Abstract
Background
Resection of large tumors of the proximal tibia may be reconstructed with endoprostheses or allografts with fixation. Endoprosthetic replacement is associated with high failure rates and complications. Proximal tibia osteoarticular allografts after tumor resection allows restoration of bone stock and reconstruction of the extensor mechanism, but the long-term failure rates and complications are not known.
Questions/purposes
We therefore determined (1) the middle- and long-term survival of proximal tibia osteoarticular allografts, (2) their complications, and (3) functional (Musculoskeletal Tumor Society score) and radiographic (International Society of Limb Salvage) outcomes in patients treated with this reconstruction.
Patients and Methods
We retrospectively reviewed 52 patients (58 reconstructions including six repeat reconstructions) who underwent osteoarticular proximal tibia allograft reconstructions after resection of a bone tumor. The minimum followup of the 46 surviving patients was 72 months (mean, 123 months; range, 10–250 months). Survival of the allograft was estimated using the Kaplan-Meier method. We documented outcomes using the Musculoskeletal Tumor Society functional scoring system and the International Society of Limb Salvage radiographic scoring system.
Results
Six patients died from tumor-related causes without allograft failure before the 5-year radiographic followup. At last followup, 32 of the 52 remaining allografts were still in place; 20 failed owing to infections, local recurrences, or fractures. Overall allograft survival was 65% at 5 and 10 years, with an average Musculoskeletal Tumor Society functional score of 26 points and an average radiographic result of 87%.
Conclusions
Based on these data we believe proximal tibia osteoarticular allograft is a valuable reconstructive procedure for large defects after resection of bone tumors.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Tumor excision with wide surgical margins is the primary goal of treatment for some aggressive or recurrent benign bone tumors and malignant bone sarcomas. This requires an aggressive surgical resection, with potentially large osseous defects. Functional reconstructive options for such large defects include structural allograft transplantation [5, 6, 13, 16, 17, 19, 23, 28], endoprosthetic arthroplasty [4, 5, 12, 18, 22, 25, 26], and composite reconstruction using allografts and metal prostheses [2, 3, 7]. Limb salvage surgery of the proximal tibia is one of the most demanding reconstructions owing to difficulties with soft tissue coverage [14, 24], a high rate (12% to 36%) of infection [3, 6, 7, 12, 14, 21], and the necessity of restoring the knee extensor mechanism [1, 4, 5, 12, 17, 22], all of which lead to high failure rates (27% to 55%) [6, 7, 12, 13, 26]. As an alternative, the use of proximal tibia osteoarticular allografts after tumor resection may restore bone stock and reconstruct the extensor mechanism [6, 13, 16, 19].
As diagnostic and therapeutic techniques improve, patients with musculoskeletal sarcomas should expect increased survival, decreased complications and side effects, and an improved quality of life. Functional longevity of reconstructions becomes a major concern, especially in young and physically active patients, who place high demands on the reconstructions. Although the longer-term survival of endoprosthetic reconstructions has been described [12, 21], that for allografts has not.
We therefore determined (1) the middle- and long-term survival of proximal tibia osteoarticular allografts, (2) their complications, and (3) functional (Musculoskeletal Tumor Society score) and radiographic (International Society of Limb Salvage) outcomes in patients treated with this reconstruction.
Patients and Methods
We retrospectively reviewed all 52 patients (58 reconstructions including six with repeat reconstructions) with bone tumors or secondary to a previous allograft failure who underwent a proximal tibia osteoarticular allograft reconstruction from February 1982 to March 2002. The primary diagnoses were giant cell tumor in 21 patients, osteosarcoma in 22, Ewing’s sarcoma in six, chondrosarcoma in one, recurrent osteoblastoma in one, and recurrent chondroblastoma in one. The mean age of the patients at the time of the reconstruction was 24 years (range, 10–54 years). Twenty-six of the patients were females and 26 were males (Table 1). Six patients died from tumor-related causes without allograft failure before the 5-year radiographic followup. The minimum followup of the 46 surviving patients was 72 months (mean, 123 months; range, 10–250 months). This left 52 allografts for the study. No patients were lost to followup. Our study was approved by the local institutional review board.
Table 1.
