Abstract
Background
Quadriceps muscle strength, which is essential for the function and stability of the knee, has been found to be impaired even years after arthroscopic partial meniscectomy. However, the neuromuscular alterations that could account for such muscle weakness remain unclear.
Questions/purposes
We investigated (1) the side-to-side asymmetries in quadriceps muscle strength 6 months after arthroscopic partial meniscectomy, (2) the physiologic mechanisms (neural versus muscular) underlying muscle weakness, and (3) the impact of quadriceps weakness on muscle control at submaximal force levels.
Patients and Methods
We tested 14 volunteers (10 men, four women) with an average age of 44 ± 9 years (range, 24–59 years) at 6 ± 1 months after unilateral medial arthroscopic partial meniscectomy. We measured maximal voluntary strength and muscle activation during isometric, concentric, and eccentric contractions using isokinetic dynamometry and surface EMG, respectively. We assessed vastus lateralis muscle size and architecture using ultrasonography. We measured muscle control at submaximal force levels with a repositioning test (knee proprioception) and a low-force target-tracking task (steadiness, accuracy).
Results
Isometric and concentric quadriceps strength and vastus lateralis EMG activity were lower on the involved than on the uninvolved side. Muscle architecture and muscle control did not differ between the involved and uninvolved sides.
Conclusions
Our results showed quadriceps weakness exists 6 months after arthroscopic partial meniscectomy. As suggested by the EMG results, this is likely attributable to neural impairments (activation failure) that affect muscle control at maximal but not submaximal force outputs.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The time for recovery of quadriceps strength after arthroscopic partial meniscectomy (APM) surgery was described by Gapeyeva et al. [15]. Concentric peak torque was found to be significantly lower on the involved than on the uninvolved quadriceps (ie, side-to-side asymmetry) at 1 (30%), 3 (18%), and even 6 months (13%) postsurgery. Such asymmetry in quadriceps strength could be significant even years after meniscectomy [12]. This is an important message because muscle function recovery from arthroscopic surgery generally is considered relatively fast, and in some cases, return to work and sports can occur a few days and a few weeks after surgery [20, 26, 29]. Particularly for the quadriceps, an essential muscle for daily and sports’ activities, significant muscle weakness implies a greater percentage of residual force capacity is needed to perform job- or sport-related tasks even months after APM. The involved side therefore will be exposed to conditions that overload the knee and increase the risk of reinjury, which in turn may result in abnormal joint function and joint injury associated with the beginning of knee osteoarthritis [7].
Unfortunately, the mechanisms underlying quadriceps weakness after APM have been poorly investigated. Such knowledge would help to identify neural and/or muscle impairments in patients undergoing APM, with the ultimate goal to optimize their postoperative rehabilitation strategies. Assessment of quadriceps EMG activity during maximal voluntary contractions can be used to assess the completeness of quadriceps recovery because it estimates the extent of muscle activation [3]. In a prospective pilot study [11], quadriceps concentric strength and EMG activity of the vastus medialis muscle were found to be reduced in patients having APM compared with control subjects. Additionally, a MRI study provided experimental evidence of significant asymmetries in quadriceps muscle mass (ie, muscle atrophy) after APM [4]. As an alternative to the expensive MRI, ultrasonography is a comfortable and innocuous technique that could be used for quantitative assessment of muscle atrophy and architecture (muscle thickness, pennation angle, fascicle length) [1, 14, 31], although to our knowledge, its use is rare in orthopaedic research [6].
In addition to maximal strength, adequate quadriceps control at submaximal muscle forces is essential for daily activities, including job-related tasks, which normally are performed at a fraction of the available maximal muscle strength [18]. Knee repositioning and target-tracking tasks are commonly used in orthopaedic practice and research, for rehabilitation purposes and for evaluation of different forms of muscle control (eg, proprioception, accuracy, steadiness) [19, 32]. It remains to be determined whether patients undergoing APM have, in addition to quadriceps weakness, impaired muscle control at submaximal force levels.
We therefore investigated (1) quadriceps peak torque asymmetries after APM in maximal isometric, eccentric, and concentric conditions, (2) EMG activity and muscle architecture, to explore the neural and muscular mechanisms underlying quadriceps muscle weakness, and (3) steadiness, accuracy and proprioception, to examine the impact of quadriceps weakness on muscle control at submaximal force levels.
