Summary
Background
Many randomised controlled trials have investigated the effect of adjuvant chemotherapy in operable non-small-cell lung cancer. We undertook two comprehensive systematic reviews and meta-analyses to establish the effects of adding adjuvant chemotherapy to surgery, or to surgery plus radiotherapy.
Methods
We included randomised trials, not confounded by additional therapeutic differences between the two groups and that started randomisation on or after Jan 1, 1965, which compared surgery plus adjuvant chemotherapy versus surgery alone, or surgery plus adjuvant radiotherapy and chemotherapy versus surgery plus adjuvant radiotherapy. Updated individual patient data were collected, checked, and included in meta-analyses stratified by trial. The primary endpoint was overall survival, defined as time from randomisation until death by any cause. All analyses were by intention to treat.
Findings
The first meta-analysis of surgery plus chemotherapy versus surgery alone was based on 34 trial comparisons and 8447 patients (3323 deaths). We recorded a benefit of adding chemotherapy after surgery (hazard ratio [HR] 0·86, 95% CI 0·81–0·92, p<0·0001), with an absolute increase in survival of 4% (95% CI 3–6) at 5 years (from 60% to 64%). The second meta-analysis of surgery plus radiotherapy and chemotherapy versus surgery plus radiotherapy was based on 13 trial comparisons and 2660 patients (1909 deaths). We recorded a benefit of adding chemotherapy to surgery plus radiotherapy (HR 0·88, 95% CI 0·81–0·97, p=0·009), representing an absolute improvement in survival of 4% (95% CI 1–8) at 5 years (from 29% to 33%). In both meta-analyses we noted little variation in effect according to the type of chemotherapy, other trial characteristics, or patient subgroup.
Interpretation
The addition of adjuvant chemotherapy after surgery for patients with operable non-small-cell lung cancer improves survival, irrespective of whether chemotherapy was adjuvant to surgery alone or adjuvant to surgery plus radiotherapy.
Funding
UK Medical Research Council, Institut Gustave-Roussy, Programme Hospitalier de Recherche Clinique (AOM 05 209), Ligue Nationale Contre le Cancer, and Sanofi-Aventis.
Introduction
Around 1·5 million new cases of lung cancer are diagnosed every year,1 and about 85% of tumours are non-small-cell lung cancer.2 Although surgery is regarded as the best possible treatment, only 20–25% of tumours are suitable for potentially curative resection.3
Our previous meta-analyses of individual patient data4 provided evidence that cisplatin-based chemotherapy after surgery might increase survival (hazard ratio [HR] 0·87, 95% CI 0·74–1·02, p=0·08). With fewer trials and patients, the value of chemotherapy after surgery plus postoperative radiotherapy was less clear in our previous meta-analyses.4 Meta-analyses5–10 showing significant survival benefits with adjuvant chemotherapy have included many trials and patients (webappendix p 1). We aimed to assess the effects of adjuvant chemotherapy, with or without postoperative radiotherapy, in two new comprehensive meta-analyses of individual patient data. By comparison with our previous meta-analyses, this study was restricted to patients with early stage disease.
Methods
Study design, search strategy, and study selection
Before data collection, two protocols were developed: one for the meta-analysis of chemotherapy plus surgery and the other for the meta-analysis of chemotherapy plus surgery and radiotherapy.
To be included, trials had to be randomised, not confounded by additional therapeutic differences between the two groups, and have started randomisation on or after Jan 1, 1965. Trials should have aimed to include patients who had undergone a potentially curative resection and not received previous chemotherapy. For the first meta-analysis, trials should have compared surgery plus adjuvant chemotherapy versus surgery alone. For the second, trials should have compared surgery plus adjuvant radiotherapy and chemotherapy versus surgery plus adjuvant radiotherapy. We excluded trials using long-term alkylating agents for more than 1 year, because these agents are no longer used to treat non-small-cell lung cancer and are harmful.4
To limit publication bias, we included published and unpublished trials with no restriction by language. Searches of Medline and CancerLit (with an amended version of the Cochrane Collaboration optimal search strategy11) and trial registers, with additional MESH and free text terms for non-small-cell lung cancer and chemotherapy, were supplemented by hand searches of conference proceedings and reference lists of trial publications and review articles. Our collaborators were asked whether they knew of additional trials. Initial searches were undertaken in 2003 and were regularly updated until September, 2009.
Data collection
For the 15 trials included in our previous meta-analysis undertaken in 1995, we sought only updated follow-up. For new trials, we gathered data for age, sex, extent of resection, pathological tumour stage, histology, performance status, treatment group, date of randomisation, recurrence, survival, and follow-up for all patients randomly assigned.
We used standard checks to identify missing data, assess data validity, and consistency. We verified the amount of missing data, checked the order of dates, and assessed data validity and consistency. To assess randomisation integrity, we checked patterns of treatment allocation and balance of baseline characteristics by treatment group. Follow-up of surviving patients was checked to ensure that it was balanced by treatment group and was up-to-date. Any queries were resolved and the final database verified by each trial investigator or statistician
Definition of outcome measures
The primary outcome of overall survival was defined as the time from randomisation until death by any cause. Living patients were censored on the date of last follow-up. Recurrence-free survival, a secondary outcome, was defined as the time from randomisation until first recurrence or death by any cause. Patients alive without disease were censored on the date of last follow-up. To avoid bias from under-reporting of subsequent events, time to locoregional recurrence was defined as the time from randomisation until first locoregional recurrence, and patients with previous distant recurrences were censored at the time of distant recurrence. Similarly, for time to distant recurrence, patients with previous locoregional recurrences were censored on that date.
Statistical analysis
Unless otherwise stated, all analyses were prespecified in the protocols, and undertaken on an intention-to-treat basis. For every outcome, we used the log-rank expected number of events and variance to calculate individual trial HRs, which were pooled across trials with the fixed-effect model. Survival is also presented with simple (non-stratified) Kaplan-Meier curves. We calculated the median follow-up for all patients with the reverse Kaplan-Meier method.12
For survival, to explore any effect of trial and patient characteristics on the effect of chemotherapy, pooled HRs were calculated for every prespecified trial group or patient subgroup. We used χ2 tests for interaction to investigate differences in the treatment effect across trial groups. To investigate differences in the treatment effect across patient subgroups, we undertook Cox regressions including the relevant treatment by subgroup interaction term within trials and the interaction coefficients (HRs) pooled across trials. χ2 tests and the I2 statistic were used to assess heterogeneity in the treatment effect or patient subgroup interactions across trials.
We calculated absolute differences in overall survival at 5 years using overall HRs and survival in the control group. If a difference in effect by trial group or patient subgroup was identified, we used HRs and control group survival for the relevant groups to calculate absolute differences; otherwise the overall HR was used.
Since two trials compared two chemotherapy regimens with one control group,13,14 we compared every treatment group with the control group and analysed as separate trial comparisons in different chemotherapy categories. However, to avoid double-counting the control groups in the overall and subgroup analyses, the treatment groups were combined and compared with the relevant control group. For other trials that belonged in different chemotherapy categories15 or different meta-analyses,16–18 or both,19,20 we compared relevant patients from the treatment group with the corresponding patients in the control group, and analysed them as separate trial comparisons. This method of analysis provides a greater number of trial comparisons than there are trials.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
For the meta-analysis of surgery and chemotherapy versus surgery, we identified 35 eligible trials, of which 26 were included: nine from the previous meta-analysis done in 1995, and 17 additional trials. Nine trials could not be included because: data were not available for three published21–23 and two small unpublished trials (NCCTG 852451, EORTC 08922), adequate contact with the investigators could not be established for two trials,24,25 and two trials have only recently been presented.26,27 Therefore, data from 26 published trials13–20,28–45 were included, allowing 34 trial comparisons (table 1).
