Abstract
Objectives. Clinical and laboratory markers in current use have limited specificity and sensitivity for predicting the development of renal disease in lupus patients. In this longitudinal study, we investigated whether urinary neutrophil gelatinase-associated lipocalin (uNGAL) predicts active nephritis and renal flares in lupus patients with and without a history of biopsy-proven lupus nephritis.
Methods. Renal disease activity and flare status was determined by SLEDAI and BILAG scores. Random effects models were used to determine whether uNGAL was a significant predictor for renal disease activity in SLE patients, and for renal flares in patients with established nephritis. To assess the predictive performance of uNGAL, receiver operating characteristic (ROC) curves were constructed using the previous visit’s uNGAL level. These curves were then compared with curves constructed with currently used biomarkers. Cut-offs determined by ROC curves were tested in an independent validation cohort.
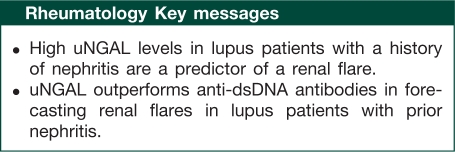
Results. uNGAL was found to be a significant predictor of renal disease activity in all SLE patients, and a significant predictor for flare in patients with a history of biopsy-proven nephritis, in multivariate models adjusting for age, race, sex and anti-double-stranded (ds)DNA antibody titres. As a predictor of renal flare in patients with biopsy-proven nephritis, uNGAL outperformed anti-dsDNA antibody titres. These results were confirmed in an independent validation cohort.
Conclusions. uNGAL predicts renal flare in patients with a history of biopsy-proven nephritis with high sensitivity and specificity. Furthermore, uNGAL is a more sensitive and specific forecaster of renal flare in patients with a history of lupus nephritis than anti-dsDNA antibody titres.
Keywords: Systemic lupus erythematosus, Lupus nephritis, Neutrophil gelatinase-associated lipocalin, Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group, Biomarkers
Introduction
While disease-modifying drugs have decreased the morbidity and mortality of SLE, renal involvement or lupus nephritis (LN) still occurs in up to 50% of SLE patients [1]. Severe LN has been reported to result in end-stage kidney disease at a rate of 10–26% [2], which may be a result of the difficulty in recognizing a flare early enough to affect the course of the disease—since prompt diagnosis and earlier treatment lead to better outcomes [3].
Currently, the laboratory tools used to measure kidney damage are crude and neither specific nor sensitive to nephritis flares [4]. Physicians determine the level of disease, or the presence of a flare, by using validated activity ‘tools’ such as SLEDAI and BILAG and by assessing changes in other SLE disease activity markers and the need for treatment. Novel putative biomarkers in LN include a variety of inflammatory cytokines/chemokines; however, these have yet to be widely implemented in clinical practice [5, 6].
Renal biopsy is considered more powerful and informative than currently used laboratory tests in determining nephritis activity. Though some clinicians recommend serial kidney biopsies [7, 8], the role of such an aggressive approach in the management of LN remains controversial [8] and is not common practice. The advent of accurate biomarkers to predict, diagnose and monitor LN would be a major achievement in the management of SLE patients. In addition, biomarkers may have the advantage of providing a more global view of the kidney than a small sample of tissue. Furthermore, if biomarkers are fundamental mediators in the pathogenesis of the disease, their detection may precede changes in currently used laboratory measurements of renal function [9].
Neutrophil gelatinase-associated lipocalin (NGAL) was originally found to be highly expressed in renal tissue of mice following ischaemia/reperfusion injury [10]. Subsequently, high levels of NGAL were shown in a variety of kidney diseases such as haemolytic uraemic syndrome [11], acute tubular necrosis [12], acute renal failure following cardio-pulmonary bypass [13] and chronic kidney disease [14]. Moreover, NGAL seems to be intricately related to inflammation, by preventing the auto-degradation of MMP-9 [15], and through a possible link with apoptosis [16, 17]. For these reasons, there has been significant interest in using NGAL as a possible marker for kidney injury [6].