Patients and Methods
To detect a significant and clinically relevant side-to-side asymmetry in quadriceps peak torque of 13% [15], with a statistical power of 0.8 (beta = 0.2) and a two-sided alpha of 0.05, and assuming a correlation of 0.9 between the paired values, 13 subjects were needed. Fourteen volunteers (10 men, four women) were tested 6 ± 1 months (mean ± SD) after unilateral medial APM performed at our institution. Their age, weight, and height averaged 44 ± 9 years (range, 24–59 years), 77 ± 11 kg (range, 58–93 kg), and 176 ± 9 cm (range, 159–190 cm), respectively. The main reasons for surgery were degenerative changes of the meniscus (five men, one woman) or accident/sports trauma (five men, three women). Duration of symptoms before surgery ranged between 4 months and 2 years for patients with a degenerative meniscus tear and between 2 days and 10 months for patients with a traumatic tear. Patients had no concomitant lower limb injury (eg, cruciate ligament rupture), disorder (eg, gonarthrosis), and/or previous hip, knee, or ankle surgery. All surgical procedures were performed under tourniquet control, with an average pressure of 350 mm Hg and an average pressure time of 28 ± 8 minutes. The amount of meniscal tissue (corpus and posterior horn) removed at meniscectomy was between 30% and 60% of the medial meniscus. Ten patients were referred for nonstandardized (nonaggressive) physical therapy after surgery, and they received 13 ± 6 exercise sessions. The remaining four patients were not referred for physical therapy. At the time of followup, all patients were physically active and performed one to two weekly sessions of sport activities such as cycling and running. The study was approved by the local ethical committee (specialized subcommittee for orthopaedics, Zurich, Switzerland) and patients signed written consent forms before participation.
Patients were tested using a series of validated assessments, which included a self-report questionnaire and tests for (1) muscle strength (peak torque) using isokinetic dynamometry, (2) muscle activation using surface EMG recordings, (3) muscle size and architecture using ultrasonography, and (4) muscle control using a repositioning test (knee proprioception) and a low-force target-tracking task (steadiness, accuracy). These assessments are described in detail below. Before each test trial, patients received standardized verbal instructions and completed two to three familiarization trials. All tests were performed separately for the involved and uninvolved sides (ie, unilateral assessments) in a randomized fashion.
For muscle strength measurements, patients were seated on the chair of an isokinetic dynamometer (Biodex™ System 2; Biodex Medical Systems, Shirley, NY), with belts applied across the shoulders and pelvis to minimize body movements during thigh muscle contractions. The tested leg was fixed to the dynamometer lever arm with a strap 2 to 3 cm proximal to the lateral malleolus. The alignment between the knee rotation axis (lateral femoral condyle) and the dynamometer rotational axis was consistently checked. Maximal strength tests were completed and analyzed according to the methodology proposed by Maffiuletti et al. [21]. Briefly, maximal isometric, concentric, and eccentric strength of the quadriceps and concentric strength of the hamstring muscles were investigated using 1-minute rest periods between series of measurements. Isometric trials were completed at a knee angle of 60° (0°: knee fully extended) because this corresponds to the joint position where maximal quadriceps force-generating capacity is observed [36]. Patients completed two 5-second maximal isometric knee extensions while they were asked to produce their maximal force as fast as possible. For the isokinetic trials, ROM was 75°, from 90° to 15° knee flexion, and vice versa. Concentric tests were performed at two angular velocities: 60°/second and 180°/second. Patients were asked to complete the full ROM during three consecutive extension-flexion trials. Finally, three eccentric contractions of the quadriceps were performed using an angular velocity of −60°/second. For isometric, concentric, and eccentric strength tests, the main outcome was peak torque [9, 27], as provided by the Biodex™ software.
