Abstract
Background
Minimally invasive knee arthroplasty seeks to diminish the problems of traditional extensile exposures aiming for more rapid rehabilitation of patients after surgery.
Questions/purposes
To determine if the subvastus approach results in less perioperative pain and blood loss, shorter hospital stay, and improved function at both early and long-term followup.
Methods
One hundred patients were enrolled in a prospective, randomized trial. Fifty were operated on using a minimally invasive subvastus approach and the other 50 by a conventional, peripatellar approach. Minimum followup was 3 years. A repeated-measures analysis of variance was used to compare the Knee Society score and range of motion during followup.
Results
The minimally invasive approach resulted in greater perioperative bleeding but no increase in transfusions. No differences were found in postoperative pain between groups nor did hospital stay show any differences. The range of motion on the third day after surgery was greater in the minimally invasive group. No differences were found in surgical time, femoral or tibial component orientation or outliers, or complication rates. Both Knee Society score and range of motion were superior using the minimally invasive subvastus approach during followup out to 36 months.
Conclusions
The minimally invasive subvastus approach can result in improved long-term Knee Society scores and range of motion of total knee arthroplasty without increased risk of component malalignment, surgical time, or complication rate.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
No universally accepted definition for minimally invasive surgery exists. It is defined as a minimally invasive technique in which the skin incision and surgical dissection have been modified to reduce the surgical morbidity associated with any procedure [11, 16]. This definition discriminates between techniques that involve only a smaller skin incision (mini-incision techniques) and those techniques properly called minimally invasive techniques. In minimally invasive techniques the skin incision is decreased but, in addition, the subvastus insertion is conserved, the patella is not everted, and the joint is not dislocated or hyperflexed until definitive placement of the tibial component. Less tissue injury with minimally invasive techniques has been demonstrated in other orthopaedic surgery fields through acute phase reactant analysis [6]. This decrease could produce advantages in terms of less bleeding, and postoperative pain, as well as shortened recovery time. However, poor exposure and visualization of important landmarks could precipitate inadequate orientation of the components or increase complication rates.
Some consensus exists regarding the surgical modifications that are required to provide a minimally invasive TKA [1, 2, 7, 13, 22, 23]. The skin incision should be reduced (8–13 cm). The vastus medialis insertion should be preserved. The patella should not be everted and tibiofemoral dislocation avoided. Knee hyperflexion should be minimized and used principally for placing the tibial component. Moreover, specially designed retractors, guides, and cutting blades are recommended to minimize soft tissue damage.
During the last decade, several minimally invasive knee approaches using some of these features have been described by several authors. These include a minimally invasive subvastus approach [4]; a minimally invasive midvastus approach [14]; a medial miniparapatellar approach [21]; and the quadriceps-sparing approach [23]. Some cadaveric studies have suggested that, among these approaches, only the minimally invasive subvastus approach preserves the vastus medialis attachment to the medial side of the patella [17, 24]. From this point of view, we argue that it is the only approach that could be strictly named a minimally invasive approach.
The advantages of minimally invasive techniques in TKA are still debated in all orthopaedic forums. Despite the large number of existing papers, there are hardly any well-designed trials capable of providing high levels of evidence [6]. This study was designed to address this need. Three research questions were raised: (1) Is the minimally invasive subvastus approach capable of providing advantages over traditional exposure with respect to decreasing perioperative bleeding, diminishing postoperative pain, or accelerating early recovery? (2) Does this approach introduce disadvantages compared with traditional exposures such as increasing surgical time, component malalignment, or complication rates? (3) Are the Knee Society score and ROM improved with the minimally invasive subvastus approach compared with traditional exposures?
Patients and Methods
A prospective, randomized trial was designed. One hundred patients were enrolled in this trial. To calculate the appropriate number of patients, a meaningful difference of 10% in each variable was supposed with statistical power of 80% (β type risk 0.8). This power analysis provided different sample sizes from 84 to 98 so a sample size of 100 was chosen. Patients were selected according to the following inclusion criteria: knee osteoarthritis and willingness to participate in the trial. Patients with knee flexions contracture greater than 10º, varus greater than 20º, valgus greater than 15º, body mass index greater than 40 kg/m2, or those who previously had knee surgery were excluded. Using a table of randomized numbers, the patients were divided into two groups of 50 members; in one group, TKAs were implanted using a minimally invasive subvastus approach (MIS Group). In the other group, the patients were operated on through a classic parapatellar approach (Control Group). No differences were found between the groups related to the following preoperative parameters: age, gender, weight, body mass index, preoperative hemoglobin values, preoperative global Knee Society score, objective or functional scores, preoperative ROM, and preoperative long-leg knee axis (Table 1).