Table 1.
Characteristics of trials of surgery plus chemotherapy versus surgery
| Years of accrual | Number of patients | Country | Drug used (dose per cycle [mg/m2]) | Number of cycles | Stage | Extent of resection | ||
|---|---|---|---|---|---|---|---|---|
| Without tegafur and uracil/tegafur | ||||||||
| Platinum+vinca alkaloid/etoposide | ||||||||
| IPCR Chiba28 | 1985–91 | 29 | Japan | Cisplatin (80), vindesine (3), mitomycin (8) | >2 | NK | Complete and incomplete | |
| JLCSSG31 | 1986–88 | 209 | Japan | Cisplatin (80), vindesine (6) | 2–3 | III | NK | |
| Mineo36 | 1988–94 | 66 | Italy | Cisplatin (100), etoposide (120) | 6 | IB | Complete | |
| Park141 | 1989–98 | 118 | South Korea | Cisplatin (100), mitomycin (10), vinblastine (6) | 3–4 | I | Complete | |
| Park243 | 1989–98 | 108 | South Korea | Cisplatin (100), mitomycin (10), vinblastine (6) | 3–4 | IIIA | Complete | |
| ALPI116 | 1994–99 | 618* | European | Cisplatin (100), vindesine (6), mitomycin (8) | 3 | I–IIIA | Complete | |
| IALT118 | 1995–2001 | 1001* | International | Cisplatin (80, 100, or 120) and vindesine (3; weekly then twice weekly); or vinblastine (8; weekly then twice weekly) or etoposide (300) | 3 or 4 | I–III | Complete | |
| BLT119 | 1995–2001 | 136* | International | Cisplatin (50), mitomycin (6), vinblastine (6); or cisplatin (80), vindesine (6) | 3 | I–III | Complete | |
| JCOG 930439 | 1994–99 | 119 | Japan | Cisplatin (80), vindesine (3) | 3 | I–III | Complete and incomplete | |
| Platinum+vinorelbine | ||||||||
| ANITA117 | 1994–2000 | 463* | International | Cisplatin (100), vinorelbine (120) | 4 | IB–IIIA | Complete | |
| JBR.1042 | 1994–2001 | 482 | Canada, USA | Cisplatin (50), vinorelbine (25; initial patients received 30) | 4 | IB–II | Complete | |
| IALT218 | 1995–2001 | 294* | International | Cisplatin (80, 100, or 120), vinorelbine (30; weekly) | 3 or 4 | I–III | Complete | |
| BLT219 | 1995–2001 | 65* | International | Cisplatin (80), vinorelbine (60) | 3 | I–III | Complete | |
| Platinum+taxane | ||||||||
| CALGB 963344 | 1996–2003 | 344 | USA | Carboplatin (6 mg/mL over 45–60 min), paclitaxel (200) | 4 | IB | Complete | |
| Other platinum regimens | ||||||||
| LCSG 80130 | 1980–86 | 283 | USA, Canada | Cisplatin (60), doxorubicin (40), cyclophosphamide (400) | 4 | I | Complete | |
| FLCSG145 | 1980–86 | 110 | Finland | Cisplatin (40), doxorubicin (40), cyclophosphamide (400) | 6 | I–III | NK | |
| LCSG 85332 | 1985–89 | 188 | USA, Canada | Cisplatin (60), doxorubicin (40), cyclophosphamide (400) | 4 | II–III | Complete | |
| BLT319 | 1995–2001 | 118* | International | Cisplatin (50), mitomycin (6), ifosphamide (3) | 3 | I–III | Complete | |
| With tegafur and uracil/tegafur | ||||||||
| Platinum+vinca alkaloid+tegafur and uracil/tegafur | ||||||||
| SGACLC ACTLC129 | 1982–85 | 306 | Japan | Cisplatin (0·08 mg/kg), mitomycin (2 mg/kg); tegafur (12 mg/kg) | 10; daily treatment >6 months | NK | NK | |
| OLCSG1c20 | 1983–88 | 28* | Japan | Cisplatin (80); tegafur (600–800 total) | 1; daily treatment >1 year | II | Complete | |
| SGACLC ACTLC233 | 1985–87 | 332 | Japan | Cisplatin (66), doxorubicin (26); tegafur and uracil (8 mg/kg) | 1; daily treatment >6 months | I–III | Complete and incomplete | |
| WJSG2 (1+3)13 | 1985–89 | 215* | Japan | Cisplatin (50); vindesine (2–3 mg/kg); tegafur and uracil (400) | 1; 3; daily treatment for 1 year | I–III | Complete | |
| WJSG335 | 1988–89 | 225 | Japan | Cisplatin (80), vindesine (2–3; once or twice), mitomycin (8); tegafur and uracil (400 total) | 2; daily treatment for 1 year | I–II | Complete | |
| Xu34 | 1989–92 | 70 | China | Cisplatin (100), cyclophosphamide (300), vincristine (1·4), doxorubicin (50), lomustine (50); then oral tegafur (600–900 total) | 4; daily treatment for 1 year | I–III | Complete | |
| ACTLC4a14 | 1992–95 | 104* | Japan | Cisplatin (80); vindesine (6); tegafur and uracil (400) | 1; 2; daily treatment for 2 years | I | Complete | |
| OLCSG2b15 | 1992–94 | 95* | Japan | Cisplatin (80), vindesine (6); tegafur and uracil (400 total) | 2; daily treatment for 1 year | II–III | Complete | |
| Tegafur and uracil/tegafur+other agent | ||||||||
| OLSCG1b20 | 1982–86 | 83* | Japan | Doxorubicin (100), mitomycin (20); tegafur (600–800); followed by tegafur (600–800) | 3; daily treatment; daily treatment >1 year | II–III | Complete and incomplete | |
| Tegafur and uracil/tegafur | ||||||||
| OLCSG1a20 | 1982–88 | 321 | Japan | Tegafur (600–800 total) | Daily treatment >1 year | I | Complete | |
| WJSG2 (2+3)13 | 1985–88 | 208* | Japan | Tegafur and uracil (400) | Daily treatment for 1 year | I–III | Complete | |
| WJSG440 | 1991–94 | 367 | Japan | Tegafur and uracil (400 total) | Daily treatment for 1 year | I–II | Complete | |
| NJSGLCS37 | 1992–94 | 219 | Japan | Tegafur and uracil (260 total or 400 total) | Daily treatment for 2 years | I–II | Complete | |
| OLCSG2a15 | 1992–94 | 172* | Japan | Tegafur and uracil (400 total) | Daily treatment for 1 year | I | Complete | |
| ACTLC4b14 | 1992–95 | 104* | Japan | Tegafur and uracil (400 total) | Daily treatment for 2 years | I | Complete | |
| JLCRG38 | 1994–97 | 999 | Japan | Tegafur and uracil (250 total) | Daily treatment for 2 years | I | Complete and incomplete | |
NK=not known. LCSG=Lung Cancer Study Group. FLCSG=Finnish Lung Cancer Study Group. IPCR=Institute of Pulmonary Cancer Research, Chiba. JLCSSG=Japan Lung Cancer Surgical Study Group. ALPI=Adjuvant Lung Cancer Project Italy. JCOG=Japan Clinical Oncology Group. ANITA=Adjuvant Navelbine International Trialist Association. IALT=International Adjuvant Lung Trial. BLT=Big Lung Trial. CALGB=Cancer and Leukemia Group B. OLCSG=Osaka Lung Cancer Study Group. SGACLC=Study Group of Adjuvant Chemotherapy for Lung Cancer. WJSG=West Japan Study Group for Lung Cancer Surgery. ACTLC=Study Group of Adjuvant Chemotherapy for Lung Cancer. NJSGLCS=North-east Japan Study Group for Lung Cancer. JLCRG=Japan Lung Cancer Research Group.