Surface EMG activity of the vastus medialis and vastus lateralis muscles was recorded during all maximal strength tests. Silver chloride circular recording electrodes were fixed over the middle of respective muscle bellies [10], with an interelectrode (center-to-center) distance of 30 mm. The electrodes had a recording diameter of 10 mm. The reference electrode was fixed approximately 10 cm away from the recording area. Low impedance between the electrodes was obtained by shaving and cleaning the skin with an alcohol swab (Con-Zellin®, isopropyl alcohol; Paul Hartmann, Heidenheim, Germany). EMG signals were amplified with a bandwidth frequency ranging from 8 Hz to 1.2 kHz (common mode rejection ratio: 90 dB; input impedance: 1012 ohms; gain at 100 Hz: 1000). EMG signals were digitized at a sampling frequency of 100 Hz and stored with MuscleLab® software (Version 7.16; Ergotest Technology, Langesund, Norway). The software calculated directly the root mean square EMG amplitude for respective muscles, from which the maximal value was retained.
Vastus lateralis muscle size and architecture (muscle thickness, fascicle pennation angle, fascicle length) were investigated using B-mode ultrasound (MyLab® 25; Esaote, Florence, Italy). This muscle was selected because it generally is considered to be the best surrogate of the quadriceps muscle [28] and its architectural properties are commonly studied using the same methodology used here [1, 14, 31]. Patients were seated on a physical therapy treatment table with the hip and knees at 90° and the quadriceps muscles completely relaxed. Femur length was measured as the distance from the greater trochanter to the lateral condyle of the femur. Three longitudinal ultrasonic pictures (width: 4 cm; depth: 7–9 cm) then were recorded at 50% of the femur length over the lateral aspect of the vastus lateralis muscle. The probe (4 cm × 0.7 cm) was consistently used at a frequency of 15 MHz. Conductive gel was used to improve the quality of the images. Vastus lateralis muscle thickness and fascicle pennation angle subsequently were calculated with an image-editing program (ImageJ 1.36b; National Institutes of Health, Bethesda, MD). Muscle thickness was measured as the distance from the superficial to the deep aponeurosis of the vastus lateralis muscle at three different positions of the 4-cm picture (left, middle, right). Pennation angle was quantified as the angle between the deep aponeurosis and a clearly visible fascicle [1]. Because the angle between the superficial aponeurosis and the fascicle might be altered by the pressure of the ultrasonic probe, we always considered the angle between the deep aponeurosis and the fascicle. For each picture, three fascicles were selected and the three corresponding pennation angles were measured. Because fascicles were not visible over their whole length, fascicle length was estimated using the measured muscle thickness and pennation angle according to the formula: fascicle length (cm) = muscle thickness (cm) × (sin [pennation angle (rad)])−1 [5]. For each side, the average muscle thickness, pennation angle, and fascicle length of the three pictures were retained.
Multijoint motor coordination was examined using a lower limb tracking-trajectory test. The assessment was conducted according to reliable procedures [22] using a horizontal leg press machine (Functional Squat System; Monitored Rehab Systems, Haarlem, The Netherlands) connected to a computer (sampling rate: 50 Hz). The knee ROM during the target-tracking task was approximately 90°, from 90° knee flexion (starting position) to complete knee extension, and vice versa. The test was conducted using a load of approximately 10% of individual body weight. The duration of one trial was 60 seconds and each side was tested twice with 60-second rest periods between. Patients were supine on the leg press apparatus and were asked to move a cursor (actual trajectory) over a moving target trajectory. The patients could move the cursor to the right or left side of the screen by extending and flexing the lower limb, respectively. Patients were asked to follow the moving trajectory with the cursor as accurately as possible to minimize the difference between their actual trajectory and the criterion trajectory. The main outcome measures were accuracy (in cm), which is the average of actual trajectory minus criterion trajectory for each data point (ie, the average tracking error), and steadiness (in cm), which is the SD of the average actual trajectory (ie, the SD error). The software automatically calculated accuracy and steadiness for the concentric and eccentric phases.
Knee proprioception was assessed using a knee repositioning test, as described by Hortobagyi et al. [17]. Patients were seated on the isokinetic dynamometer chair and wore a knee brace (SofTec Genu, Bauerfeind, Germany) equipped with an electrogoniometer (MuscleLab®). The test always started at 90° knee flexion. The leg was moved passively by the investigator to 30° or 60° knee flexion (target knee angle). This position was held for 5 seconds by the investigator while the patient was asked to memorize the position. Subsequently, the leg was moved back to the starting position (90° knee flexion) and the patient was asked to reproduce the memorized position for 5 seconds with the eyes closed and with no feedback regarding knee angle (actual knee angle). Two trials were performed at each of the two experimental positions (30° and 60°). The absolute error of repositioning, which is the difference between the target and the actual knee angle (average of the 5-second trial), was recorded for the two sides and joint positions.