Demographics and clinical data
| Case | Gender | Age (years) | Diagnosis | Chemotherapy | Followup (months) | Allograft status | Reason for removal | Procedure | Limb status | Patient status | Internal fixation | Functional score | Radiographic score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 30 | GCT | No | 240 | Preserved | Preserved | ANED | Plate | 26 | 86% | ||
| 2 | Male | 34 | GCT | No | 200 | Preserved | Preserved | ANED | Plate | 20 | 49% | ||
| 3 | Female | 28 | GCT | No | 250 | Preserved | Preserved | ANED | Plate | 26 | 49% | ||
| 4 | Female | 29 | GCT | No | 200 | Preserved | Preserved | ANED | Plate | 28 | 80% | ||
| 5 | Male | 31 | GCT | No | 200 | Preserved | Preserved | ANED | Plate | 26 | 86% | ||
| 6 | Male | 44 | GCT | No | 22 | Not preserved | Infection | Knee arthrodesis | Preserved | ANED | Plate | ||
| 7 | Female | 36 | GCT | No | 220 | Preserved | Preserved | ANED | Plate | 27 | 80% | ||
| 8 | Male | 19 | GCT | No | 166 | Preserved | Preserved | ANED | Plate | 26 | 94% | ||
| 9 | Male | 27 | GCT | No | 210 | Preserved | Preserved | ANED | Plate | 29 | 91% | ||
| 10 | Female | 16 | OS | Yes | 12 | Not preserved | Infection | Knee arthrodesis | Preserved | ANED | Plate | ||
| 11 | Male | 18 | OS | Yes | 200 | Preserved | Preserved | ANED | Plate | 28 | 94% | ||
| 12 | Male | 13 | OS | Yes | 10 | Not preserved | Local recurrence | Amputation | Not preserved | D | Plate | ||
| 13 | Female | 12 | Ewing | Yes | 12 | Not preserved | Infection | Second OA | Preserved | ANED | Plate | ||
| 14 | Male | 15 | OS | Yes | 24 | Not preserved | Infection | Amputation | Not preserved | ANED | Plate | ||
| 15 | Female | 14 | AI | No | 200 | Preserved | Preserved | ANED | Plate | 27 | 91% | ||
| 16 | Female | 18 | GCT | No | 4 | Not preserved | Infection | Knee arthrodesis | Preserved | ANED | Plate | ||
| 17 | Female | 11 | OS | Yes | 150 | Preserved | Preserved | ANED | Plate | 29 | 89% | ||
| 18 | Male | 15 | OS | Yes | 144 | Preserved | Preserved | ANED | Plate | 22 | 86% | ||
| 19 | Male | 19 | OS | Yes | 144 | Preserved | Preserved | ANED | Plate | 26 | 89% | ||
| 20 | Female | 13 | OS | Yes | 25 | Not preserved | Infection | Alloprosthesis | Preserved | ANED | Nail | ||
| 21 | Male | 13 | OS | Yes | 13 | Preserved | Preserved | D | Plate | ||||
| 22 | Female | 35 | GCT | No | 130 | Preserved | Preserved | ANED | Plate | 27 | 97% | ||
| 23 | Male | 20 | OS | Yes | 12 | Not preserved | Local recurrence | Amputation | Not preserved | D | Plate | ||
| 24 | Male | 17 | OS | Yes | 24 | Not preserved | Infection | Alloprosthesis | Preserved | ANED | Plate | ||
| 25 | Female | 20 | Ewing | Yes | 150 | Preserved | Preserved | ANED | Plate | 28 | 89% | ||
| 26 | Female | 35 | GCT | No | 22 | Not preserved | Local recurrence | Amputation | Not preserved | ANED | Plate | ||
| 27 | Male | 37 | CS | No | 24 | Not preserved | Infection | Alloprosthesis | Preserved | ANED | Nail | ||
| 28 | Male | 16 | RC | No | 144 | Preserved | Preserved | ANED | Plate | 26 | 94% | ||
| 29 | Male | 13 | OS | Yes | 28 | Not preserved | Infection | Knee arthrodesis | Preserved | ANED | Nail | ||
| 30 | Female | 18 | GCT | No | 6 | Not preserved | Infection | Knee arthrodesis | Preserved | ANED | Plate | ||
| 31 | Female | 14 | OS | Yes | 130 | Preserved | Preserved | ANED | Nail | 19 | 89% | ||
| 32 | Female | 46 | OS | Yes | 16 | Not preserved | Local recurrence | Amputation | Not preserved | D | Nail | ||
| 33 | Female | 35 | Ewing | Yes | 36 | Preserved | Preserved | D | Plate | ||||
| 34 | Male | 21 | OS | Yes | 24 | Not preserved | Fracture | Second OA | Preserved | ANED | Nail | ||
| 35 | Male | 35 | GCT | No | 120 | Preserved | Preserved | ANED | Plate | 29 | 91% | ||
| 36 | Male | 15 | Ewing | Yes | 15 | Preserved | Preserved | D | Plate | ||||