Only patients relevant to the particular meta-analysis and/or chemotherapy category.
Platinum-based chemotherapy without a combination of tegafur and uracil or tegafur alone was used in 18 trial comparisons, and with tegafur and uracil or tegafur in eight (table 1). In all but one trial,44 cisplatin was the platinum agent. Tegafur and uracil or tegafur alone was used in combination with other agents in one trial comparison and alone in seven (table 1). Data for histology and stage were provided for all 34 trial comparisons, age and sex for 33, and performance status for 24 (webappendix p 2). Patients were mostly men with a median age of 61 years (range 18–84). They tended to have good performance status and tumours that were predominantly stage I–II adenocarcinomas or squamous cell carcinomas (webappendix p 2). The few patients with stage IIIB and IV tumours included—eg, because of misclassification at diagnosis—were combined with stage IIIA patients for analysis; this group is subsequently referred to as stage III. The median follow-up was 5·5 years (IQR 4·4–6·6).
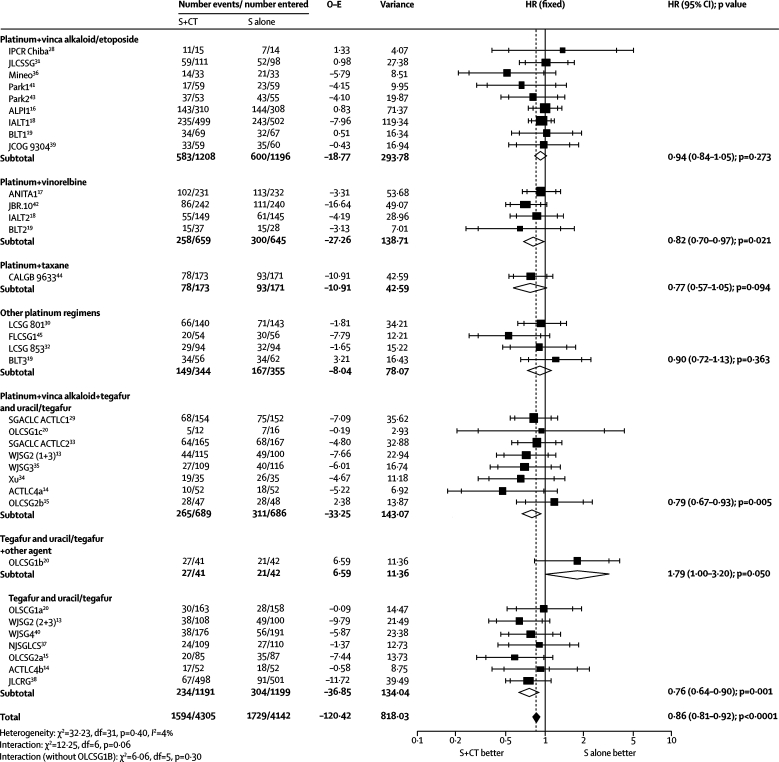
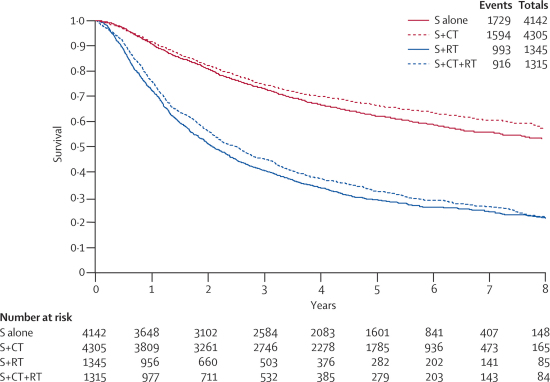
Survival results for the first meta-analysis were based on 34 trial comparisons and 8447 patients (3323 deaths), representing 92% of patients who were randomly assigned. The results show a benefit of chemotherapy (HR 0·86, 95% CI 0·81–0·92, p<0·0001; figure 1), with minimum heterogeneity (p=0·40, I2=4%). This finding represents an absolute improvement of 4% (95% CI 3–6) at 5 years, increasing survival from 60% to 64% (figure 2). We noted a difference in effect by chemotherapy category (interaction p=0·06, figure 1), largely driven by the result of the OLCSG1b trial20 that alone constituted the chemotherapy category for tegafur and uracil or tegafur plus other agent. A sensitivity analysis excluding this trial did not suggest that this drug regimen affects the effect of chemotherapy (data not shown; interaction p=0·30).
Figure 1.
Effect of surgery (S) and chemotherapy (CT) versus surgery on survival, by type of chemotherapy
Every trial is represented by a square, the center of which denotes the hazard ratio (HR) for that trial comparison with the horizontal lines showing the 95% and 99% CIs. The size of the square is directly proportional to the amount of information contributed by the trial. The open diamonds represent pooled HRs for the trial groups, with the centre denoting the HR and the extremities the 95% CI. The black diamond gives the pooled hazard ratio from the fixed effect model, without double counting the control groups of the three-grouped trials WJSG2 and ACTLC4. The centre of this diamond denotes the HR and the extremities the 95% CI. The control groups of the three-grouped trials WJSG2 and ACTLC4 are included only once in the total events and patients and in the overall analysis. O–E=observed minus expected. IPCR=Institute of Pulmonary Cancer Research, Chiba. JLCSSG=Japan Lung Cancer Surgical Study Group. ALPI=Adjuvant Lung Cancer Project. IALT=International Adjuvant Lung Trial. BLT=Big Lung Trial. JCOG=Japan Clinical Oncology Group. ANITA=Adjuvant Navelbine International Trialist Association. CALGB=Cancer and Leukemia Group B. LCSG=Lung Cancer Study Group. FLCSG=Finnish Lung Cancer Study Group. SGACLC=Study Group of Adjuvant Chemotherapy for Lung Cancer. OLCSG=Osaka Lung Cancer Study Group. WJSG=West Japan Study Group for Lung Cancer Surgery. ACTLC=Study Group of Adjuvant Chemotherapy for Lung Cancer. NJSGLCS=North-east Japan Study Group for Lung Cancer. JLCRG=Japan Lung Cancer Research Group.
Figure 2.
Simple (non-stratified) Kaplan-Meier curves for trials of surgery (S) and chemotherapy (CT) versus surgery alone and for trials of surgery and chemotherapy and radiotherapy (RT) versus surgery and radiotherapy
In view of the differences in the types of chemotherapy used over time and by geographical region, we grouped trial comparisons by these characteristics for exploratory analyses. We noted no clear evidence of a difference in the effect between trial comparisons included in the 1995 meta-analysis, and those included since this time (interaction p=0·76), by accrual decade (interaction p=0·61), or by geographical region (North America, Europe, Asia; interaction p=0·25; data not shown). Trial comparisons using tegafur and uracil or tegafur all originated in Asia, and recruited more women (n=1293 of 3465 [37%]) and more patients with stage I tumours (3003/3673 [82%]) of adenocarcinoma histology (2505/3676 [68%]) than those that did not use tegafur and uracil or tegafur alone (1093/4745 [23%], 2613/4727 [55%], 1910/4744 [40%], respectively). However, we recorded no clear evidence of a difference in treatment effect between trial comparisons that did (3848 [45%]; HR 0·80, 95% CI 0·71–0·90) and those that did not (4751 [55%]; HR 0·89, 0·82–0·97) use tegafur and uracil or tegafur (overall HR 0·86, 0·81–0·92, interaction p=0·16; webappendix p 3), even when we excluded the OLCSG1b trial comparison20 (data not shown; interaction p=0·07).