The different outcome measures were verified for normal distribution using the Kolmogorov-Smirnov test. Paired Student’s t tests (one-tailed) were used to test for differences in outcome measures between the involved and uninvolved sides. Side-to-side asymmetries for muscle strength, EMG activity, and muscle architecture were calculated using the formula proposed by Wrigley and Strauss [37]: asymmetry = (involved/uninvolved × 100) − 100. Statistical procedures were performed using SigmaStat® 2.0 (Systat Software Inc, San Jose, CA) and STATISTICA® 6.0 (StatSoft, Inc, Tulsa, OK) softwares. Statistical significance was set at p < 0.05 for all procedures.
Results
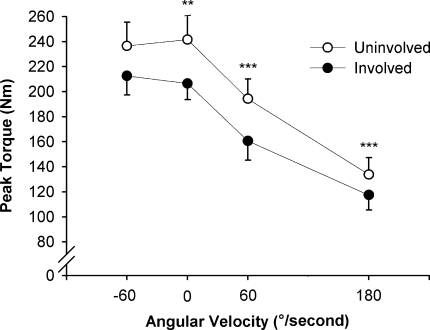
Quadriceps peak torque was lower on the involved than on the uninvolved side in isometric (11.6%; p = 0.004) and concentric conditions (14.5%; p < 0.001), whereas eccentric values did not differ between sides (p = 0.065) (Fig. 1). Concentric peak torque of the hamstring muscles was not different between the involved and uninvolved sides at the two angular velocities (60°/second: 81 ± 29 versus 87 ± 29 Nm, respectively; p = 0.079; 180°/second: 67 ± 26 versus 67 ± 25 Nm, respectively; p = 0.469).
Fig. 1.
A graph shows the peak torque-angular velocity relation for the involved and uninvolved quadriceps in eccentric (−60°/second), isometric (0°/second), and concentric (60° and 180°/second) conditions. Peak torque was lower for the involved side in isometric (** = p < 0.01) and concentric (*** = p < 0.001) conditions, whereas eccentric torque did not show any side-to-side differences. Group mean values and standard errors are shown.
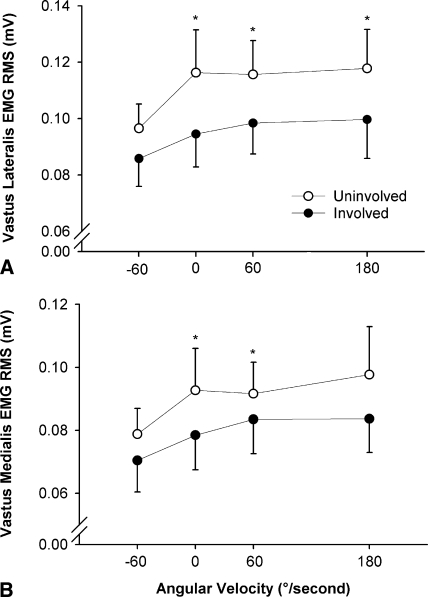
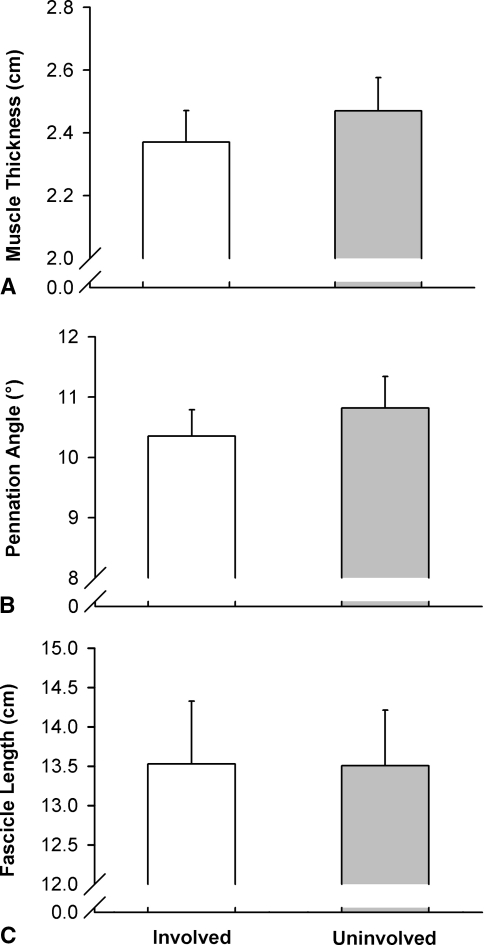
As observed for peak torque, EMG activity of the vastus lateralis muscle was lower for the involved than for the uninvolved side in isometric (10.0%; p = 0.024) and concentric conditions (13.2%; p = 0.021), whereas no difference was observed in the eccentric mode (p = 0.097) (Fig. 2A). For the vastus medialis muscle (Fig. 2B), a side-to-side asymmetry was observed in isometric (14.1%; p = 0.028) and concentric (60°/second) conditions (10.0%; p = 0.038). Small, nonsignificant asymmetries were observed for vastus lateralis muscle thickness (2.9%; p = 0.137), fascicle pennation angle (3.6%; p = 0.086), and fascicle muscle length (−1.9%; p = 0.374) (Fig. 3).
Fig. 2A–B.
The graphs show (A) vastus lateralis and (B) vastus medialis maximal EMG-angular velocity relation for the involved and uninvolved quadriceps in eccentric (−60°/second), isometric (0°/second), and concentric (60° and 180°/second) conditions. EMG activity was lower for the involved side in isometric and concentric conditions (* = p < 0.05), except for the vastus medialis at 180°/second. No side-to-side differences were observed in eccentric conditions. Group mean values and standard errors are shown. RMS = root mean square.