| 37 | Female | 25 | GCT | No | 100 | Preserved | Preserved | ANED | Plate | 30 | 94% | ||
| 38 | Female | 10 | OS | Yes | 24 | Not preserved | Infection | Second OA | Preserved | ANED | Nail | ||
| 39 | Male | 13 | OS | Yes | 110 | Preserved | Preserved | ANED | Nail | 25 | 74% | ||
| 40 | Male | 18 | OS | Yes | 36 | Preserved | Preserved | D | Plate | ||||
| 41 | Female | 30 | GCT | No | 24 | Not preserved | Fracture | Second OA | Preserved | ANED | Plate | ||
| 42 | Male | 23 | AF | No | 35 | Preserved | Preserved | D | Plate | ||||
| 43 | Male | 54 | OS | Yes | 12 | Not preserved | Infection | Alloprosthesis | Preserved | ANED | Plate | ||
| 44 | Male | 35 | GCT | No | 100 | Preserved | Preserved | ANED | Plate | 30 | 100% | ||
| 45 | Female | 10 | AI | No | 100 | Preserved | Preserved | ANED | Plate | 25 | 94% | ||
| 46 | Female | 46 | GCT | No | 100 | Preserved | Preserved | ANED | Plate | 30 | 100% | ||
| 47 | Male | 36 | GCT | No | 96 | Preserved | Preserved | ANED | Plate | 28 | 94% | ||
| 48 | Male | 16 | OS | Yes | 90 | Preserved | Preserved | ANED | Plate | 29 | 74% | ||
| 49 | Female | 32 | RO | No | 16 | Not preserved | Infection | Second OA | Preserved | ANED | Plate | ||
| 50 | Male | 24 | GCT | No | 90 | Preserved | Preserved | ANED | Plate | 30 | 100% | ||
| 51 | Female | 16 | OS | Yes | 24 | Not preserved | Fracture | Second OA | Preserved | ANED | Plate | ||
| 52 | Female | 18 | Ewing | Yes | 85 | Preserved | Preserved | ANED | Plate | 25 | 71% | ||
| 53 | Female | 23 | Ewing | Yes | 36 | Preserved | Preserved | D | Plate | ||||
| 54 | Female | 26 | GCT | No | 84 | Preserved | Preserved | ANED | Plate | 27 | 100% | ||
| 55 | Female | 32 | AF | No | 70 | Preserved | Preserved | ANED | Plate | 27 | 94% | ||
| 56 | Female | 12 | OS | Yes | 78 | Preserved | Preserved | ANED | Plate | 28 | 80% | ||
| 57 | Female | 30 | AI | No | 75 | Preserved | Preserved | ANED | Plate | 27 | 89% | ||
| 58 | Female | 18 | AF | No | 72 | Preserved | Preserved | ANED | Plate | 26 | 89% |
GCT = giant cell tumor; OS = osteosarcoma; CS = chondrosarcoma; RC = recurrent chondroblastoma; RO = recurrent osteoblastoma; AI = allograft infection; AF = allograft fracture; OA = osteoarticular allograft; ANED = alive with no evidence of disease; D = deceased.
We performed staging studies in all patients, including plain radiography, CT, and MRI of the affected limb. CT studies of the chest also were performed in all patients. According to the grading system of Enneking [8], the 29 malignant tumors were Stage II-B, and the benign lesions were all Stage III lesions.
The nonirradiated allografts were harvested under sterile conditions and stored frozen in the bone bank at the study institution, according to a technique described previously [20]. No attempt was made to preserve the viability of the articular cartilage, and bacteriologic and viral tests available at the time were performed in accordance with the recommendations of the American Association of Tissue Banks. Fifty-two transplants were primary procedures (Fig. 1) and six were secondary procedures (after three infections (Fig. 2) and three fractures of an earlier transplant). Thirty-one allografts were performed in conventionally ventilated operating rooms (OR) and 27 were performed in a vertical laminar airflow OR, established at the institution in 1996. The allografts were selected by comparing the age, gender, height, and radiographs of the patient with corresponding data from the donor to achieve the closest anatomic match. The grafts were taken out of their packaging and placed directly in a warm normal saline solution. After being thawed, the donor bone was cut to the proper length and soft tissue structures such as the cruciate ligaments, medial collateral ligament, and posterior capsule were prepared for implantation.