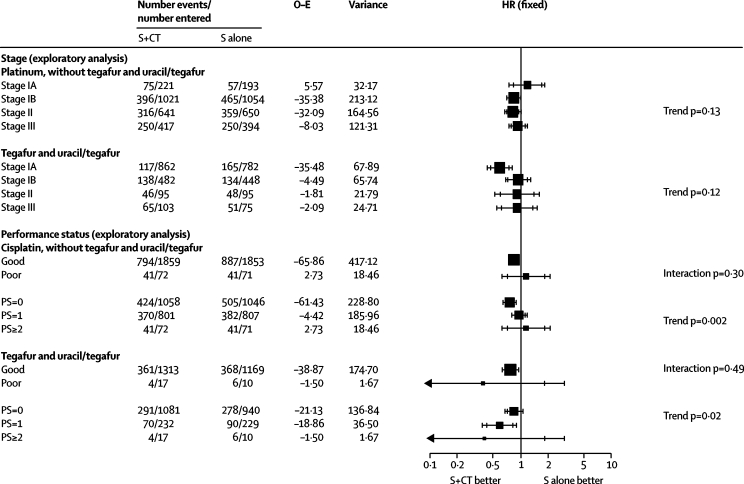
We recorded no significant evidence (p≥0·10) that any patient subgroup defined by age, sex, histology, performance status, or stage benefited more or less from chemotherapy (webappendix p 4). However, because of the geographical differences in the types of patients and chemotherapy used, we undertook exploratory subgroup analyses separately for trial comparisons using platinum, without tegafur and uracil or tegafur, and those using these drugs. Stage I disease was also split into IA and IB for all but four trials,15,29,33,41 which had to be excluded since this information was not available.
For the platinum without tegafur and uracil or tegafur alone group, although there was no evidence of difference in the effect of chemotherapy between patients with good and poor performance status (interaction p=0·30; figure 3), we noted an increasing relative effect of chemotherapy with improving performance status (trend p=0·002; figure 3), which was consistent across trials (data not shown; p=0·32). However, a few patients had a poor performance status (figure 3). The relative effect of chemotherapy did not differ significantly by other patient subgroups, including stage (trend p=0·13; figure 3). Therefore, application of the overall hazard ratio to survival in the control group by stage suggests absolute improvements in 5-year survival of 3% (95% CI 2–5) for stage IA (from 70% to 73%), 5% (2–7) for stage IB (from 55% to 60%), 5% (3–8) for stage II (from 40% to 45%), and 5% (3–8) for stage III disease (from 30% to 35%). The suggested survival benefit of 3% for stage IA and the HR of 1·19 (95% CI 0·84–1·68) for that subgroup seemed to be contradictory. However, data are scarce for this group of patients, the CIs are very wide, and the result is not significant (p=0·33).
Figure 3.
Exploratory analyses of the effect of surgery (S) and chemotherapy (CT) versus surgery on survival, by use of tegafur plus uracil or tegafur and by stage and performance status
HR=hazard ratio. O–E=observed minus expected. PS=performance status.
In the tegafur and uracil or tegafur alone group, we noted no clear difference in the effect of chemotherapy between patients with good or poor performance status (interaction p=0·49; figure 3), but did record a suggestion of an increasing relative effect of chemotherapy with worsening performance status (trend p=0·02; figure 3). This trend varies substantially across trials (data not shown; p=0·01), and few patients had a poor performance status. We noted no significant difference in the relative effect of chemotherapy by age, sex, histology, or stage, and application of the overall HR gave absolute improvements in 5-year survival of 2% (95% CI 1–3) for stage IA (from 80% to 82%), 3% (1–4) for stage IB (from 75% to 78%), 5% (2–7) for stage II (from 45% to 50%), and 5% (3–8) for stage III disease (from 25% to 30%).
Data for recurrence-free survival were available for 18 trial comparisons (2519 events; 5379 patients) and data for locoregional (936 events; 5226 patients) and distant recurrence (1267 events; 5224 patients) for 16 trial comparisons, mostly from newer trials of platinum-based chemotherapy without tegafur and uracil or tegafur alone. Results for recurrence-free survival (HR 0·83, 95% CI 0·77–0·90, p<0·0001), time to locoregional recurrence (0·75, 0·66–0·85, p<0·0001), and time to distant recurrence (0·80, 0·72–0·89, p=0·0007) all significantly favoured chemotherapy. Exclusion of the four trial comparisons that included tegafur and uracil or tegafur alone14,34,35,38 showed similar results (data not shown).
For the second meta-analysis of surgery plus radiotherapy and chemotherapy versus surgery plus radiotherapy, we identified 15 eligible trials, of which 12 were included: six from the previous meta-analysis in 1995 and six additional trials. Three could not be included because: data were not available for one trial,22 and adequate contact with investigators could not be made for two trials.46,47 Therefore, nine published16–20,48–51 and three unpublished (EORTC 08861, MDA DM 87045, FLCSG3) trials were included, allowing 13 trial comparisons (table 2).
Table 2.