In a prior cross-sectional study, we found that patients with LN had higher levels of urinary NGAL (uNGAL) than either SLE patients without a history of LN or normal controls [18]. These results corroborated the initial study of NGAL in paediatric LN by Brunner et al. [4], which showed that in childhood-onset SLE uNGAL correlated with disease activity and renal damage. Recently, the same group reported that increases in uNGAL in this paediatric lupus cohort corresponded to worsening renal disease [19]. Considering the prior evidence that uNGAL correlates with lupus renal disease activity, we set out in the current study to investigate the predictive ability of uNGAL—whether preceding uNGAL levels can inform clinicians of future disease activity in adult patients, both with and without a prior history of LN. The ability of uNGAL to predict future renal disease activity and flares would be extremely useful in determining its clinical utility as a biomarker.
Patients and methods
After study approval by the Committee on Clinical Investigations, the federally designated Institutional Review Board for Jacobi and Montefiore Medical Centers, subjects were recruited from rheumatology clinics at Jacobi and Montefiore Medical Centers (Bronx, NY, USA) between January 2005 and March 2008 (‘Bronx cohort’). Written informed consent according to the Declaration of Helsinki was obtained from all recruited patients. To meet inclusion criteria for the study, subjects must have met four ACR criteria for SLE diagnosis. For the subgroup analysis of LN patients, inclusion into the group required evidence of WHO Class II, III, IV or V on biopsy. Urine samples were collected from each subject during multiple visits within the above time period and stored at −80°C. One hundred and eighty subjects were successfully recruited, of whom 107 had multiple study visits.
At each study visit, urine samples were collected for measurement of uNGAL as well as for standard urinalysis and a urine protein/creatinine ratio. All visits were morning visits in an effort to approximate urine protein/creatinine ratios of first morning voids. In addition to urine sample, clinical data were collected for each subject, including SLEDAI-2K scores, complete blood count, serum chemistry, serum C3 and C4, and anti-double-stranded (ds)DNA antibody titres. The presence of renal disease activity was defined as a renal SLEDAI score of ⩾4, corresponding to the presence of any one of the following on urinalysis: haematuria, proteinuria, pyuria or urinary red cell casts [20]. Haematuria and pyuria were defined as ⩾5 cells per high powered field and proteinuria was defined as a urine protein/creatinine ratio ⩾0.5. In the Bronx cohort, renal flare was defined as an increase in the renal SLEDAI score of ⩾4 points from the previous visit [18]. SLEDAI scores, including renal SLEDAI scores, were determined prospectively and not informed by renal biopsy. It should be noted that unlike with renal flare, for an individual to be categorized as having renal disease activity at a specific visit, no change was required from the individual’s previous clinic visit status.
uNGAL levels were measured in quadruplicate by ELISA, as described [18]. In brief, 96-well polystyrene plates were coated overnight at 4°C with mouse anti-human NGAL mAb (Antibody Shop, Genofte, Denmark). All subsequent steps were performed at room temperature. Plates were blocked with 1% BSA in phosphate buffered saline, and serum samples diluted 1: 300 in blocking buffer were incubated for 2 h. Serial dilutions of recombinant human NGAL (R&D Systems, Minneapolis, MN, USA) were used on each plate to generate a standard curve. The plates were then washed and incubated with a biotinylated anti-human NGAL monoclonal antibody (Antibody Shop, Genofte, Denmark), followed by streptavidin conjugated to horseradish peroxidase. Tetramethylbenzidine was used for colour development (BD Biosciences, San Jose, CA, USA). After the addition of stop solution, the plates were read at 450 nm in a microplate reader. Our ELISA technique to measure uNGAL levels was previously validated using western blotting [18].
Since previous studies by us [18] and others [19] found a strong, significant correlation in lupus patients between uNGAL levels, whether or not these were normalized to urine creatinine concentrations, only uNGAL concentrations (in nanograms per millilitre) were calculated for this study.
All samples were measured together to minimize batch effects. Each sample was measured in duplicate on a plate and each plate was duplicated. Concentrations of three control samples were measured against a standard for each set of plates, and samples for each plate were measured against the three control samples.
Power calculation
For an estimated frequency of renal disease activity of 33%, a sample size of 107 patients yields 80% power, with a two-sided alpha set at 0.05, to detect an odds ratio (OR) of 2.18. A sample size of 25 patients with biopsy-proven nephritis and an estimated frequency of renal flare of 33% yields the same power to detect an OR of 5.13. These are conservative estimates of the minimal detectable odds, since they do not account for the multiple samples provided by each patient. Power calculations were made using PS Power and Sample Size Calculation 2.1.30.