Table 1.
Preoperative parameters
| Preoperative data | Age (years) | Gender | Weight | Body mass index (kg/m2) | Preoperative hemoglobin (g/dL) | Preoperative Knee Society score | Objective Knee Society score | Functional Knee Society score | Preoperative ROM | Preoperative knee deviation | Preoperative knee angle |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIS Group | 68.02 (8.14) | 14 M 36 F | 79.30 (14.20) | 30.97 (5.25) | 14.11 (1.13) | 82.44 (19.16) | 35.28 (11.90) | 47.16 (12.10) | 93.70º (16.74) | 45 VR 5 VL | 173.5º (3.51) |
| Standard Group | 70.64 (7.88) | 13 M 37 F | 78.13 (7.50) | 30.62 (3.42) | 13.75 (1.00) | 89.08 (31.55) | 47.96 (15.02) | 41.12 (20.70) | 99.29º (13.26) | 45 VR 5 VL | 174.1º (4.34) |
| Significance (p) | 0.102 | 0.822 | 0.912 | 0.693* | 0.064* | 0.093 | 0.465 | 0.436* | 0.079* | 1.000 | 0.157 |
Standard deviations in parentheses; *Student’s t-test has been applied; the remainder had the Mann-Whitney U test applied; MIS = minimally invasive surgery; M = male; F = female; VR = varus; VL = valgus.
All procedures were performed by the same surgeon (JRVG), who has broad experience in both conventional and minimally invasive TKA. A cemented Nexgen® (Zimmer®, Winterthur, Switzerland) prosthetic model was implanted in all patients. Minimally invasive procedures were performed using the modified subvastus technique described by Boerger and colleagues [4]. The patient was placed in a standard fashion with the tourniquet placed around the upper thigh. The tourniquet was inflated at the start of the procedure and released after placement of definitive components to secure hemostasis. A longitudinal patella-centered skin incision was created with a slight medial inclination from the superior pole of the patella to below the joint line. An inverted L-shaped arthrotomy was made along the medial side of the patella and the inferior border of the vastus medialis. The tibial osteotomy was performed first using a specifically designed extramedullary guide that incorporates a 7° posterior slope. Second, femoral preparation was performed using the specifically designed minimally invasive intramedullary guide with the previously measured valgus (on long-leg radiograph) and 3° of external rotation. The patella was resurfaced in all cases. In some cases, the patellar cut was made at the start of the bone resections to provide improved exposure. In no cases was the patella everted or was the joint dislocated or overflexed except during the definitive placement of the tibial component. The patients included in the standard surgery group were operated on using the classic medial parapatellar approach with patellar eversion.
All patients received prophylaxis to prevent infection by administration of 2 g of cefazolin 1 hour before surgery and 1 g every 8 hours for 24 hours. Deep venous prophylaxis consisted of administration of 40 or 60 mg enoxaparinus 12 hours before surgery and then each 24 hours for 6 weeks. All patients followed the same postoperative protocol. Ambulation and knee ROM exercise were started the day after surgery. No rapid recovery protocol was applied in any patient. Pain medication was administered only if the patient required it according to the following protocol: 1 g metamyzol each 8 hours and 50 mg meperidine if metamyzol was not enough. After hospital discharge, the patients were reviewed at 1 month, 3 months, 1 year, and 3 years.
Perioperative bleeding was assessed as follows: hemoglobin values at 6 and 48 hours after surgery were measured. Postoperative hemoglobin was compared with the preoperative value at 6 and 48 hours after surgery. Postoperative drainage, measured by calibrated bottle, number of patients transfused, and number of units of packed blood cells transfused per patient, were also compared. The criteria for blood transfusion were the same for both groups: hemoglobin levels lower than 8 mg/100 mL or lower than 10 mg/100 mL and signs or symptoms of heart insufficiency.
For the study of postoperative pain, we (MFV, VGS) retrospectively collected and then compared the number or patients who needed any pain medications, milligrams of metamyzol per kilogram administered per patient during the first 24 and 48 hours after surgery, and the number of patients who needed opioids to relieve pain. In the evaluation of early recovery, the day the patient was able to walk 20 meters and climb two stairs (walking start day) and the ROM on the third day after surgery were collected (JRVG). To measure the ROM, the same goniometer was used in all patients.