Fig. 3A–C.
The graphs show (A) muscle thickness, (B) fascicle pennation angle, and (C) fascicle length in the vastus lateralis muscle for the involved and uninvolved sides. No side-to-side differences were observed. Group mean values and standard errors are shown.
Outcome measures associated with knee proprioception (p range = 0.055–0.184) and target-tracking (p range = 0.265–0.500) tests did not show any significant side-to-side asymmetry (Table 1).
Table 1.
Outcomes of the muscle control tests for the involved and uninvolved sides
| Test | Involved | Uninvolved |
|---|---|---|
| Knee proprioception | ||
| 60°-knee angle error (°) | 3.3 ± 1.8 | 4.1 ± 2.8 |
| 30°-knee angle error (°) | 2.6 ± 1.6 | 3.2 ± 1.9 |
| Target tracking | ||
| Concentric accuracy (cm) | 0.16 ± 0.09 | 0.16 ± 0.11 |
| Concentric steadiness (cm) | 0.65 ± 0.20 | 0.65 ± 0.16 |
| Eccentric accuracy (cm) | 0.15 ± 0.08 | 0.15 ± 0.09 |
| Eccentric steadiness (cm) | 0.62 ± 0.20 | 0.65 ± 0.14 |
Values are expressed as group means ± SDs.
Discussion
We reexamined quadriceps muscle strength asymmetries after APM and determined whether muscle weakness was attributable to neural and/or muscular impairments and the impact of quadriceps weakness on muscle control at submaximal force levels.
A limitation of our retrospective study is that we considered a small group of patients who had APM regardless of gender, type (traumatic versus degenerative tears) and severity of meniscal damage, amount of meniscus removed at meniscectomy, duration of symptoms, and referral to physical therapy, despite comparable strength and EMG asymmetries in respective subgroups. All patients underwent surgery with tourniquet use, although its use could affect postoperative neuromuscular function. However, we controlled individual tourniquet time and pressure with caution, as recommended by Graf et al. [16], and consistently applied the tourniquet for short durations [25]. Graf et al. [16] clearly showed that recovery of quadriceps strength after meniscectomy was not influenced by tourniquet use if tourniquet times and pressures were carefully controlled. Another limitation is that we made only within-patient involved-uninvolved comparisons (interlimb asymmetries) and no attempt was made to compare the outcomes of patients who had APM with those of healthy matched control subjects. However, muscle function of the uninvolved quadriceps has been suggested to deserve consideration because it plays an important role in patients’ functional ability [23].