Statistical analysis
Student’s t-tests were used to compare laboratory and clinical characteristics of patients with and without renal disease, or with and without renal flare. A random effects logistic regression model (to account for the variability between subjects who contribute multiple observations) with the previous visit’s uNGAL level as a predictor and renal disease activity in the concurrent visit as the outcome was constructed to determine whether uNGAL was a significant predictor for renal disease activity in the total population of SLE patients. A multivariate random effects logistic regression model was also constructed to examine whether uNGAL remains a significant predictor of renal disease activity after adjusting for sex, age, race, history of biopsy-proven nephritis and the previous visit’s anti-dsDNA antibody titres. Another random effects model was constructed with the outcome of renal flare in patients with biopsy-proven LN, and a multivariate model including the confounders of age, race, sex and the previous visit’s anti-dsDNA antibody titres.
An ROC curve was constructed using the previous visit’s uNGAL level for each follow-up visit’s outcome of either the presence or absence of renal disease activity among SLE patients. A second ROC curve was constructed for the binary outcome of renal flare in follow-up visits among those patients with biopsy-proven nephritis. Similar curves were constructed with currently used biomarkers for LN for comparison with uNGAL curves: C3, C4 and anti-dsDNA antibody titres. Cut-offs for ROC curves that optimized the number of correctly predicted outcomes were determined by sensitivity and specificity tables. Statistical analysis was performed using STATA 10 (Stata Corp, College Station, TX, USA).
Validation study in a second patient population
uNGAL levels were similarly assessed on banked urine samples obtained from a population of patients treated by the Rheumatology Division of University College London (UCL; London, UK) (‘London cohort’), as approved by the UCL Research Ethics Committee. Written informed consent according to the Declaration of Helsinki was obtained from all recruited patients. Eligibility criteria were similar to the primary study. Urine samples and clinical data were collected during each visit. Urine dipstick data were collected for all patients and for those with proteinuria found on dipstick a urine protein/creatinine ratio was measured.
In the London cohort, in lieu of flare and renal activity determined by SLEDAI, the BILAG index was used, which is considered a more sensitive scoring system for gradations in disease activity and flares [21]. The presence of active (non-stable) renal disease corresponding to either the onset of new renal disease or a flare was determined by a renal BILAG score of ‘A’ (severe renal disease requiring immunosuppressants, or prednisone >20 mg daily), or a score of ‘B’ (moderate disease activity requiring medication). The global BILAG score was calculated using the formula A = 9, B = 3, C = 1, D/E = 0 and this formula was used to create a renal BILAG score [21, 22]. In addition, SLICC damage index data were collected for total global damage and for renal damage specifically.
Two random effects models were constructed with uNGAL as the predictor for renal disease activity in all SLE patients, and separately in LN patients, to examine whether the findings in the original Bronx cohort could be reproduced in a population of differing ethnicities and using an alternative scoring system for renal disease activity. Cut-offs determined from the ROC curves constructed from the Bronx study population data were tested on the validation London cohort to evaluate the sensitivity and specificity of high uNGAL for detecting renal disease activity.
Results
There were 107 patients in the Bronx cohort seen for two or more study visits (265 total observations). Of these, 103 patients had complete SLEDAI data for multiple study visits (257 total observations). The demographics of our study sample reflected our clinic population (Table 1): 91% female; 47% Hispanic, 46% African American, 2% white and 4% others. The mean age was 41 (12) years (ranging from 16 to 67 years). Renal disease activity (any visit with a renal SLEDAI ⩾4) was seen in 32% of visits (83/257 visits). The 25 patients with biopsy-proven nephritis had similar demographics to the patients overall, but with a higher percentage of Hispanic patients (Table 1). Most of the LN patients had Class IV (60%), and a renal flare was seen in 38% of visits (29/77 visits), with repeat renal flares seen in eight individuals (mean of 2.3 flares/participant).
Table 1.