Characteristics of trials surgery plus radiotherapy and chemotherapy versus surgery plus radiotherapy
| Years of Accrual | Number of patients | Country | Drug used (dose per cycle [mg/m2]) | Number of cycles | RT dose (Gy)/fraction | Stage | Extent of resection | ||
|---|---|---|---|---|---|---|---|---|---|
| Without tegafur and uracil/tegafur | |||||||||
| Platinum+vinca alkaloid/etoposide | |||||||||
| MSKCC 80-5351 | 1981–87 | 72 | USA | Cisplatin (120), vindesine (9) | 4 | 46/NK; concomitant CT-RT | III | Complete and incomplete | |
| GETCB 01CB8248 | 1982–86 | 267 | France | Cisplatin (75), doxorubicin (40), vincristine (1·2), lomustine (80 total) alternating with cyclophosphamide (600) | 3 | 60–65/30–33; CT before RT | I-III | Complete and incomplete | |
| EORTC 08861 (unpublished) | 1986–90 | 24 | International | Cisplatin (100), vindesine (6) | 4 | 56/28; CT for 2 cycles then concomitant CT-RT | IIB–IIIA | Complete | |
| MDA DM 87045 (unpublished) | 1987–93 | 34 | USA | Cisplatin (50–100), etoposide (60–120), cyclophosphamide (300–600) | NK | 50–60/25–33; CT before RT | NK | Incomplete | |
| Int 011549 | 1991–97 | 488 | USA | Cisplatin (60), etoposide (360) | 4 | 50·4/28; concomitant CT-RT | II, IIIA | Complete | |
| ALPI216 | 1994–99 | 470* | Italy | Cisplatin (100), vindesine (6), mitomycin C (8) | 3 | 50–54/25–27; CT before RT | I–IIIA | Complete | |
| IALT318 | 1995–2001 | 366* | International | Cisplatin (80, 100, or 120) and vindesine (3; weekly then twice weekly) or vinblastine (8; weekly then twice weekly) or etoposide (300) | 3 or 4 | <60; CT before RT | I–III | Complete | |
| BLT419 | 1995–2001 | 49* | UK | Cisplatin (50), mitomycin (6), vinblastine (6); or cisplatin (80), vindesine (6) | 3 | 40–60/15–30; CT before RT | I–III | Complete and incomplete | |
| Platinum+vinorelbine | |||||||||
| ANITA217 | 1994–2000 | 377* | International | Cisplatin (100), vinorelbine (120) | 4 | 45–60/23–30; CT before RT | IB–IIIA | Complete | |
| IALT418 | 1995–2001 | 206* | International | Cisplatin (80,100, or 120), vinorelbine (30 weekly) | 3 or 4 | <60; CT before RT | I–III | Complete | |
| Other platinum regimens | |||||||||
| LCSG 79150 | 1979–85 | 172 | USA, Canada | Cisplatin (40), cyclophosphamide (400), doxorubicin (40) | 6 | 40/10†; concomitant CT-RT for first 2 cycles | I-III | Incomplete | |
| FLCSG3 (unpublished) | 1982–87 | 86 | Finland | Cisplatin (40), cyclophosphamide (400), doxorubicin (40) | 8 | 55/20†; 2 cycles of CT before RT | I–III | Incomplete | |
| With tegafur and uracil/tegafur | |||||||||
| OLCSG1d20 | 1983–87 | 49 | Japan | Cisplatin (80), tegafur (600–800 given orally; daily treatment) | NK | 50/25; CT before RT | III | Complete and incomplete | |
RT=radiotherapy. CT=chemotherapy. NK=not known. LCSG=Lung Cancer Study Group. MSKCC=Memorial Sloan Kettering Cancer Center. GETCB=Groupe d'Etude et de Traitement des Cancers Bronchiques. FLCSG=Finnish Lung Cancer Study Group. EORTC=European Organization for Research and Treatment of Cancer. MDA DM=MD Anderson Department of Medicine. Int=Intergroup. ALPI=Adjuvant Lung Cancer Project Italy. IALT=International Adjuvant Lung Trial. BLT=Big Lung Trial. ANITA=Adjuvant Navelbine International Trialist Association. OLCSG=Osaka Lung Cancer Study Group.
Only patients relevant to the particular meta-analysis and/or chemotherapy category.
Split-course radiotherapy.
In nine trial comparisons chemotherapy was given before radiotherapy, and in four it was given concurrently with radiotherapy (table 2). Platinum and a vinca alkaloid or etoposide was used in ten trial comparisons, platinum and tegafur and uracil or tegafur alone in one, and other platinum regimens in two (table 2). Cisplatin was the sole platinum agent. Data for age, sex, and histology were supplied for all trial comparisons, stage and extent of resection for 12, and performance status for 11 (webappendix p 2). On the basis of these data, patients were mostly men, with good performance status, a median age of 59 years (range 27–81), and stage III, squamous carcinomas (webappendix p 2). The few patients with stage IV tumours were combined with stage III patients for analyses, and referred to as stage III. The median follow-up was 6·4 years (IQR 4·6–8·3).
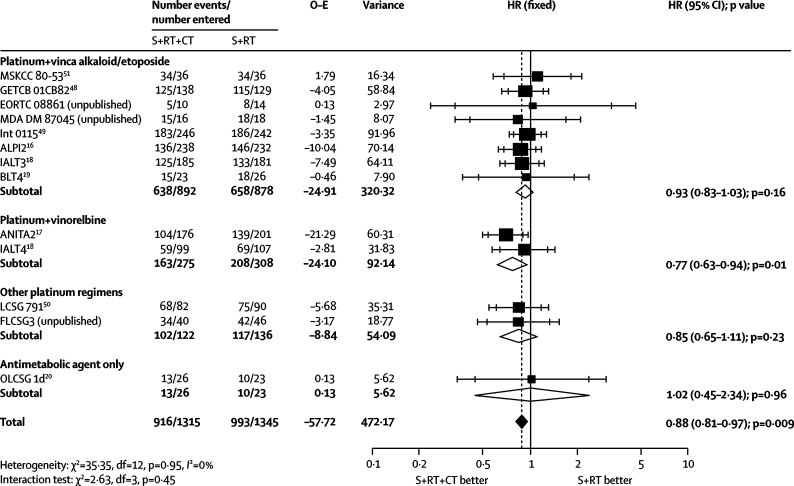
Survival analyses were based on 13 trial comparisons and 2660 patients (1909 deaths), representing 86% of patients who were randomly assigned. The results showed a clear benefit of chemotherapy (HR 0·88, 95% CI 0·81–0·97, p=0·009; figure 4), with little heterogeneity (p=0·95, I2=0%). This finding represents an absolute benefit of 4% (95% CI 1–8) at 5 years, increasing survival from 29% to 33% (figure 2). We recorded no evidence of a differential effect by chemotherapy category (interaction p=0·45; figure 4) or the extent of resection achieved (interaction p=0·54; data not shown), although few patients had incomplete resections. Furthermore, an exploratory analysis suggests that the timing of chemotherapy in relation to radiotherapy is unimportant (chemotherapy before radiotherapy, concomitant chemoradiotherapy; interaction p=0·28; data not shown).
Figure 4.
Effect of surgery (S) and radiotherapy (RT) and chemotherapy (CT) versus surgery and radiotherapy on survival by type of chemotherapy
HR=hazard ratio. O–E=observed minus expected. MSKCC=Memorial Sloan Kettering Cancer Center. GETCB=Groupe d'Etude et de Traitement des Cancers Bronchiques. MDA DM=MD Anderson Department of Medicine. Int=Intergroup. ALPI=Adjuvant Lung Cancer Project Italy. IALT=International Adjuvant Lung Trial. BLT=Big Lung Trial. ANITA=Adjuvant Navelbine International Trialist Association. LCSG=Lung Cancer Study Group. FLCSG=Finnish Lung Cancer Study Group. OLCSG=Osaka Lung Cancer Study Group.
The relative effect of chemotherapy did not differ significantly by age, sex, histology, performance status, or stage (webappendix p 6). Data for recurrence-free survival, and locoregional and distant recurrence, were available for eight trial comparisons (2247 patients). Results for recurrence-free survival (1673 events, 2247 patients; HR 0·85, 95% CI 0·77–0·93, p=0·0006), time to locoregional recurrence (533 events; 0·79, 0·67–0·94, p=0·008), and time to distant recurrence (806 events; 0·75, 0·66–0·87, p<0·0001) all showed a significant benefit of chemotherapy.
Discussion
Our results show a benefit of adjuvant chemotherapy after surgery, which has been already shown in some large trials but not in others (eg, ALPI15 and CALGB 963343). They also show a benefit of chemotherapy in the presence of postoperative radiotherapy. The absolute survival improvements of 4% at 5 years are fairly modest, but might result in 10 000 more patients alive at 5 years.3 The results of the two meta-analyses are based on data from 47 comparisons in 33 trials and 11 107 patients with non-small-cell lung cancer, which is more than three times that available in 1995.4 In these meta-analyses, we have an opportunity to bring together most trials undertaken during the past few decades, and to assess the effectiveness of adjuvant chemotherapy in patients with non-small-cell lung cancer worldwide.