Maximal strength of the involved quadriceps was less than that of the uninvolved side in isometric and concentric but not in eccentric conditions. The asymmetry in concentric strength measured in this study (15%) fits well with the deficits previously reported in similar populations at different postoperative times (29% after 1 month, 20% after 3 months, 6%–9% after 1–4 years) [12, 15]. We also observed a significant isometric strength asymmetry (12%), whereas eccentric peak torque asymmetry (6%) did not reach significance. According to the benchmarks proposed by Sapega [34], the extent of the side-to-side strength differences we observed in isometric and concentric conditions (ie, higher than 10%) must be considered “probably abnormal.” Postoperative assessment of quadriceps force-generating capacity is essential for patients who had APM to detect and eventually adjust possible asymmetries in muscle strength. More importantly, determination of the etiology of quadriceps muscle weakness is required to act on any specific neural and/or muscular impairment through optimized rehabilitation strategies.
Quadriceps weakness was accompanied by significant side-to-side asymmetries in muscle activation, as evidenced by maximal EMG activity results, whereas muscle size and architecture did not differ between the involved and uninvolved sides. The fact that EMG activity of the vastus lateralis and vastus medialis muscles was significantly lower on the involved than on the uninvolved side lends support to the theory that quadriceps weakness after APM was attributable to suboptimal neural drive to the agonist muscle during maximal concentric and isometric contractions. Despite surface EMG limitations [13], such neural impairment, which has been observed 2 months after APM [11], is probably the result of suboptimal motor unit firing frequency and/or recruitment, which could affect spinal and/or supraspinal processes of voluntary muscle activation. Deficits in quadriceps muscle activation have been reported after ACL injury [8] and TKA [24] using techniques other than surface EMG (eg, burst superimposition technique); interestingly, it has even been suggested activation failure is by far the largest impairment after TKA [24]. The similarities between peak torque and EMG asymmetries we observed and the lack of significant side-to-side differences in ultrasonographic findings (see below) suggest quadriceps weakness was attributable to neural rather than muscular impairment. For the first time in a meniscectomy study, we used a more recently developed and validated methodology [1, 14, 31] to investigate possible differences in muscle size and architecture between the involved and uninvolved vastus lateralis, mainly attributable to muscle atrophy. This alteration would have modified not only muscle thickness but also pennation angle. Muscle atrophy is accompanied by a decrease in pennation angle, and the smaller the pennation angle, the fewer number of sarcomeres can be arranged in parallel and therefore less force can be generated during a contraction [33]. Muscle thickness and fascicle pennation angle did not differ between the involved and uninvolved sides, contrary to the significant alterations reported as occurring 8 months after tibia/femur fracture [6]. It also is possible atrophy occurred in muscles other than the vastus lateralis and/or B-mode ultrasonography is not sensitive enough to detect small changes in muscle architecture, as significant side-to-side asymmetries in quadriceps muscle mass (8%–15%) were observed after APM using MRI [4].
Quadriceps muscle control, as assessed with a repositioning test (knee proprioception), and a low-force target-tracking task (steadiness, accuracy) showed no difference between the involved and uninvolved sides, in line with the findings of Jerosch et al. [19]. These authors compared the outcomes of a knee repositioning test between healthy subjects and patients with a medial meniscus lesion, preoperatively and postoperatively, and showed a significant proprioceptive impairment in the patient group, but only preoperatively. The fact that steadiness and accuracy in concentric and eccentric conditions did not differ between the two sides in our patients who had APM suggests the neural mechanisms governing low-force quadriceps control, eg, sensitivity of joint and muscle receptors sensing motion and position [30], discharge behavior of motor units [4], and ability to coordinate multiple agonist and antagonist muscles [11], were not affected 6 months after APM, contrary to maximal-force neural control (see above).