Patient characteristics in the Bronx and London cohorts
| Bronx cohort |
London cohort | ||
|---|---|---|---|
| SLE patients (n = 107) | Biopsy-proven LN study population (n = 25) | SLE patients (n = 35) | |
| Race, n (%) | Hispanic, 45 (47) Black, 44 (46) White, 2 (2) Others, 4 (4) | Hispanic, 16 (62) Black, 7 (27) Asian/South Asian, 2 (8) | White, 17 (49) Black, 8 (23) Asian, 5 (14) Southeast Asian, 3 (9) Others, 2 (6) |
| Age, years | 41 (12) | 37 (13) | 41 (13) |
| Sex, female, n (%) | 97 (91) | 22 (88) | 34 (97) |
| History of biopsy-proven LN, n (%) | 25 (23) | 25 (100) | 15 (43) |
| WHO Class of LN, n (%) | Class III, 7 (28) Class IV, 15 (60) Class V, 3 (12) | Class II, 1 (7) Class III, 1 (7) Class IV, 8 (53) Class V, 5 (33) | |
| Average follow-up time in between visits, weeks | 27 (26) | 24 (27) | 7.1 (3.6) |
| Average number of visits | 4.3 (2.2) | 4.0 (1.7) | 2.2 (0.5) |
| Renal disease activity prevalence, n (%) | 83/257 visits (32) | NA | 13/70 visits (19) |
| Renal flare prevalence among biopsy-proven nephritis, n (%) | NA | 29/77 visits (38) | 8/31 visits (26) |
| Years since SLE diagnosis | Not available | 5.66 (3.66) | |
| GFR | 86.3 (22.3) | ||
| Medications (during first follow-up visit) | |||
| Angiotensin inhibitors, n (%) | 9 (25.7) | ||
| MTX, n (%) | 2 (5.7) | ||
| Rituximab (ever use), n (%) | 9 (25.7) | ||
| Mycophenolate mofetil, n (%) | 6 (17.1) | ||
| AZA, n (%) | 11 (31.4) | ||
| Ciclosporin, n (%) | 1 (2.9) | ||
| HCQ, n (%) | 19 (54.3) | ||
| NSAIDs, n (%) | 4 (11.4) | ||
| Oral steroids, n (%) | 28 (80) | ||
| Mean dose of oral steroids, mg/day | 7.76 (5.64) | ||
The subgroup of biopsy-proven LN participants in the Bronx cohort (n = 25) is included in the total SLE population of the Bronx cohort (n = 107). Values are represented as means (s.d.), unless otherwise noted. GFR: glomerular filtration rate; NA: not applicable.
uNGAL and renal disease activity in SLE patients
In previous studies, it was determined that the performance of uNGAL as a lupus biomarker is clearly superior to that of plasma or serum NGAL [18, 19]. In fact, we found that levels of urinary and serum NGAL are not associated in lupus patients [18]. Therefore, for the purpose of the current analysis, we did not measure plasma/serum levels of NGAL, but rather focused on analysis of uNGAL concentrations.
Patients with renal disease activity had significantly higher urine protein/creatinine ratios and anti-dsDNA antibody titres than lupus patients without renal disease activity (Table 2). Mean uNGAL level among all lupus study subjects was found to be 17.3 ± 12.6 ng/ml, and patients with active renal disease had a significantly higher level of uNGAL in the preceding visit than those with no renal disease (Table 2).
Table 2.