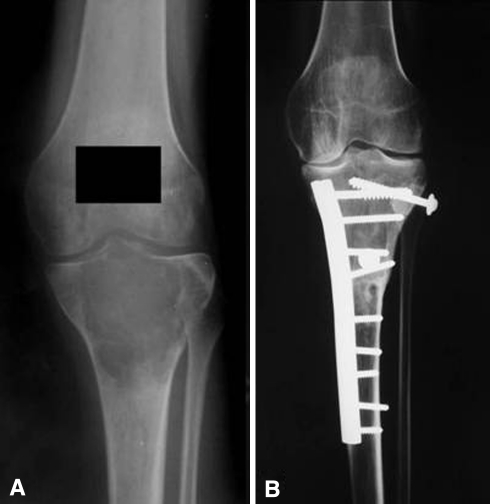
Fig. 1A–B.
The radiographs illustrate the case of a 25-year-old woman with a diagnosis of giant cell tumor who had a proximal tibia osteoarticular allograft after resection of the tumor (Case 37). (A) A preoperative AP radiograph shows the extension of the tumor that compromises the proximal end of the tibia. (B) The 8-year radiographic control shows a solid union of the osteotomy.
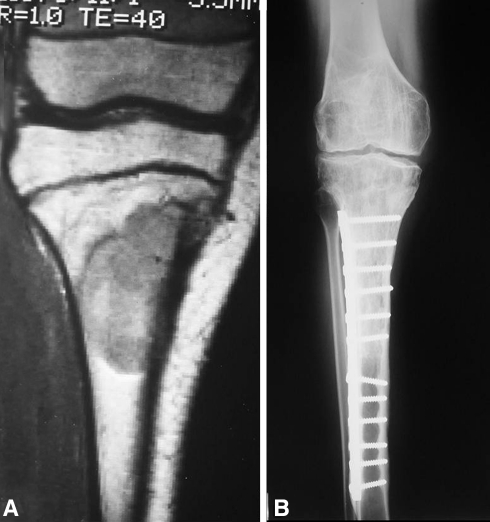
Fig. 2A–B.
The images illustrate the case of a 12-year-old girl with a diagnosis of Ewing’s sarcoma who received chemotherapy and had an osteoarticular allograft after tumor resection of the proximal tibia (Cases 13 and 15). Owing to a deep infection, the osteoarticular allograft was removed (Case 13) and antibiotic-impregnated polymethylmethacrylate spacer cement was placed. After achieving infection control, a second osteoarticular allograft was performed (Case 15). (A) A coronal MR image obtained after chemotherapy but before the first surgery was performed shows the tumor extension. (B) An AP radiograph of the second osteoarticular allograft after 16 years of followup shows joint deterioration.
Through an extended anteromedial approach to the knee, we released the extensor mechanism by sectioning the host patellar tendon and resected the tumor at the proximal tibia with an adequate margin of normal tissue according to preoperative staging studies. All resections were intraarticular and intracompartmental. A transverse osteotomy was used in every case. At the time of allograft implantation, rigid fixation of the host-donor junction was always obtained. In 50 transplants, a dynamic compression plate was used for fixation whereas, in the remaining eight, the fixation was obtained with an intramedullary interlocking nail.
After internal fixation was achieved, soft tissues from the allograft were attached to corresponding host tissues to obtain the greatest possible stability. Soft tissue reconstructions included repair of the posterior capsule, the anterior and posterior cruciate ligaments, and the medial collateral ligament. Allograft tissue flaps overlapped the corresponding host tissues and were sutured to restore knee stability. We attempted to preserve the host menisci and reattach them to the osteoarticular allograft tissue. The extensor mechanism then was reconstructed to the corresponding tissue of the allograft using a previously described technique [1]. A medial gastrocnemius flap then was performed in all patients to provide soft tissue coverage to the proximal tibia allograft.
We prescribed 1 g cefazolin intravenously for the first postoperative week unless there was a history of penicillin or cephalosporin allergy, in which case the patient received 600 mg clindamycin. In recent years, a splint has been applied to the knee until the wound has healed. Ice or a cryotherapy device was used to help minimize postoperative swelling and discomfort. Postoperatively, a physical therapist instructed patients on brace use, crutched walking, and quadriceps contractions. The goals during the first postoperative week were to minimize swelling and obtain passive complete extension. Passive flexion exercises were started 2 weeks postoperatively with the goal of obtaining at least 60° flexion. At 4 weeks postoperatively, active assisted knee motion was initiated until full active extension and 90° flexion were obtained.