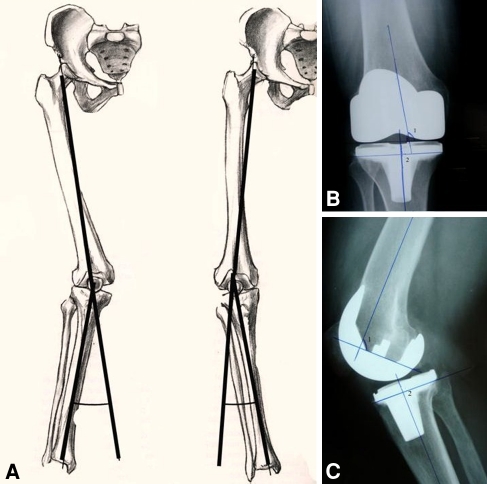
The skin-to-skin surgical time and complications during early and late followup were registered. For the evaluation of the component orientation (JRVE), the following measurements were made: long-leg mechanical angle of the knee drawing a line from the center of the femoral head to the intercondylar notch and from here to the center of the talus. We classified the alignment as varus when this angle was lower than 177°, neutral when this angle was between 177° to 183°, and valgus when it was higher than 183°. Similarly, the angles formed by the femoral component with the femoral mechanical axis and with the femoral diaphyseal axis in AP and lateral views were evaluated. In the same way, the angles formed by the tibial component with the tibial mechanical axis in AP and lateral views were measured (Fig. 1).
Fig. 1A–C.
The method used to measure component orientation is illustrated including (A) knee deviation and knee angle measurement, left valgus, right varus. (B) The method used to measure the angles formed by the femoral component (1) and diaphyseal axis in the AP view and by tibial component (2) and mechanical axis of the tibia in the AP view are shown. (C) This method was used to measure the angles formed by the femoral component (1) and diaphyseal axis in the lateral view and by the tibial component (2) and mechanical axis of the tibia in the lateral view.
Functional results between the groups were compared using the Knee Society score (KSS) assessed at 1 month, 3 months, 1 year, and 3 years. Global KSS and its subparts, objective and functional, were analyzed. Finally, the ROM (measured by goniometer, JRVG) at 1 month, 3 months, and 1 year was evaluated independently for its particular relevance in knee function.
All categorical variables were analyzed by chi square test. Kolmogorov-Smirnov and Levene test were applied to each numeric variable if the variable fit to normal distribution and if it showed variance homogeneity, a Student’s t test was used. Otherwise, a Mann-Whitney U test was applied. For the evaluation of the progress of the global KSS and ROM, a repeated-measures analysis of variance was used. The difference was considered statistically significant when p < 0.05.
Results
The results of the evaluation of the perioperative bleeding showed greater postoperative drainage and reduced hemoglobin levels in the first 6 hours in the MIS Group (Table 2). No differences were found in the hemoglobin values at 6 and 48 hours after surgery (p = 0.511 and p = 0.203, respectively). However, the decrease of hemoglobin values from preoperatively to 6 hours after surgery was higher in the MIS Group (p = 0.025). Moreover, the total postoperative drainage was greater in the MIS Groups (p = 0.001). On the other hand, the number of patients who received transfusions and packed blood cells transfused per patient did not show differences between the groups (p = 0.817).
Table 2.
Perioperative bleeding
| Perioperative bleeding | Hemoglobin levels at 6 hours (g/dL) | Hemoglobin fall from preoperative to 6 hours after surgery (g/dL) | Hemoglobin levels at 48 hours after surgery (g/dL) | Hemoglobin fall from preoperative to 48 hours after surgery (g/dL) | Patients transfused | Packed red blood cells transfused per patient | Drainage (mL) |
|---|---|---|---|---|---|---|---|
| MIS Group | 11.33 (1.17) | 2.87 (0.91) | 9.99 (1.02) | 4.19 (1.14) | 12 | 0.38 (0.75) | 389.5 (290.7) |
| Standard Group | 11.17 (1.07) | 2.41 (0.95) | 9.72 (0.90) | 3.86 (1.03) | 13 | 0.44 (0.88) | 193.8 (154.6) |
| Significance (p) | 0.511* | 0.025* 95% CI (−0.86/−0.06) | 0.203* | 0.165* | 0.817 | 0.817† | 0.001† |
Standard deviations in parentheses; *Student’s t-test was applied; †Mann-Whitney U test was used; MIS = minimally invasive surgery; 95% CI = 95% confidence interval.