Laboratory values and clinical scores for SLE patients with and without renal disease, and biopsy-proven nephritis patients with and without flare
| SLE patients (n = 107) |
SLE patients with biopsy-proven nephritis (n = 25) |
||||
|---|---|---|---|---|---|
| Visits with renal disease (83 visits; n = 56) | Visits with no renal disease (174 visits; n = 96) | Visits with renal flare (29 visits; n = 18) | Visits with no renal flare (48 visits; n = 23) | All SLE patients (n = 107) | |
| Renal SLEDAI | 5.86 (2.92)**** | 0 | 7.86 (3.78)**** | 2.72 (3.45) | 1.87 (3.19) |
| Non-renal SLEDAI | 2.61 (2.80) | 2.48 (2.75) | 2.19 (2.33) | 2.18 (2.16) | 2.52 (2.76) |
| Total SLEDAI | 8.38 (4.34)*** | 2.48 (2.75) | 9.58 (5.47)*** | 4.91 (4.78) | 4.33 (4.31) |
| Proteinuria (urine protein/creatinine ratio) | 1.57 (2.43)*** | 0.16 (0.13) | 2.80 (3.89) | 1.22 (1.56) | 0.79 (1.75) |
| C3 | 98.51 (32.66) | 102.85 (29.74) | 98.42 (26.26) | 92.70 (32.39) | 101.52 (30.68) |
| C4 | 23.19 (11.23) | 23.23 (11.32) | 24.88 (10.25) | 23.05 (10.80) | 23.22 (11.27) |
| Anti-dsDNA antibody titres | 169.26 (351.65)*** | 57.84 (100.86) | 147.33 (207.62) | 126.65 (279.11) | 89.83 (212.14) |
| uNGAL, ng/ml | |||||
| Concurrent | 17.59 (12.89) | 16.78 (12.34) | 15.82 (7.68) | 13.65 (6.78) | 17.04 (12.89) |
| Previous visit | 19.62 (15.56)* | 16.28 (10.77) | 18.68 (7.30)** | 13.41 (6.10) | 17.26 (12.57) |
*P < 0.05; **P = 0.006; ***P < 0.005; ****P < 0.0001. Columns 3 and 4 indicate visits for the subgroup of patients with biopsy-proven LN (these patients are included in calculations for columns 1 and 2). SLEDAI scores were determined by the SLEDAI 2K. Renal SLEDAI is scored on a urinalysis that indicates the presence of proteinuria, haematuria, pyuria and urinary casts, each receiving a score of up to four points for a total possible ranging from 0 to 16. Non-renal SLEDAI is scored based on clinical indicators of systemic disease or disease in other organ systems including: neurological, skin, cardiac, pulmonary and immunological. Data were analysed using Student’s t-test. Means are reported and s.d.s are shown in parentheses.
Using a random effects logistic regression model, with renal disease activity as the outcome and uNGAL level as a predictor, we found that uNGAL was a significant predictor for renal disease activity (P < 0.05) with an OR of 1.54 (1.00, 2.35) for each s.d. increase of uNGAL (Table 3). The random effects model showed that there was a significant correlation between observations from the same subjects (P for ρ < 0.001). uNGAL remained a significant predictor after adjusting for sex, race, age, biopsy-proven nephritis and anti-dsDNA antibody titres, with an OR of 1.70 (1.11, 2.62) for each s.d. increase in uNGAL (Table 3). In contrast, the previous visit’s anti-dsDNA titres were not found to be significant predictors in a univariate model for renal disease activity (P = 0.144). Neither C3 nor C4 complement levels were found to be significant predictors of renal activity (P = 0.513 and 0.965, respectively). Moreover, a multivariate model containing anti-dsDNA antibody titres and C3 levels did not significantly predict renal disease (P = 0.366), nor did a univariate model with a combined variable that reflected high anti-dsDNA antibody titres and low C3 levels (P = 0.166).
Table 3.
uNGAL as a predictor of renal disease activity and renal flare
| OR (95% CI) for renal disease activity by s.d. (12.6 ng/ml) increase in uNGAL for SLE patients | OR (95% CI) by s.d. increase in uNGAL for renal flare in SLE patients with biopsy-proven nephritis | |
|---|---|---|
| Crude OR | 1.54 (1.00, 2.35) | 4.49 (1.37, 14.69) |
| Adjusted OR | 1.70 (1.11, 2.62)a | 4.63 (0.99, 21.80)b |
aAdjusted for sex, race (Hispanic vs non-Hispanic), age, history of biopsy-proven nephritis, previous visit’s anti-dsDNA antibody titres. bAdjusted for sex, race (Hispanic vs non-Hispanic), age, previous visit’s anti-dsDNA antibody titres.
To confirm that high uNGAL is specific for renal disease rather than reflecting higher levels of global disease activity, we compared the ability of uNGAL to forecast increases in the renal SLEDAI score with its ability to predict a rise in the non-renal components of the SLEDAI. We found that uNGAL was a significant predictor for the following visit’s renal SLEDAI score (P < 0.0001), whereas it was not a significant predictor for the non-renal SLEDAI (P = 0.860). Results were similar in multivariate models that adjusted for age, sex and race (Table 4).