The patients were seen postoperatively at 1 week, 2 weeks, 1 month, 2 months, 3 months, every 3 months thereafter until 2 years, and then annually. Plain radiographs were obtained at every visit, beginning 1 month after the operation. At final followup, a revised 30-point functional classification system established by the Musculoskeletal Tumor Society [10] was used and active extension and extensor lag were measured with a goniometer. This functional score measures six parameters: pain, function, emotional acceptance, use of walking supports, walking ability, and gait. Each parameter is given a value ranging from 0 to 5, according to specific criteria. The individual scores are added together to obtain an overall functional score, with a maximum of 30 points possible.
Two of us (GF, LAAT) independently evaluated plain radiographs of all patients and disagreement was resolved by consensus. We used the system established by the International Society of Limb Salvage [11], which is based on eight criteria: healing of proximal or distal osteotomies, contour of the graft, fixation of the graft, density of the graft, stability of the joint, diameter of the graft, and degeneration of the joint. The score is calculated by adding the value for each criterion and dividing by the maximum attainable score overall. The score is expressed as a percentage, with a maximum possible score of 100%.
We considered an allograft failure to have occurred when the allograft was removed during either a revision procedure or an amputation, and we considered a joint failure to have occurred when the allograft was not removed but resurfacing with a knee prosthesis was needed owing to articular fracture or symptomatic degeneration of the joint. Survival of the allograft and the joint surface were estimated using the Kaplan-Meier method [15], starting on the date of the operation and ending on the date of removal, joint resurfacing, death without failure, or the latest followup.
Results
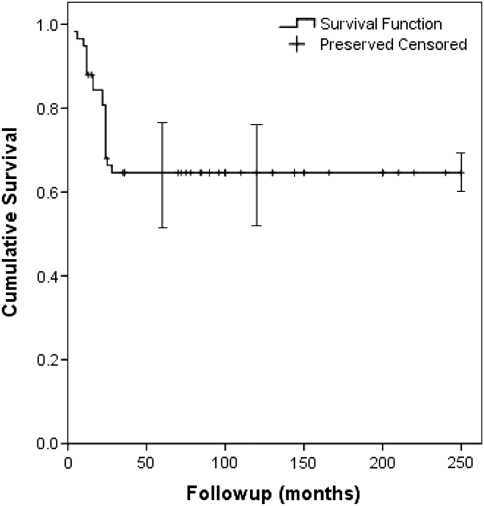
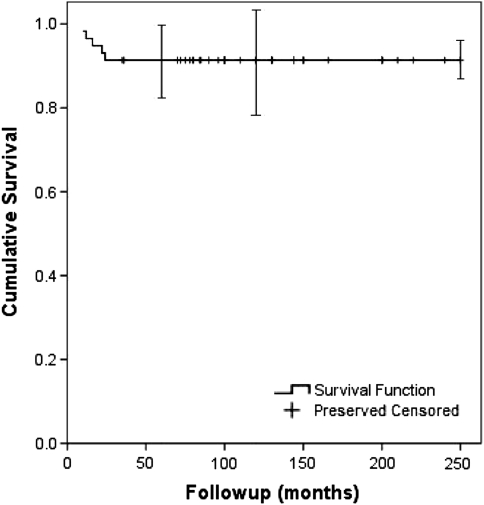
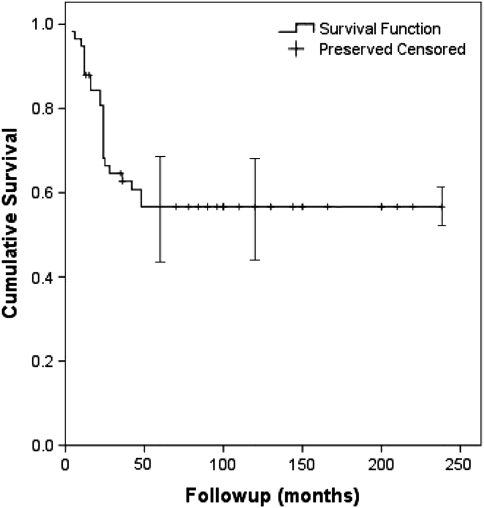
The Kaplan-Meier osteoarticular allograft survival rate was 65% (95% confidence interval [CI], 53%–78%) at 5 and 10 years (Fig. 3). The limb preservation rate was 91% (95% CI, 84%–98%) at 5 and 10 years (Fig. 4). Six patients died from tumor-related causes without allograft failure before the 5-year radiographic followup. Among the remaining 52 allografts, 20 failed owing to infection (13), local recurrence (four), and fracture (three). At the time of the latest evaluation, 32 of the original 58 allografts (55%) were still in place; 18 of these 32 allografts were in patients who were followed up at least 10 years.