Analyzing the postoperative pain, patients in the MIS Group required less pain medication during the early postoperative period (Table 3). The number of patients who needed any medication to relieve pain during the first 24 hours after surgery was lower in the MIS Group (p = 0.002). Furthermore, fewer patients of the MIS Group needed opioids for pain control in the first 24 hours (p = 0.007) and the first 48 hours (p = 0.007). Lastly, patients involved in the MIS Group consumed less metamyzol during the first 24 hours after surgery in comparison with patients operated on using the standard technique (p = 0.014).
Table 3.
Postoperative pain and recovery analysis
| Postoperative pain and early recovery | Patients consumed analgesic 24 hours | Patients consumed opioids 24 hours | Patients consumed analgesic 48 hours | Patients consumed opioids 48 hours | Milligrams metamyzol per kilogram per patient 24 hours | Milligrams metamyzol per kilogram per patient 48 hours | Walking start (days) | Hospital stay (days) | Third day ROM |
|---|---|---|---|---|---|---|---|---|---|
| MIS Group | 20 | 2 | 41 | 1 | 11.1 (10.2) | 25.98 (18.9) | 2.68 (0.62) | 8.3 (3.33) | 66.5º (14.91) |
| Standard Group | 35 | 11 | 36 | 9 | 25.9 (17.4) | 29.04 (19.5) | 4.47 (1.37) | 8.4 (2.05) | 55.4º (24.79) |
| Significance (p) | 0.002 | 0.007 | 0.307 | 0.007 | 0.002† 95% CI (5.71–23.9) | 0.568† | < 0.001* 95% CI (1.36–2.21) | 0.361 | 0.036† 95% CI (−19.64/−2.59 |
Standard deviations in parentheses; *Student’s t-test was applied; †Mann-Whitney U test was used; MIS = minimally invasive surgery; 95% CI = 95% confidence interval.
Regarding early recovery, the walking start day was earlier in the MIS Group compared with the Standard Group (p = 0.001). The MIS Group averaged the walking start day at 2.68 versus 4.47 days in the Standard Group. Moreover, the ROM on the third day after surgery was higher in the MIS Group, which achieved an average 66° of flexion compared with 55° of flexion in the Standard Group (p = 0.036) (Table 3). On the other hand, no difference was found in hospital stay.
There was no difference in the average surgical time between the MIS Group (69.2 minutes) and the Standard Group (60.6 minutes) (p = 0.090).
No differences were found in any of the component orientation parameters defined: postoperative long-leg mechanical axis of the knee, number of outliers, angles formed by the femoral component with the femoral mechanical axis and with the femoral diaphyseal axis in AP and lateral views, or angles formed by the tibial component with the tibial mechanical axis in AP and lateral views (Table 4).
Table 4.
Component orientation
| Radiographic measures | Knee deviation outliers | Postoperative knee angle | Fem-mech-axis angle | Fem-diaphyseal-axis AP view | Fem-diaphyseal-axis L view | Tibial-mech-axis angle AP view | Tibial-mech-axis angle L view |
|---|---|---|---|---|---|---|---|
| MIS Group | 5 VR 45 N | 178.9º (1.74) | 89.6º (0.82) | 97.2º (1.85) | 89.3º (1.28) | 89.08º (1.44) | 84.8º (1.92) |
| Standard Group | 9 VR 41 N | 178.8º (2.08) | 89.6º (1.13) | 96.7º (1.99) | 89.7º (1.35) | 89.1º (1.67) | 84.5º (3.52) |
| Significance (p) | 0.249* | 0.706† | 0.881† | 0.312† | 0.096† | 0.475† | 0.402† |
Standard deviations in parentheses; *Student’s t-test was applied; †Mann-Whitney U test was used; Fem-mech-axis angle = angle formed by femoral component and femoral mechanical axis; Fem-diaphyseal-axis AP view = angle formed by femoral component and diaphyseal axis in AP view; Fem-diaphyseal-axis L view = angle formed by femoral component and diaphyseal axis in L view; Tibial-mech-axis angle AP view = angle formed by tibial component and mechanical axis in AP view; Tibial-mech-axis angle L view = angle formed by tibial component and mechanical axis in L view; MIS = minimally invasive surgery; VR = varus; N = neutral.
No differences were found in complications rate. In the MIS Group, the following complications were detected: one case of superficial infection, which healed with only antibiotic treatment, and one case of scar dehiscence, which needed surgical revision. In the Standard Group, one patient presented a superficial infection and healed with only antibiotic treatment and another patient needed manipulation under sedation for knee stiffness 1 month after surgery. No differences were found in complication rate (p = 0.342).