Although we noted no significant difference in effect between chemotherapy categories in the first meta-analysis, results for the trials that used older vinca alkaloids (vinblastine, vindesine, vincristine), etoposide, or other platinum combinations were somewhat uncertain, whereas trials using a combination of platinum and vinorelbine provided slightly more reliable evidence of benefit to inform present clinical practice (figures 1 and 4). The results for chemotherapy with tegafur and uracil or tegafur alone are similar to those for platinum-based regimens. However, results come largely from older studies in Asian populations, which are increasingly showing differences in their response to treatment,52 and so cannot be extrapolated to modern practice in non-Asian patients. A trial of tegafur and uracil or tegafur alone in patients with stage IA, adenocarcinoma from non-Asian countries would be beneficial in this context. Results of an ongoing trial might establish the relative merits of carboplatin-paclitaxel and tegafur and uracil in Asian patients.53
Guidelines from Cancer Care Ontario and American Society of Clinical Oncology (ASCO)17–19,42,54 recommend that adjuvant cisplatin-based chemotherapy is given to patients with stage II and IIIA non-small-cell lung cancer. These guidelines state that evidence is insufficient to make recommendations for patients with stage IA disease, and one meta-analysis10 reported a significant decrease in the effect of cisplatin-based chemotherapy by stage, largely driven by the stage IA result. This meta-analysis does not show significant differences in the effect of platinum chemotherapy (without tegafur and uracil or tegafur alone) by stage or significantly poorer survival in patients with stage IA disease (figure 3). The evidence in stage IA tumours remains scarce until results from further trials are available.
The ASCO guidelines also state that none of the studies reviewed showed a significant benefit of adjuvant chemotherapy in patients with stage IB tumours. By contrast, our estimate of the effect of platinum-based chemotherapy in patients with stage IB tumours is based on a substantial number of events and is similar to estimates for patients with stage II and III tumours (figure 3). Since we did not collect data for tumour size, patients with larger stage IB tumours, who would be classed as stage II in the 7th edition of the TNM staging system55 and might achieve a greater benefit from chemotherapy,44 are potentially included. In the absence of comorbidities and contraindications to chemotherapy, adjuvant platinum-based chemotherapy should be considered for patients at high risk of recurrence—ie, those with stage IB, II, or III disease. Whether cisplatin-based chemotherapy should be used in patients with stage IA disease remains uncertain, since the scarcity of data did not allow us to distinguish reliably between a benefit, a detriment, or no effect.
Most patients had good performance status and the benefit was clear in this group. A small increasing effect of platinum-based chemotherapy with better performance status was also apparent in this and another meta-analysis,10 but was not confirmed in trials using tegafur and uracil or tegafur alone or those that included postoperative radiotherapy. Nevertheless, these results could suggest cautious use of platinum-based chemotherapy in less fit patients. Despite the amount of data collected, some of the subgroup analyses lacked power.
The benefits of adjuvant chemotherapy have been reported to be attenuated in long-term results;56,57 however, we do not have much data beyond 5 years. The potential benefit of chemotherapy should always be balanced with possible toxic effects for the individual patient. We were unable to assess toxic effects of treatment in this study. Moreover, extrapolation of the results to patients with comorbidities is uncertain because most of the patients included in these meta-analyses had mild or no comorbidities.
Addition of chemotherapy to surgery and postoperative radiotherapy gave a 4% improvement in 5-year survival from 29% to 33%. This increase does not seem to vary with the timing of chemotherapy in relation to radiotherapy, extent of surgery, or by patient subgroup (table 2, webappendix p 5). The lower survival rates than those in the surgery and chemotherapy meta-analysis are most likely because patients with stage III tumours predominate and the incomplete resection rate is higher (table 2). A previous meta-analysis58,59 has shown that postoperative radiotherapy has a detrimental effect on survival, particularly for early stage tumours, but old radiotherapy techniques were used. This meta-analysis was not designed to study the effect of postoperative radiotherapy, but has shown that the effect of chemotherapy is similar irrespective of what locoregional treatment is used: surgery alone or surgery plus postoperative radiotherapy. Randomised trials are needed to assess whether modern radiotherapy is effective as an adjuvant treatment.
Acknowledgments
Acknowledgments
The MRC Project Management Group was funded by the UK Medical Research Council, and IGR Project Management Group was supported by Institut Gustave-Roussy, Programme Hospitalier de Recherche Clinique (AOM 05 209), Ligue Nationale Contre le Cancer, and Sanofi-Aventis (unrestricted grants). We thank all patients who took part in the trials and contributed to this research. The meta-analysis would not have been possible without their participation or without the collaborating institutions that provided their trial data. We thank Estelle Rolland and Audrey Mauguen for their help in the analysis of the meta-analysis of adjuvant chemotherapy plus surgery and radiotherapy; and Catherine Hill for her comments on the report.
Contributors
A Auperin, S Burdett, T Le Chevalier, C Le Pechoux, J-P Pignon, L A Stewart, J F Tierney, and R J Stephens, with the help of the members of the Advisory Group, contributed to the conception of the study. S Burdett, J-P Pignon, J F Tierney, and H Tribodet collected and checked the data, with the help of the trial investigators who validated the re-analysis of their trials. S Burdett, J-P Pignon, J F Tierney, and H Tribodet did the statistical analysis. The report was drafted by S Burdett, J-P Pignon, H Tribodet, and J F Tierney, and was submitted for comments to the members of the Project Management Group and the Advisory Group. The investigators contributed to the interpretation of the results during the investigator meeting and revision of the report.
NSCLC Meta-analysis Collaborative Group
Project Management Group A Auperin, T Le Chevalier, C Le Pechoux, J P Pignon, H Tribodet (Institut Gustave-Roussy, Villejuif, France); S Burdett, L A Stewart, J F Tierney, R J Stephens (MRC Clinical Trials Unit, London, UK). International Advisory Group R Arriagada (Karolinska Institutet, Stockholm, Sweden; Institut Gustave-Roussy, Villejuif, France); J P Higgins (MRC Biostatistics Unit, Cambridge, UK); D H Johnson (Vanderbilt-Ingram Cancer Center, Nashville, USA); J van Meerbeeck (University Hospital, Ghent, Belgium); M K B Parmar (MRC Clinical Trials Unit, London, UK); R L Souhami (Cancer Research UK, London, UK). Writing group (Project Management Group and International Advisory Group) R Arriagada, A Auperin, S Burdett, J P Higgins, D H Johnson, T Le Chevalier, C Le Pechoux, M K B Parmar, J P Pignon, R L Souhami, R J Stephens, L A Stewart, J F Tierney, H Tribodet, J van Meerbeeck. Collaborators who supplied individual patient data B Bergman, Sahlgrenska Academy, Gothenburg, Sweden (IALT); B Dautzenberg, Groupe Hospitalier Pitié Salpêtrière, Paris (GETCB 01CB82); J Y Douillard, Centre Rene Gauducheau, Cedex, France (ANITA); A Dunant, Institut Gustave-Roussy, Villejuif, France (IALT); C Endo, Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan (NJSGLCS); D J Girling (Retired), MRC Clinical Trials Unit, London, UK (MRC LU02); M Imaizumi, Nagoya University School of Medicine, Nagoya, Japan (SGACLC ACTLC1, ACTLC2, ACTLC4); H Kato, Tokyo Medical University, Tokyo, Japan (JLCSSG, JLCRG); S M Keller, Montefiore Medical Center, NY, USA (INT 0115); H Kimura, Chiba Cancer Center, Chiba City, Japan (IPCR CHIBA); A Knuuttila, Helsinki University Central Hospital, Helsinki, Finland (FLCSG1, FLCSG2); K Kodama, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan (OLCSG1); R Komaki, University of Texas MD Anderson Cancer Center, Houston, TX, USA (MDA DM 87045); M G Kris, Memorial Sloan-Kettering Cancer Center, New York, NY, USA (MSKCC 8053); T Lad, Cook County Hospital, Chicago, IL, USA (LCSG 791, 801, 853); T Mineo, Policlinico Tor Vergata University, Rome, Italy (Mineo); J H Park, Korea Cancer Center Hospital, Seoul, South Korea (PARK1, PARK2); S Piantadosi, Cedars Sinai Medical Centre, Samuel Oschin Comprehensive Cancer Inst, Los Angeles, CA, USA (LCSG 791, 801, 853); S Pyrhönen, Turku University Central Hospital, Turku, Finland (FLCSG1, FLCSG3); R Rosell, Catalan Institute of Oncology, Hospital Germans Trias i Pujol, Barcelona, Spain (ANITA); G V Scagliotti, S. Luigi Hospital, Torino, Italy (ALPI); L W Seymour, Queen's University, NCIC Clinical Trials Group, Kingston, ON, Canada (JBR.10); F A Shepherd, Princess Margaret Hospital, Toronto, ON, Canada (JBR.10); S G Spiro, University College Hospital, London, UK (BLT); G M Strauss, Tufts Medical Center, Boston, MA, USA (CALGB 9633); R Sylvester, EORTC Headquarters, Brussels, Belgium (EORTC 08861); H Tada, Osaka City General Hospital, Osaka, Japan (JCOG 9304, OLCSG2); F Tanaka, Hyogo College of Medicine, Nishinomiya, Japan (WJSG2, WJSG3, WJSG4); V Torri, Istituto di Ricerche Farmacologiche “Mario Negri”, Milan, Italy (ALPI); H Wada, Kyoto University, Kyoto, Japan (WJSG2, WJSG3, WJSG4); D Waller, Glenfield Hospital, Groby Road, Leicester (BLT); G C Xu, Sun Yat-Sen University Cancer Center, Guangzhou, China (XU).