Taken as a whole, these findings indicate quadriceps weakness after APM mainly was attributable to neural impairment (activation failure), which markedly affected force-generating capacity at maximal but not submaximal levels. Patients having meniscectomy often are prescribed a home exercise program but also may undergo supervised rehabilitation programs. Standardized physical therapy may be useful after APM, but only if it is aggressive enough. Based on our findings, high-load progressive strength training of the quadriceps muscle may be indicated [2], with the general objective to increase muscle activation and therefore reduce the strength asymmetries below abnormal levels (less than 10%) [34, 37], particularly in concentric actions. This form of resistance exercise, which can be performed easily with specific machines (leg extension, leg press, etc) and free weights (squat, lunges, etc), has proven its effectiveness for quadriceps strengthening via neural adaptations in healthy individuals [2], provided training intensity is sufficiently high (> 70%–80% of one repetition maximal) and training volume is increased progressively throughout the program. Specifically, we believe more emphasis is needed on neural rather than muscular recovery after APM. Exercise modalities such as neuromuscular electrical stimulation [35] and visual feedback of muscle activation and strength also may be useful for maximizing neural adaptations. Our findings also suggest it probably is unnecessary to focus on the recovery of hamstring muscle function and quadriceps control at low force levels in a supervised rehabilitation program after APM.
Acknowledgments
We thank the patients for participation in the study; Katharina Widler for helping with some of the experiments; and the following orthopaedic surgeons: Drs. Tomas Drobny and Stefan Preiss.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Neuromuscular Research Laboratory, Schulthess Clinic.
References
- 1.Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92:2309–2318. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- 3.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Halkjaer-Kristensen J, Dyhre-Poulsen P. Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol. 2000;89:2249–2257. doi: 10.1152/jappl.2000.89.6.2249. [DOI] [PubMed] [Google Scholar]
- 4.Akima H, Furukawa T. Atrophy of thigh muscles after meniscal lesions and arthroscopic partial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2005;13:632–637. doi: 10.1007/s00167-004-0602-9. [DOI] [PubMed] [Google Scholar]
- 5.Alegre LM, Jimenez F, Gonzalo-Orden JM, Martin-Acero R, Aguado X. Effects of dynamic resistance training on fascicle length and isometric strength. J Sports Sci. 2006;24:501–508. doi: 10.1080/02640410500189322. [DOI] [PubMed] [Google Scholar]
- 6.Bleakney R, Maffulli N. Ultrasound changes to intramuscular architecture of the quadriceps following intramedullary nailing. J Sports Med Phys Fitness. 2002;42:120–125. [PubMed] [Google Scholar]
- 7.Bolano LE, Grana WA. Isolated arthroscopic partial meniscectomy: functional radiographic evaluation at five years. Am J Sports Med. 1993;21:432–437. doi: 10.1177/036354659302100318. [DOI] [PubMed] [Google Scholar]
- 8.Chmielewski TL, Stackhouse S, Axe MJ, Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004;22:925–930. doi: 10.1016/j.orthres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Davies GJ, Heiderscheit B, Brinks K. Test interpretation. In: Brown LE, editor. Isokinetics in Human Performance. Champaign, IL: Human Kinetics; 2000. pp. 1–24. [Google Scholar]
- 10.Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;12:135–163. [Google Scholar]
- 11.Durand A, Richards CL, Malouin F. Strength recovery and muscle activation of the knee extensor and flexor muscles after arthroscopic meniscectomy: a pilot study. Clin Orthop Relat Res. 1991;262:210–226. [PubMed] [Google Scholar]
- 12.Ericsson YB, Roos EM, Dahlberg L. Muscle strength, functional performance, and self-reported outcomes four years after arthroscopic partial meniscectomy in middle-aged patients. Arthritis Rheum. 2006;55:946–952. doi: 10.1002/art.22346. [DOI] [PubMed] [Google Scholar]
- 13.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga T, Ichinose Y, Ito M, Kawakami Y, Fukashiro S. Determination of fascicle length and pennation in a contracting human muscle in vivo. J Appl Physiol. 1997;82:354–358. doi: 10.1152/jappl.1997.82.1.354. [DOI] [PubMed] [Google Scholar]