Fig. 3.
A Kaplan-Meier survival curve shows the osteoarticular allograft survival rate was 65% at 5 and 10 years. Error bars = confidence intervals.
Fig. 4.
A Kaplan-Meier survival curve shows the limb preservation rate was 91% at 5 and 10 years. Error bars = confidence intervals.
Complications included infection (13), local recurrence (four), fracture (three), and articular collapse (four). For the 13 infected grafts (eight patients received chemotherapy and five did not), the patients were treated with allograft resection, and the length of the limb was maintained with an antibiotic-impregnated polymethylmethacrylate spacer. According to the microorganisms recovered from the site of the infected allograft, appropriate antibiotics were administered for 1 to 3 months. After achieving infection control, 12 of the 13 patients underwent a second limb salvage procedure (five had a knee allograft arthrodesis, four had an allograft prosthetic arthroplasty, and three had a second osteoarticular allograft). Ten of these procedures took place in a conventional OR and the remaining three were done in a vertical laminar airflow OR. One patient required an amputation owing to inability to control the infection. In the four patients with local tumor recurrence, the treatment was an above-the-knee amputation. Removal of the allograft was necessary in the three patients with an allograft fracture, and a second osteoarticular allograft was performed. In four of the 32 allografts that were not removed, a knee resurfacing standard prosthesis with stemmed components was implanted for symptomatic articular collapse. The rate of allograft survival without the need for subsequent knee prosthesis resurfacing was 57% (95% CI, 45%–69%) at 5 and 10 years (Fig. 5).
Fig. 5.
A Kaplan-Meier survival curve shows the rate of allograft survival without the need for a subsequent knee prosthesis resurfacing was 57% at 5 and 10 years. Error bars = confidence intervals.
The 32 patients who had retained the allograft at their latest followup had an average functional score of 26 points (range, 17–30 points). Twenty patients had no pain in the involved knee, and 12 had modest pain. Six patients had no functional restrictions, and 26 had restrictions in recreational activities. Twenty-five patients were enthusiastic about the result, and seven were satisfied with the result. Thirty-one patients walked without the use of supports, and one walked with a cane. Thirteen patients could walk an unlimited distance, and 19 had some limitations in walking. Twenty-four patients had no discernible limp, seven had a minor limp, and one had a major limp. Active knee extension was restored in all patients. Ten patients had an average residual extensor lag of 6.5°, and the remaining 22 evaluated patients had no extensor lag. The mean radiographic score for the 32 allografts evaluated was 87%, which represents an excellent radiographic result, with 27 grafts having scores between 80% and 100%. According to the radiographic evaluation, the joint space was rated as unchanged or with minor deterioration in 28% (nine of 32) of the allografts. Twenty-three of the allografts (72%) had some articular deterioration; five (16%) had joint narrowing of 2 mm, seven (22%) had joint narrowing of 4 mm, and 11 (34%) had some form of subchondral bone collapse.
Discussion
Limb salvage surgery of the proximal tibia is one of the most demanding reconstructions. It is important to consider the availability of each procedure (allograft, endoprosthesis, or a combination of both), and the level of surgical difficulty, morbidity, incidence of complications, and prognosis for survival of the various reconstructions in this anatomic area. Implantation of proximal tibia osteoarticular allografts after tumor resection is one biologic option, especially in young and physically active patients, who place high demands on the reconstructions. However, the survival rate of this procedure is not known. We therefore determined (1) the middle- and long-term survival of proximal tibia osteoarticular allografts, (2) their complications, and (3) functional (Musculoskeletal Tumor Society score) and radiographic (International Society of Limb Salvage) outcomes in patients treated with this reconstruction.
Our study has certain limitations. First, we had no control group with alternate approaches. Second, the group had some inherent heterogeneity in terms of diagnosis, chemotherapy, and type of internal fixation, which could have affected the incidence of complications. Although this is a large series for the type of reconstruction, the subcohorts are too small and heterogeneous (with various confounding and uncontrolled variables) to have adequate power to identify whether and how these influence graft survival.