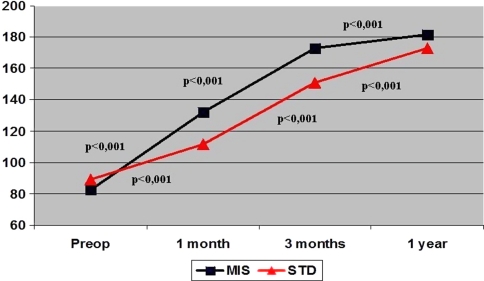
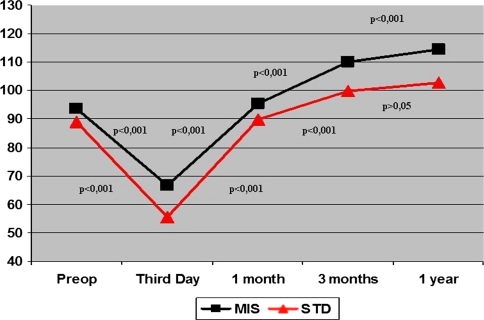
The global KSS at 1 month, 3 months, 1 year, and 3 years after surgery was higher in the MIS Group (p = 0.001). Furthermore, the objective score was greater in the MIS Group at 3 months, 1 year, and 3 years, whereas functional score was higher only at 1 and 3 months (Table 5). ROM was higher in the MIS Group at all followup time points out to 3 years of followup (Table 6). The timing of the improvement in the post-operative KSS was similar in both groups. Improved KSS from preoperative to 1 year was detected in both groups (p < 0.001; 95% confidence interval, 92.1–106.1 MIS Group; 72.5–94.9 Standard Group) (Fig. 2). Improvement in ROM occurred from 1 month to 3 months and then from 3 months to 1 year (Fig. 3). However, the analysis of ROM by repeated-measures analysis of variance revealed different progress of knee flexion in each group (Fig. 3). Both groups experienced a notable decrease of ROM from preoperatively to the third day after surgery, then a substantial improvement from the third day after surgery to 1 month and from 1 month to the third month. However, the progress from the third month to 1 year differs in each group. Whereas the MIS Group experienced major upgrowth during this time, the Standard Group did not. Moreover, the MIS Group presented an increase (p < 0.001) of ROM from preoperative values to the 1-year evaluation (95% confidence interval, 13.8–27.3), whereas the Standard Group did not show differences from preoperative to 1-year followup (p = 1.000).
Table 5.
Knee Society score functional results
| Postoperative Knee Society score | KSS 1 m | Objective score 1 month | Functional score 1 month | KSS 3 months | Objective score 3 months | Functional score 3 months | KSS 12 months | Objective score 12 months | Functional score 12 months | KSS 3 years | Objective score 3 years | Functional score 3 years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIS Group | 131.83 (23.23) | 69.31 (11.43) | 62.52 (15.18) | 173.03 (16.67) | 84.09 (7.88) | 88.94 (10.97) | 181.60 (10.99) | 86.98 (6.17) | 94.62 (7.18) | 182.42 (11.43) | 87.38 (4.57) | 95.04 (6.97) |
| Standard Group | 111.63 (28.53) | 67.85 (11.01) | 43.78 (20.44) | 151.00 (24.89) | 77.20 (9.80) | 73.80 (20.31) | 172.71 (20.28) | 82.37 (6.34) | 90.34 (16.54) | 172.52 (15.99) | 82.27 (6.24) | 90.25 (10.65) |
| Significance (p) | < 0.001 95% CI (30.5–9.8) | 0.517 | < 0.001 95% CI (25.8–11.5) | < 0.001 95% CI (13.6– 30.4) | < 0.001 95% CI (3.3–10.4) | < 0.001 95% CI (8.6–21.6) | < 0.001 95% CI (2.42–15.3) | < 0.001 95% CI (2.1–7.1) | 0.083 | < 0.001 95% CI (2.56–16.2) | < 0.001 95% CI (2.4–7.5) | 0.073 |
Standard deviations in parentheses; Mann-Whitney U test has been applied to all variables; MIS = minimally invasive surgery; KSS = Knee Society score; 95% CI = 95% confidence interval.
Table 6.
