and concluded that osteochondral allografting was not recommended in this group. They thought steroid use might interfere with revascularization of the osseous portion of the allograft. In our study, only two of 22 patients continued to take steroids and both had a good or excellent result. However, it is likely that with longer followup more patients will show radiographic signs of graft failure. We did not observe radiographic signs of failure of the chondral portion, which we defined as 50% loss of the joint space.
The second question we sought to answer was related to clinical outcome. Eighteen of 19 patients who did not have revision of their allografts (and therefore were considered to have clinical failure) had significant improvement in pain and function. Particularly notable is the marked improvement in pain, as this is a key feature of the clinical presentation of osteonecrosis and an important reason that patients seek treatment. The clinical outcomes of our patients appear to be better than that reported by Bayne et al. [2]. In their study, successful outcome, defined as improved clinical outcome and no reoperation, was reported as 46% to 74% of 21 patients, depending on the etiology of osteonecrosis. The four patients with steroid-associated osteonecrosis had unsatisfactory results. Bayne et al. concluded revascularization of the allografts was poor owing to the continuous use of high doses of steroids, leading to graft collapse. The difference in our results may be attributable to a different patient population or surgical technique. At the time of allografting, 90% of our patients were not taking steroids; therefore, the biologic milieu in which the graft was placed may have been better despite the presence of large areas of necrosis. The extent of necrotic bone also may be important. Both of our revisions and TKA conversion involved either bicondylar involvement or large necrotic areas (reflected in total graft area). Because of the small number of patients, we were unable to definitively conclude this was an independent risk factor for failure. Flynn et al. [5] used frozen allografts in treating knee osteonecrosis with good or excellent results, defined as functional ROM, unlimited walking, and no or mild pain, in 70% of patients (12/17) at an average followup of 4.2 years. Their technique included using relatively larger and thicker fragment frozen allografts with various fixation techniques. They did not report graft size, but the grafts were able to accommodate two or three cancellous screws. It is possible that surgical technique may influence outcome. Our technique of using smaller fragment fresh (rather than frozen) grafts, supplemental bone grafting, and careful attention to the mechanical environment of the knee (low body mass index and no limb malalignment) also may provide a more protected environment for the grafts. Overall, our clinical results of treatment of patients with steroid-associated osteonecrosis of the femoral condyle are comparable to those reported for treatment of other knee disorders [6].
The patients in this study specifically were seeking an alternative treatment to TKA. Our findings support the concept that fresh osteochondral allografting can postpone the need for arthroplasty in the majority of patients with steroid-associated osteonecrosis of the femoral condyle. Of the three patients undergoing subsequent major surgery, two had another allograft and one had an uneventful arthroplasty after 6.5 years. Flynn et al. [5] reported three of 17 patients underwent conversion surgery to arthroplasty and one allograft was revised. Bayne et al. [2] reported the use of arthroplasty as salvage surgery, but did not report a conversion rate. These results should be analyzed in the context of the outcome of primary knee arthroplasty for knee osteonecrosis. One of the most common surgical treatments for advanced osteonecrosis of the knee is TKA. However, results of traditional TKA for treatment of osteonecrosis historically have not been able to match the outcomes of TKA for osteoarthritis. Mont et al. [12] reported on 31 TKAs for secondary osteonecrosis in young patients with a mean age of 36 years. At a mean of 9 years, 34 of 48 (71%) were good or excellent. In a study by Seldes et al. [15], 24 of 31 knees (77.3%) were improved at a mean of 5 years. However, the use of cemented techniques and the advent of modern implant designs have improved the success rates for arthroplasty in treating osteonecrosis to approach outcomes for other indications. In a subsequent study by Mont et al. [14], 31 of 32 (97%) patients (average age, 44 years) had successful outcomes with cemented TKA at 9 years average followup. It is difficult to compare these results with the outcomes for patients in our study as the patient populations are different. The average age of patients in our study was 24.3 years and most were healthy, active, and free of disease that originally required steroids. We know of no studies of TKA on patients this young. Therefore, we cannot conclude if our patients would have been better suited with TKA as opposed to allografting. Our patients declined arthroplasty and chose to pursue an alternate surgical treatment. Although this may introduce a selection bias, it does reflect the perceived risk of arthroplasty in young individuals.
Steroid-associated osteonecrosis of the femoral condyle is a challenging clinical entity, often afflicting young individuals and causing significant pain and disability. Fresh osteochondral allografting is a useful procedure for improving pain and function, and also appears to be an appropriate alternative to TKA in this group. Longer-term followup and larger numbers of patients are necessary to better define the indications for osteochondral allografting in the setting of osteonecrosis and the long-term durability of this procedure.
Acknowledgments
We thank Judy Blake for help in preparation of this manuscript.
Appendix 1


Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Scripps Green Hospital, Scripps Clinic, University of California San Diego, and Shiley Center for Orthopaedic Research and Education at Scripps Clinic.
References
- 1.Anderton JM, Helm R. Multiple joint osteonecrosis following short-term steroid therapy: case report. J Bone Joint Surg Am. 1982;64:139–141. [PubMed] [Google Scholar]
- 2.Bayne O, Langer F, Pritzker KP, Houpt J, Gross AE. Osteochondral allografts in the treatment of osteonecrosis of the knee. Orthop Clin North Am. 1985;16:727–740. [PubMed] [Google Scholar]
- 3.D’Aubigne RM, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed] [Google Scholar]
- 4.Fisher DE, Bickel WH. Corticosteroid-induced avascular necrosis: a clinical study of seventy-seven patients. J Bone Joint Surg Am. 1971;53:859–873. [PubMed] [Google Scholar]
- 5.Flynn JM, Springfield DS, Mankin HJ. Osteoarticular allografts to treat distal femoral osteonecrosis. Clin Orthop Relat Res. 1994;303:38–43. [PubMed] [Google Scholar]
- 6.Görtz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374–1384. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 8.Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 9.Koshino T. The treatment of spontaneous osteonecrosis of the knee by high tibial osteotomy with and without bone-grafting or drilling of the lesion. J Bone Joint Surg Am. 1982;64:47–58. [PubMed] [Google Scholar]
- 10.Koshino T, Okamoto R, Takamura K, Tsuchiya K. Arthroscopy in spontaneous osteonecrosis of the knee. Orthop Clin North Am. 1979;10:609–618. [PubMed] [Google Scholar]
- 11.Marulanda G, Seyler TM, Sheikh NH, Mont MA. Percutaneous drilling for the treatment of secondary osteonecrosis of the knee. J Bone Joint Surg Br. 2006;88:740–746. doi: 10.1302/0301-620X.88B6.17459. [DOI] [PubMed] [Google Scholar]
- 12.Mont MA, Baumgarten KM, Rifai A, Bluemke DA, Jones LC, Hungerford DS. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82:1279–1290. doi: 10.2106/00004623-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Mont MA, Glueck CJ, Pacheco IH, Wang P, Hungerford DS, Petri M. Risk factors for osteonecrosis in systemic lupus erythematosus. J Rheumatol. 1997;24:654–662. [PubMed] [Google Scholar]
- 14.Mont MA, Rifai A, Baumgarten KM, Sheldon M, Hungerford DS. Total knee arthroplasty for osteonecrosis. J Bone Joint Surg Am. 2002;84:599–603. doi: 10.2106/00004623-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Seldes RM, Tan V, Duffy G, Rand JA, Lotke PA. Total knee arthroplasty for steroid-induced osteonecrosis. J Arthroplasty. 1999;14:533–537. doi: 10.1016/S0883-5403(99)90073-6. [DOI] [PubMed] [Google Scholar]