Prosthetic reconstruction has advantages, such as maintenance of motion and immediate functional restoration [4, 5, 12, 18, 21, 22, 25–27]. Although high survival rates have been reported with this type of reconstruction [18, 21], complication and failure rates also have been high [25, 26] (Table 2). A group of 44 consecutive patients who underwent cemented proximal tibial arthroplasty had a prosthetic failure rate of 34% [26]. Custom-designed prostheses and longer length of resection were associated with prosthesis survival [26]. In another report that analyzed 194 patients who underwent a proximal tibial endoprosthetic arthroplasty, only 115 patients remained alive at final followup. The risk of revision for any reason was 32% at 5 years, 61% at 10 years, and 75% at 15 and 20 years in the fixed-hinge group and 12% at 5 years, 25% at 10 years, and 30% at 15 years in the rotating-hinge group [25]. Increased emphasis has been placed on biologic reconstructive alternatives owing to concerns involving the durability of prosthetic materials and the increasing survivorship of patients with sarcomas. We reviewed the middle- to long-term results of reconstructions with osteoarticular allografts after resection of proximal tibia tumors. The reconstructive survivorship analysis showed an allograft survival rate of 65% (95% CI, 53%–78%) and a joint survival rate of 57% (95% CI, 45%–69%), with a limb preservation survival rate of 91% (95% CI, 84%–98%) at 5 and 10 years. Although a high number of failures occurred during the first 4 years, no additional failures occurred after that time, similar to reported results [16, 17, 19, 28].
Table 2.
Comparison of survival and function of different types of proximal tibial reconstruction
| Study | Number of cases | Type of reconstruction | Mean followup (years) | 5-year survival | 10-year survival | Failure of reconstruction | Functional MSTS score |
|---|---|---|---|---|---|---|---|
| Donati et al. [7] | 62 | Alloprosthesis | 6 | 73% | 68% | 17 (27%) | 27 |
| Grimer et al. [12] | 151 | Endoprosthesis | 7 | 60% | 30% | 95 (63%) | 25 |
| Biau et al. [3] | 26 | Alloprosthesis | 10 | 68% | 33% | 14 (54%) | |
| Myers et al. [21] | 194 | Endoprosthesis | 15 | 78% | 57% | 88 (45%) | |
| Current study | 58 | Osteoarticular | 10 | 65% | 65% | 20 (34%) | 26 |
MSTS = Musculoskeletal Tumor Society.
More recently, the combination of allograft and prosthetic components (APC) has been advocated as an optional solution [3, 7] (Table 2). However, as the authors found a 27% fracture rate [3] and an infection rate of 24% [3, 7], they recommended this procedure for young patients with benign aggressive or malignant low-grade tumors who will not receive chemotherapy [7]. One of the major disadvantages in revision of an APC in the proximal tibia is that it is difficult to maintain the original graft in the procedure [2]. Osteoarticular allografts had reported fracture and infection rates similar to those of APCs [6, 13] (Table 2). Consistent with reported results [3, 6, 7, 13, 14, 21], infection was a main cause of failure in our series. Different factors have been reported as related to these complications: long surgeries owing to reconstructive technical difficulties with prolonged wound exposure, dead space after tumor resection, and insufficient soft tissue coverage. The fracture rate in our series was 5%, which is lower than previously reported rates [6, 13] (Table 3). However, in those reports, fractures included diaphyseal and intraarticular collapse. In our series, only diaphyseal fractures are included because articular collapse is a complication that is solved with a knee arthroplasty without the need to remove the allograft. Although 72% of the retained allografts (23 of 32) had some articular deterioration, most of the patients were not symptomatic. However, in four of the 32 retained allografts, a knee resurfacing prosthesis was needed. Anatomic and dimensional matching of the articular surface, adequate joint stability obtained by host-donor soft tissue repair, and joint alignment have been associated with only minor degenerative changes at the articular surface [1, 9, 16, 20].
Table 3.