Knee ROM
| Postoperative ROM | ROM 1 month | ROM 3 months | ROM 12 months | ROM 3 years |
|---|---|---|---|---|
| MIS Group | 95.5º (12.43) | 110º (10.92) | 114.4º (6.97) | 113.7º (3.46) |
| Standard Group | 89.7º (15.99) | 99.9º (13.07) | 102.4º (10.65) | 102.7º (9.57) |
| Significance (p) | 0.049 95% CI (0.1–11.4) | < 0.001 95% CI (5.3–14.88) | < 0.001 95% CI (8–15.1) | < 0.001 95% CI (7.8–16.3) |
Standard deviations in parentheses; Mann-Whitney U test has been applied to all variables; MIS = minimally invasive surgery; 95% CI = 95% confidence interval.
Fig. 2.
Knee Society score development analyzed using repeated-measures analysis of variance is shown. Note how both groups presented significant upgrowth of Knee Society score in each time segment. MIS = minimally invasive; STD = standard.
Fig. 3.
Knee ROM improvements over time have been analyzed using repeated-measures analysis of variance and are shown in this figure. Both groups presented a significant decrease of ROM from preoperatively to the third day and then a substantial upgrowth from the third day to 1 month and from 1 month to the third month. However, the development from the third month to 1 year was different in both groups; when no notable increase was found in the Standard (STD) Group, a substantial upgrowth was detected in the minimally invasive (MIS) Group. Moreover, when no notable increase was detected in the STD Group from preoperative to 1 year, a substantial increase was found in the MIS Group.
Discussion
Minimally invasive TKA has been developed during the last decade. Today, it is easy to find hundreds of scientific works related to these techniques. Unfortunately, few well-designed trials exist capable of providing conclusions of high evidence about the real benefits and complications of minimally invasive TKA [6]. This study was performed to provide a higher level of evidence in comparison of MIS with a conventional approach in TKA. We found patients treated by MIS TKA had less pain early after surgery, achieved and sustained better ROM, but did not experience clinically important greater blood loss, component malpositioning, longer hospital stay, or incidence of complications.
This study presents several limitations. First, the study compares two different approaches: MIS subvastus approach and traditional medial parapatellar approach. The conventional subvastus approach has been demonstrated to be less aggressive than the medial parapatellar approach so probably not all the differences found in this study can be attributed to the MIS technique. Another limitation is the evaluation of postoperative pain by the painkiller intake instead of Visual Analog Scale (VAS). We decided to use the medication intake rather than a VAS because, in our opinion, the use of a pain VAS can be problematic. For example VAS scores do not account for administration of medication at the time the score is recorded. A patient who recently received medication will have a lower VAS score at the time the score is recorded than an unmedicated patient but the true severity of pain overall may be the same. In our opinion, it is more reliable to evaluate pain by painkiller intake. Finally, the variable of walking start day is not a validated measurement but provides an idea of faster recovery in the MIS Group. Other authors like Healy et al. [10] and Berger et al. [3] have used similar nonvalidated measurements to demonstrate faster recovery in an MIS Group.
Whereas the transfusion requirements did not show notable differences, the greater fall in hemoglobin from preoperative to 6 hours postoperatively and the greater drainage in the MIS Group have led us to conclude that the perioperative bleeding could be slightly higher in the MIS Group. Our results are in agreement with Boerger and colleagues [4] who detected 10% more bleeding with the minimally invasive subvastus approach. However, Zanasi [25] found no differences. Probably, the higher bleeding could be explained by the difficulty of making a perfect hemostasis after the components have been definitively placed and by the accumulation of blood under the vastus medialis muscle. In our opinion, although drainage may be greater, the most clinically relevant point is that no differences existed in the rate of transfusion. Nevertheless, there is a tendency toward more bleeding with MIS surgery.
Based on analgesic consumption, lower postoperative pain was detected in patients operated on by the minimally invasive subvastus approach. In our opinion, the use of the pain VAS presents several problems because the score would change depending on the moment it was applied in relation to the analgesic intake. Using the VAS of pain, Boerger and colleagues [4] found less pain in patients operated on using the MIS technique. Zanasi [25] and Lombardini and colleagues [15] also detected less postoperative pain in the MIS Group.
In our study, the early recovery was faster in the MIS Group compared with the standard approach. This is demonstrated by the earlier walking start and the greater ROM of the knee in the patients operated on using the MIS subvastus approach. On the other hand, the hospital stay showed no major differences. Although this fact may be surprising, Boerger and colleagues [4], Zanasi [25], and Kolisek and colleagues [12] also found substantial differences in early knee flexion but not in hospital stay. In contrast, Lombardini and colleagues [15] and Schroer and colleagues [20] found earlier hospital discharge with the minimally invasive subvastus approach. The application of rapid recovery protocols may accelerate the recovery of these patients and reduce the hospital stay [2, 5, 19]. However, one should not forget that Healy and colleagues [10] showed how an aggressive rapid recovery protocol was capable of reducing hospital stay and cost of TKA performed by the conventional approach as well.