Conflicts of interest
We declare that we have no conflicts of interest.
Correspondence to: Sarah Burdett, Meta-analysis Group, MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK sb@ctu.mrc.ac.uk or Jean-Pierre Pignon, Meta-Analysis Unit, Institut Gustave-Roussy, 39 Rue Camille Desmoulins, 94805 Villejuif cedex, France jean-pierre.pignon@igr.fr
Web Extra Material
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani Paola Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer facts and figures 2007. American Cancer Society; Atlanta: 2007. [Google Scholar]
- 3.Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123:2096–2103. doi: 10.1378/chest.123.6.2096. [DOI] [PubMed] [Google Scholar]
- 4.Non-small Cell Lung Cancer Collaborative Group Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 5.Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Role of adjuvant chemotherapy in patients with resected non-small cell lung cancer: reappraisal with a meta-analysis of randomised controlled trials. J Clin Oncol. 2004;22:3860–3867. doi: 10.1200/JCO.2004.01.153. [DOI] [PubMed] [Google Scholar]
- 6.Sedrakyan A, van Der Meulen J, O'Byrne K, Prendiville J, Hill J, Treasure T. Postoperative chemotherapy for non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2004;128:414–419. doi: 10.1016/j.jtcvs.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Berghmans T, Paesmans M, Meert AP. Survival improvement in resectable non-small cell lung cancer with (neo)adjuvant chemotherapy: results of a meta-analysis of the literature. Lung Cancer. 2005;49:13–23. doi: 10.1016/j.lungcan.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Bria E, Gralla RJ, Raftopoulous H. Does adjuvant chemotherapy improve survival in non-small cell lung cancer (NSCLC)? A pooled analysis of 6494 patients in 12 studies, examining survival and magnitude of benefit. J Clin Oncol. 2005;23:7140. [Google Scholar]
- 9.Hamada C, Tanaka F, Ohta M. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small cell lung cancer. J Clin Oncol. 2005;23:4999–5006. doi: 10.1200/JCO.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Pignon JP, Tribodet H, Scagliotti GV. Lung Adjuvant Cisplatin Evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre C, Clarke MJ. Identifying randomised trials. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in healthcare. 2nd edn. BMJ Publishing Group; London: 2001. pp. 69–87. [Google Scholar]
- 12.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, Hitomi S, Takashi T, the West Japan Study Group for Lung Cancer Surgery Adjuvant chemotherapy after complete resection in non-small cell lung cancer. J Clin Oncol. 1996;14:1048–1054. doi: 10.1200/JCO.1996.14.4.1048. [DOI] [PubMed] [Google Scholar]
- 14.Imaizumi M. Postoperative adjuvant cisplatin, vindesine, plus uracil-tegafur chemotherapy increased survival of patients with completely resected p-stage I non-small cell lung cancer. Lung Cancer. 2005;49:85–94. doi: 10.1016/j.lungcan.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Tada H, Akash A. Randomised study of adjuvant chemotherapy for completely resected p stage I-IIIa non-small cell lung cancer. Br J Cancer. 2006;95:817–821. doi: 10.1038/sj.bjc.6603336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scagliotti GV, Fossati R, Torri V. Randomized study of adjuvant chemotherapy for completely resected stage I, II or IIIa non-small cell lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Rosell R, De Lena M. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 18.The International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 19.Waller D, Peake MD, Stephens RJ. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Sawamura K, Mori T, Doi O. A prospective randomized controlled study of the postoperative adjuvant therapy for non-small cell lung cancer. Lung Cancer. 1988;4:A166. [Google Scholar]
- 21.Ichinose Y, Hara N, Ohta M, Motohiro A, Kuda T, Aso H. Postoperative adjuvant chemotherapy in non-small cell lung cancer: prognostic value of DNA ploidy and postrecurrent survival. J Surg Oncol. 1991;46:15–20. doi: 10.1002/jso.2930460105. [DOI] [PubMed] [Google Scholar]
- 22.Ayoub J, Vigneault E, Hanley J. The Montreal multicenter trial in operable non-small cell lung cancer (NSCLC): a multivariate analysis of the predictors of relapse. Proc Am Soc Clin Oncol. 1991;10:247. [Google Scholar]
- 23.Clerici M, Barni S, Cantaluppi G. Adjuvant chemotherapy in non-small cell lung cancer (NSCLC): a randomised trial. Eur J Cancer. 1991;27(suppl 2):S173. [Google Scholar]
- 24.Zarogoulidis K, Filippou K, Antonio C, et al. The impact of adjuvant chemotherapy on the survival of postoperative stage IIIa NSCLC patients. Proceedings of the 2nd International Congress on Lung Cancer. Crete, 1996 (abstr PO35).