Comparison of complications and survival of proximal tibial osteoarticular allografts
The life expectancy for most patients treated for a highly aggressive or malignant tumor in the proximal tibia is several decades. We found proximal tibia allograft reconstruction resulted in a survivorship rate at middle- and long-term followup similar to those of other types of reconstructions. Future developments in infection control, selection of grafts with the best anatomic matching, determination of mechanical resistance of potential allografts, and improvement in allograft-host fixation and graft incorporation enhancement will likely further improve patient outcome.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ayerza MA, Aponte-Tinao LA, Abalo E, Muscolo DL. Continuity and function of patellar tendon host-donor suture in tibial allograft. Clin Orthop Relat Res. 2006;450:33–38. doi: 10.1097/01.blo.0000229291.21722.b5. [DOI] [PubMed] [Google Scholar]
- 2.Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293. doi: 10.2106/JBJS.E.00553. [DOI] [PubMed] [Google Scholar]
- 3.Biau DJ, Dumaine V, Babinet A, Tomeno B, Anract P. Allograft-prosthesis composites after bone tumor resection at the proximal tibia. Clin Orthop Relat Res. 2007;456:211–217. doi: 10.1097/BLO.0b013e31802ba478. [DOI] [PubMed] [Google Scholar]
- 4.Bickels J, Wittig JC, Kollender Y, Neff RS, Kellar-Graney K, Meller I, Malawer MM. Reconstruction of the extensor mechanism after proximal tibia endoprosthetic replacement. J Arthroplasty. 2001;16:856–862. doi: 10.1054/arth.2001.25502. [DOI] [PubMed] [Google Scholar]
- 5.Brien EW, Terek RM, Healey JH, Lane JM. Allograft reconstruction after proximal tibial resection for bone tumors: an analysis of function and outcome comparing allograft and prosthetic reconstructions. Clin Orthop Relat Res. 1994;303:116–127. [PubMed] [Google Scholar]
- 6.Clohisy DR, Mankin HJ. Osteoarticular allografts for reconstruction after resection of a musculoskeletal tumor in the proximal end of the tibia. J Bone Joint Surg Am. 1994;76:549–554. doi: 10.2106/00004623-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Donati D, Colangeli M, Colangeli S, Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop Relat Res. 2008;466:459–465. doi: 10.1007/s11999-007-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24. [PubMed] [Google Scholar]
- 9.Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83:971–986. [PubMed] [Google Scholar]
- 10.Enneking WF, Dunham W, Gebhardt MC, Malawer M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 11.Glasser D, Langlais F. The ISOLS radiological implant evaluation system. In: Langlais F, Tomeno B, editors. Limb Salvage: Major Reconstructions in Oncologic and Nontumoral Conditions. Heidelberg, Germany: Springer-Verlag; 1991. [Google Scholar]
- 12.Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 1999;81:488–494. doi: 10.1302/0301-620X.81B3.9234. [DOI] [PubMed] [Google Scholar]
- 13.Hornicek FJ, Jr, Mnaymneh W, Lackman RD, Exner GU, Malinin TI. Limb salvage with osteoarticular allografts after resection of proximal tibia bone tumors. Clin Orthop Relat Res. 1998;352:179–186. doi: 10.1097/00003086-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Jeon DG, Kawai A, Boland P, Healey JH. Algorithm for the surgical treatment of malignant lesions of the proximal tibia. Clin Orthop Relat Res. 1999;358:15–26. doi: 10.1097/00003086-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 16.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Mankin HJ, Gebhardt MC, Tomford WW. The use of frozen cadaveric allografts in the management of patients with bone tumors of the extremities. Orthop Clin North Am. 1987;18:275–289. [PubMed] [Google Scholar]
- 18.Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, Kotz R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–177. doi: 10.1097/00003086-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Survivorship and radiographic analysis of knee osteoarticular allografts. Clin Orthop Relat Res. 2000;373:73–79. doi: 10.1097/00003086-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Massive allograft use in orthopedic oncology. Orthop Clin North Am. 2006;37:65–74. doi: 10.1016/j.ocl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2007;89:1632–1637. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan MV, Sivaseelam A, Rajkumar G, Hussain SH. Custom megaprosthetic replacement for proximal tibial tumours. Int Orthop. 2003;27:334–337. doi: 10.1007/s00264-003-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X, Wu S, Zhao J. [Limb salvage with osteoarticular allografts after resection of proximal tibia bone] [in Chinese] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:966–969. [PubMed] [Google Scholar]
- 24.Sim FH, Beauchamp CP, Chao EY. Reconstruction of musculoskeletal defects about the knee for tumor. Clin Orthop Relat Res. 1987;221:188–201. [PubMed] [Google Scholar]
- 25.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 26.Wu CC, Henshaw RM, Pritsch T, Squires MH, Malawer MM. Implant design and resection length affect cemented endoprosthesis survival in proximal tibial reconstruction. J Arthroplasty. 2008;23:886–893. doi: 10.1016/j.arth.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Wunder JS, Leitch K, Griffin AM, Davis AM, Bell RS. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99. doi: 10.1002/jso.1076. [DOI] [PubMed] [Google Scholar]
- 28.Zatsepin ST, Burdygin VN. Replacement of the distal femur and proximal tibia with frozen allografts. Clin Orthop Relat Res. 1994;303:95–102. [PubMed] [Google Scholar]