No differences were found in surgical time. Our subjective opinion is the minimally invasive subvastus TKA consumes a little more time in the placement of the prosthesis, but this time is recovered during closure. Probably, the skin-to-skin time measurement has hidden this subjective difference. This impression has been confirmed by Boerger and colleagues [7] and Zanasi [25] (IIb), both of whom have detected longer duration for the MIS procedure measuring the surgical time from skin to prosthesis placement.
Component orientation was similar in both groups with no differences in outliers or any angles measured. All studies which involve minimally subvastus approach have detected correct component orientation without differences compared with standard approach [4, 9, 12, 15, 20]. Using a mini-incision midvastus approach, Dalury and Dennis [8] found worse component orientation with the mini-incision technique; however, this study has been criticized by other authors [18]. Our experience has led us to believe the surgeon’s previous experience in knee arthroplasty is an important factor in accomplishing reliable component placement with reduced exposures.
No differences were found in complication rates and, again, no previous report exists in which the complication rate was higher in the MIS Group [4, 15].
Functional results, both KSS and ROM, were substantially better in the MIS Group and were maintained until 3 years of followup. Our results are in accordance with most prior studies [4, 15, 20, 25]. We assume this better result can be attributed to less scar formation resulting from less dissection, including the conservation of all vastus medialis attachments. In contrast, Kolisek and colleagues [12] did not find differences in KSS at 3 and 12 weeks.
Using repeated-measures analysis of variance, the KSS and ROM development were studied. Although no differences were found in the KSS development between the groups, the ROM analysis showed the minimally invasive subvastus approach is capable of achieving and sustaining better knee ROM.
In conclusion, we found the minimally invasive subvastus approach can provide advantages over conventional surgery in terms of postoperative pain and recovery, but not in terms of perioperative bleeding. When compared with traditional exposure, the minimally invasive subvastus approach did not increase surgical time, risk of component malalignment, or complication rate. The early and late functional results were substantially better with the minimally invasive subvastus approach compared with the conventional approach.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Hospital Álvarez-Buylla, Mieres, Spain.
References
- 1.Alan RK, Tria AJ., Jr Quadriceps-sparing total knee arthroplasty using the posterior stabilized TKA design. J Knee Surg. 2006;19:71–76. doi: 10.1055/s-0030-1248082. [DOI] [PubMed] [Google Scholar]
- 2.Berger RA, Deirmengian CA, Della Valle GJ, Paprosky WG, Jacobs JJ, Rosenberg AG. A Technique for minimally invasive, quadriceps-sparing total knee arthroplasty. J Knee Surg. 2006;19:63–70. doi: 10.1055/s-0030-1248081. [DOI] [PubMed] [Google Scholar]
- 3.Berger RA, Sanders S, Gerlinger T, Della Valle C, Jacobs JJ, Rosenberg AG. Outpatient total knee arthroplasty with a minimally invasive technique. J Arthroplasty. 2005;20(Suppl 3):33–38. doi: 10.1016/j.arth.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Boerger TO, Aglietti P, Mondanelli N, Sensi L. Mini-subvastus versus parapatellar approach in total knee arthroplasty. Clin Orthop Relat Res. 2005;440:82–87. doi: 10.1097/01.blo.0000185755.09777.2d. [DOI] [PubMed] [Google Scholar]
- 5.Buvanendran A, Tuman KJ, McCoy DD, Matusic B, Chelly JE. Anesthetic techniques for minimally invasive total knee arthroplasty. J Knee Surg. 2006;19:133–136. doi: 10.1055/s-0030-1248095. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan JJ, Warth LC, Liu SS, Hozack WJ, Klein GR. Internet promotion on MIS and CAOS in TKA by Knee Society members. Clin Orthop Relat Res. 2006;452:97–101. doi: 10.1097/01.blo.0000238819.33154.95. [DOI] [PubMed] [Google Scholar]