- 15.Gapeyeva H, Paasuke M, Ereline J, Pintsaar A, Eller A. Isokinetic torque deficit of the knee extensor muscles after arthroscopic partial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2000;8:301–304. doi: 10.1007/s001670000140. [DOI] [PubMed] [Google Scholar]
- 16.Graf B, Jensen K, Orwin J, Duck H, Hagen P, Keene J. The effect of tourniquet use on postoperative strength recovery after arthroscopic meniscectomy. Orthopedics. 1996;19:497–500. [PubMed] [Google Scholar]
- 17.Hortobagyi T, Garry J, Holbert D, Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 2004;51:562–569. doi: 10.1002/art.20545. [DOI] [PubMed] [Google Scholar]
- 18.Hortobagyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003;58:M453–M460. doi: 10.1093/gerona/58.5.m453. [DOI] [PubMed] [Google Scholar]
- 19.Jerosch J, Prymka M, Castro WH. Proprioception of knee joints with a lesion of the medial meniscus. Acta Orthop Belg. 1996;62:41–45. [PubMed] [Google Scholar]
- 20.Lysholm J, Gillquist J. Endoscopic meniscectomy. Int Orthop. 1981;5:265–270. doi: 10.1007/BF00271081. [DOI] [PubMed] [Google Scholar]
- 21.Maffiuletti NA, Bizzini M, Desbrosses K, Babault N, Munzinger U. Reliability of knee extension and flexion measurements using the Con-Trex isokinetic dynamometer. Clin Physiol Funct Imaging. 2007;27:346–353. doi: 10.1111/j.1475-097X.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 22.Maffiuletti NA, Bizzini M, Schatt S, Munzinger U. A multi-joint lower-limb tracking-trajectory test for the assessment of motor coordination. Neurosci Lett. 2005;384:106–111. doi: 10.1016/j.neulet.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 23.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35:424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 24.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty: the contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87:1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholas SJ, Tyler TF, McHugh MP, Gleim GW. The effect on leg strength of tourniquet use during anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy. 2001;17:603–607. doi: 10.1053/jars.2001.24854. [DOI] [PubMed] [Google Scholar]
- 26.Patel D, Fahmy N, Sakayan A. Isokinetic and functional evaluation of the knee following arthroscopic surgery. Clin Orthop Relat Res. 1982;167:84–91. [PubMed] [Google Scholar]
- 27.Perrin DH. Isokinetic Exercise and Assessment. Champaign, IL: Human Kinetics; 1993. [Google Scholar]
- 28.Place N, Maffiuletti NA, Martin A, Lepers R. Assessment of the reliability of central and peripheral fatigue after sustained maximal voluntary contraction of the quadriceps muscle. Muscle Nerve. 2007;35:486–495. doi: 10.1002/mus.20714. [DOI] [PubMed] [Google Scholar]
- 29.Prietto CA, Caiozzo VJ, Prietto PP, McMaster WC. Closed versus open partial meniscectomy: postoperative changes in the force-velocity relationship of muscle. Am J Sports Med. 1983;11:189–194. doi: 10.1177/036354658301100401. [DOI] [PubMed] [Google Scholar]
- 30.Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Prog Neurobiol. 2000;60:85–96. doi: 10.1016/S0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 31.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91:116–118. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- 32.Roberts D, Andersson G, Friden T. Knee joint proprioception in ACL-deficient knees is related to cartilage injury, laxity and age: a retrospective study of 54 patients. Acta Orthop Scand. 2004;75:78–83. doi: 10.1080/00016470410001708160. [DOI] [PubMed] [Google Scholar]
- 33.Rutherford OM, Jones DA. Measurement of fibre pennation using ultrasound in the human quadriceps in vivo. Eur J Appl Physiol Occup Physiol. 1992;65:433–437. doi: 10.1007/BF00243510. [DOI] [PubMed] [Google Scholar]
- 34.Sapega AA. Muscle performance evaluation in orthopaedic practice. J Bone Joint Surg Am. 1990;72:1562–1574. [PubMed] [Google Scholar]
- 35.Stevens JE, Mizner RL, Snyder-Mackler L. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004;34:21–29. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 36.Thorstensson A, Grimby G, Karlsson J. Force-velocity relations and fiber composition in human knee extensor muscles. J Appl Physiol. 1976;40:12–16. doi: 10.1152/jappl.1976.40.1.12. [DOI] [PubMed] [Google Scholar]
- 37.Wrigley T, Strauss G. Strength assessment by isokinetic dynamometry. In: Gore JG, editor. Physiological Tests for Elite Athletes. Champaign, IL: Human Kinetics; 2000. pp. 155–199. [Google Scholar]