- 25.Ueda H, Sakada T, Kuwahara M, Motohiro A. A small randomized phase III single-center trial on postoperative UFT administration in patients with completely resected non-small cell lung cancer. Anticancer Drugs. 2004;15:29–33. doi: 10.1097/00001813-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Ou W, Sun H, Yang HX, Fang Q. Adjuvant chemotherapy in completely resected stage III-N2 non-small cell lung cancer. Proc Am Soc Clin Oncol. 2009;27:A7563. [Google Scholar]
- 27.Wu Y, Yang X, Chen G. Adjuvant docetaxel plus carboplatin compared with surgery only in patients with completely resected stage IB-IIIA non-small cell lung cancer: final results of CSLC201/TAX210 with Chinese Society of Lung Cancer. J Thorac Oncol. 2009;4:S583–S584. [Google Scholar]
- 28.Kimura H, Yamaguchi Y, Fujisawa T, Baba M, Shiba M. A randomized controlled study of postoperative adjuvant chemoimmunotherapy of resected non-small cell lung cancer with IL2 and LAK cells. Lung Cancer. 1991;7(suppl):133. [Google Scholar]
- 29.Study Group for Adjuvant Chemotherapy for Lung Cancer A randomised controlled trial of post-operative adjuvant therapy for non-small cell lung cancer. Hai-gan. 1992;32:481–486. [Google Scholar]
- 30.Feld R, Rubinstein L, Thomas PA. Adjuvant chemotherapy with cyclophosphamide, doxorubicin and cisplatin in patients with completely resected stage I non-small-cell lung cancer. J Natl Cancer Inst. 1993;85:299–306. doi: 10.1093/jnci/85.4.299. [DOI] [PubMed] [Google Scholar]
- 31.Ohta M, Tsuchiya R, Shimoyama M. Adjuvant chemotherapy for completely resected stage III non-small cell lung cancer. J Thorac Cardiovasc Surg. 1993;106:703–708. [PubMed] [Google Scholar]
- 32.Figlin RA, Piantodosi S. A phase 3 randomized trial of immediate combination chemotherapy vs delayed combination chemotherapy in patients with completely resected stage II and III non-small cell carcinoma of the lung. Chest. 1994;106(suppl):310S–312S. doi: 10.1378/chest.106.6_supplement.310s. [DOI] [PubMed] [Google Scholar]
- 33.Study Group for Adjuvant Chemotherapy for Lung Cancer A randomized trial of postoperative adjuvant chemotherapy in non-small cell lung cancer (the second cooperative study) Eur J Surg Oncol. 1995;21:69–77. [PubMed] [Google Scholar]
- 34.Xu G, Rong T, Lin P. Adjuvant chemotherapy following radical surgery for non-small cell lung cancer: a randomized study. Zhonghua Zhong Liu Za Zhi. 1998;20:228–230. [PubMed] [Google Scholar]
- 35.Wada H, Miyahara R, Tanaka F, Hitomi S, West Japan Study Group for lung cancer surgery Post-operative adjuvant chemotherapy with PVM (cisplatin + vindesine + mitomycin c) and UFT (uracil and tegaful) in resected stage I-II NSCLC (non-small cell lung cancer): a randomised clinical trial. Eur J Cardiothorac Surg. 1999;15:438–443. doi: 10.1016/s1010-7940(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 36.Mineo TC, Ambrogi V, Corsaro V, Roselli M. Postoperative adjuvant therapy for stage IB non-small cell lung cancer. Eur J Cardiothoracic Surg. 2001;20:378–384. doi: 10.1016/s1010-7940(01)00779-5. [DOI] [PubMed] [Google Scholar]
- 37.Endo C, Saitoi Y, Iwanawi H. A randomized trial of postoperative UFT in p stage I, II non-small cell lung cancer: North-East Japan Study Group for Lung Cancer Surgery. Lung Cancer. 2003;40:181–186. doi: 10.1016/s0169-5002(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 38.Kato H, Ichinose Y, Ohta M. A randomised trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 39.Tada H, Tsuchiya R, Ichinose Y. A randomized trial comparing adjuvant chemotherapy versus surgery alone for completely resected pN2 non-small cell lung cancer (JCOG 9304) Lung Cancer. 2004;43:167–173. doi: 10.1016/j.lungcan.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa M, Tanaka F, Tsubota N, Ohta M, Takao M, Wada H. A randomised phase III trial of adjuvant chemotherapy with UFT for completely resected pathological stage I non-small cell lung cancer: the West Japan Study Group for Lung Cancer Surgery (WJSG)—the 4th study. Ann Oncol. 2005;16:75–80. doi: 10.1093/annonc/mdi008. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Lee C-T, Lee HW, Baek HJ, Zo JI, Shim YM. Postoperative adjuvant chemotherapy for stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2005;27:1086–1091. doi: 10.1016/j.ejcts.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 42.Winton T, Livingston R, Johnson D. Vinorelbine plus cisplatin vs observation in resected non-small cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 43.Park JH. Postoperative adjuvant therapy for stage IIIA non-small cell lung cancer. J Thorac Oncol. 2007;2(suppl):S651. [Google Scholar]
- 44.Strauss GM, Herndon JE, 2nd, Maddaus MA. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niiranen A, Niitamo-Korhonen S, Kouri M, Assendelft A, Mattson K, Pyrhönen S. Adjuvant chemotherapy after radical surgery for non-small cell lung cancer: a randomized study. J Clin Oncol. 1992;10:1927–1932. doi: 10.1200/JCO.1992.10.12.1927. [DOI] [PubMed] [Google Scholar]
- 46.Wolf M, Müller H, Seifart U. Randomized phase III trial of adjuvant radiotherapy versus adjuvant chemotherapy followed by radiotherapy in patients with N2 positive non-small cell lung cancer (NSCLC) Proc Am Soc Clin Oncol. 2001;20:311a. [Google Scholar]
- 47.Kim S-W, Suh CS, Lee G-W. The number of tumor (+) N2 nodes as a prognostic factor in the patients with N2 disease non-small cell lung cancer after curative resection and postoperative thoracic radiotherapy. Lung Cancer. 2003;41(suppl 2):S152. [Google Scholar]
- 48.Dautzenberg B, Chastang C, Arriagada R. Adjuvant radiotherapy versus combined sequential chemotherapy followed by radiotherapy in the treatment of resected non-small cell lung cancer. Cancer. 1995;76:779–786. doi: 10.1002/1097-0142(19950901)76:5<779::aid-cncr2820760511>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 49.Keller SM, Adak S, Wagner H. Postoperative adjuvant therapy in patients with stage II or IIIa non-small cell lung cancer. N Engl J Med. 2000;343:1217–1222. doi: 10.1056/NEJM200010263431703. [DOI] [PubMed] [Google Scholar]
- 50.Lad T, Rubinstein L, Sadeghi A. The benefit of adjuvant treatment for resected locally advanced non-small cell lung cancer. J Clin Oncol. 1988;6:9–17. doi: 10.1200/JCO.1988.6.1.9. [DOI] [PubMed] [Google Scholar]
- 51.Pisters KMW, Kris MG, Gralla RJ, Hilaris B, McCormack PM, Bains MS. Randomized trial comparing post-operative chemotherapy with vindesine and cisplatin plus thoracic irradiation with irradiation alone in stage III (N2) non-small cell lung cancer. J Surg Oncol. 1994;56:236–241. doi: 10.1002/jso.2930560407. [DOI] [PubMed] [Google Scholar]
- 52.Sekine I, Yamamoto N, Nisho K, Saijo N. Emerging ethnic differences in lung cancer therapy. Br J Cancer. 2008;99:1757–1762. doi: 10.1038/sj.bjc.6604721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toyooka S, Hotta K, Nakamura H. A multicenter, phase III study of carboplatin/paclitaxel, versus oral uracil-tegafur as the adjuvant chemotherapy in resected non-small cell lung cancer (NSCLC): planned interim analyses. Proc Am Soc Clin Oncol. 2009;27:A7560. [Google Scholar]
- 54.Pisters KMW, Evans WK, Azzoli CG. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIa resectable non-small cell lung cancer guideline. J Clin Oncol. 2007;25:5506–5518. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 55.International Association for the Study of Lung Cancer . Staging manual in thoracic oncology. Editorial Rx Press; Orange Park: 2009. [Google Scholar]
- 56.Arriagada R, Dunant A, Pignon JP. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 57.Butts CA, Ding K, Seymour L. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR.10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.PORT Meta-analysis Trialists Group Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- 59.PORT Meta-analysis Trialists Group Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2005;2:CD002142. doi: 10.1002/14651858.CD002142.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.