- 7.Cook JL, Cushner FD, Scuderi GR. Minimal-incision total knee arthroplasty. J Knee Surg. 2006;19:46–51. doi: 10.1055/s-0030-1248078. [DOI] [PubMed] [Google Scholar]
- 8.Dalury DF, Dennis DA. Mini-incision total knee arthroplasty can increase risk of component malalignment. Clin Orthop Relat Res. 2005;440:77–81. doi: 10.1097/01.blo.0000185757.17401.7b. [DOI] [PubMed] [Google Scholar]
- 9.Hart R, Janecek M, Cizmar I, Stipcak V, Kucera B, Filan P. Navigated minimally invasive total knee arthroplasty. Orthopade. 2006;35:552–557. doi: 10.1007/s00132-006-0929-7. [DOI] [PubMed] [Google Scholar]
- 10.Healy WL, Iorio R, Ko J, Appleby S, Lemos SW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84:348–353. doi: 10.2106/00004623-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Howell JR, Garbuz DS, Duncan CP. Minimally invasive hip replacement: rationale, applied anatomy, and instrumentation. Orthop Clin North Am. 2004;35:107–118. doi: 10.1016/S0030-5898(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 12.Kolisek FR, Bonutti PM, Hozack WJ, Purtill J, Sharkey PF, Zelicof SB, Ragland PS, Kester M, Mont MA, Rothman RH. Clinical experience using a minimally invasive surgical approach for total knee arthroplasty. J Arthroplasty. 2007;22:8–13. doi: 10.1016/j.arth.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Laskin RS. Reduced-incision total knee replacement through a mini-midvastus technique. J Knee Surg. 2006;19:52–57. doi: 10.1055/s-0030-1248079. [DOI] [PubMed] [Google Scholar]
- 14.Laskin RS, Beksac B, Phongjiunakorm A, Pittors K, Davis J, Shim JC, Pavlov H, Petersen M. Minimally invasive total knee replacement through a mini-midvastus incision: an outcome study. Clin Orthop Relat Res. 2004;428:74–81. doi: 10.1097/01.blo.0000148582.86102.47. [DOI] [PubMed] [Google Scholar]
- 15.Lombardini AV, Viacava AJ, Berend KR. Rapid recovery protocols and minimally invasive surgery help achieve high knee flexion. Clin Orthop Relat Res. 2006;452:117–122. doi: 10.1097/01.blo.0000238824.56024.7a. [DOI] [PubMed] [Google Scholar]
- 16.Malik A, Dorr LD. The science of minimally invasive total hip arthroplasty. Clin Orthop Relat Res. 2007;463:74–84. doi: 10.1097/BLO.0b013e3181468766. [DOI] [PubMed] [Google Scholar]
- 17.Pagnano MW, Meneghini M, Trousdale RT. Anatomy of the extensor mechanism in reference to quadriceps-sparing TKA. Clin Orthop Relat Res. 2006;452:102–105. doi: 10.1097/01.blo.0000238788.44349.0f. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez HA. Mini-incision total knee arthroplasty can increase risk of component malalignment. Clin Orthop Relat Res. 2006;449:320. doi: 10.1097/01.blo.0000229283.37862.ea. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg AG. Anesthesia and analgesia protocols for total knee arthroplasty. Am J Orthop. 2006;35(Suppl):23–26. [PubMed] [Google Scholar]
- 20.Schroer WC, Diesfeld PJ, Reedy ME, LeMarr AR. Mini-subvastus approach for total knee arthroplasty. J Arthroplasty. 2008;23:19–25. doi: 10.1016/j.arth.2006.12.100. [DOI] [PubMed] [Google Scholar]
- 21.Scuderi GR, Tenholder M, Capeci C. Surgical approaches in mini-incision total knee arthroplasty. Clin Orthop Relat Res. 2004;428:61–67. doi: 10.1097/01.blo.0000148574.79874.d0. [DOI] [PubMed] [Google Scholar]
- 22.Sporer SM. The minimally invasive subvastus approach for primary total knee arthroplasty. J Knee Surg. 2006;19:58–62. doi: 10.1055/s-0030-1248080. [DOI] [PubMed] [Google Scholar]
- 23.Tria AJ, Jr, Coon TM. Minimally invasive total knee arthroplasty: early experience. Clin Orthop Relat Res. 2003;416:185–190. doi: 10.1097/01.blo.0000093030.56370.d9. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe N, Narita W, Namura T, Ito H, Nishimura T, Kubo T. Anatomical assessment of the vastus medialis oblique muscle in patients with osteoarthritis of the knee. J Arthroplasty. 2008;23:287–292. doi: 10.1016/j.arth.2006.12.103. [DOI] [PubMed] [Google Scholar]
- 25.Zanasi S. Minimally invasive computer-assisted total knee arthroplasty through a subvastus approach. Orthopedics. 2006;29(Suppl):S142–S144. [PubMed] [Google Scholar]