Table 4.
uNGAL as a predictor of renal SLEDAI and non-renal SLEDAI scores in the following visit for SLE patients
| Renal SLEDAI OR (95% CI) by s.d. (12.6 ng/ml) increase in uNGAL | Non-renal SLEDAI OR (95% CI) by s.d. increase in uNGAL | |
|---|---|---|
| Crude OR | 1.63 (1.11, 2.40) | 1.03 (0.73, 1.45) |
| Adjusted ORa | 1.71 (1.19, 2.47) | 1.01 (0.72, 1.42) |
aAdjusted for sex, race (Hispanic vs non-Hispanic), age, history of biopsy-proven nephritis.
In agreement with our previous study [18], there was no correlation between uNGAL and proteinuria, either measured as an absolute concentration (r = −0.002, P = 0.97) or corrected for urine creatinine (r = −0.037, P = 0.57), indicating that the presence of uNGAL cannot be explained by non-specific renal protein excretion.
For the subgroup analysis of biopsy-proven LN patients, 25 patients were seen in at least one follow-up visit. As seen in the total SLE population, a trend in higher concurrent uNGAL levels was seen in LN patients with renal flare; however, patients with renal flare had significantly higher levels of uNGAL in the previous visit than patients without a renal flare (Table 2). In a model for patients with biopsy-proven nephritis, the preceding uNGAL level was a significant predictor for renal flares (P = 0.013) with an OR of 4.49 (1.37, 14.69) for each s.d. increase in uNGAL (Table 3, right). In a multivariate model with the confounders of age, sex, race and anti-dsDNA antibody titres from the preceding visit, uNGAL had a similar effect, bordering on significance as a predictor with an OR of 4.63 (0.99, 21.80) (Table 3, right). In patients with a history of biopsy-proven nephritis, a similar pattern was seen in which increases in renal SLEDAI, but not increases in the non-renal components of the SLEDAI, could be predicted by the previous visit’s uNGAL level (data not shown).
An ROC curve constructed for the previous visit’s uNGAL level and renal disease activity in all SLE patients showed that uNGAL had a moderate level of sensitivity and specificity with an area under curve (AUC) of 0.59. The ROC curves for C3, C4 and anti-dsDNA antibody titres were similar to uNGAL’s ROC curve. A cut-off value for uNGAL of 11.7 ng/ml was chosen from the ROC curve data, based on optimization of sensitivity and specificity, providing a sensitivity of 75% and a specificity of 40%. Specifically in SLE patients with biopsy-proven nephritis, an ROC curve constructed for the previous visit’s uNGAL level and renal flare showed that uNGAL had much improved sensitivity and specificity with an AUC of 0.76 (Fig. 1). The ROC curves of C3 and C4 were similar to uNGAL’s ROC curve, whereas uNGAL significantly outperformed anti-dsDNA antibody titres (P = 0.004). A cut-off value for uNGAL of 13.6 ng/ml was chosen from the ROC curve data of patients with a history of biopsy-proven nephritis. This cut-off provides a sensitivity of 89% and a specificity of 72%.
Fig. 1.
ROC curve constructed for uNGAL and the development of renal flare in patients with a history of biopsy-proven LN. The curve depicts the specificity and sensitivity of a preceding visit’s uNGAL level for renal flare (defined as an increase of ≥4 points on the renal SLEDAI score). The AUC is 0.7639, significantly greater than the AUC from an ROC curve of anti-dsDNA titres (P = 0.004) and similar to the AUC from ROC curves of C3 and C4 levels.
Validation study in second population
There were 212 SLE patients recruited from the London cohort for a validation study. Of these, 35 patients had a baseline visit with at least one follow-up visit; 15 of these 35 patients had biopsy-proven LN. The London cohort had markedly different demographics from the original study population, with higher percentages of White and Asian patients, and a higher percentage of Class V LN (Table 1). While mean follow-up time was much shorter in the London cohort (7.1 vs 27 weeks), uNGAL levels in the former were similar to the original study population’s [25.5 (25.3) vs 17.3 (12.6) ng/ml].
Patients with significant renal disease activity (renal BILAG of A or B) were found to have higher SLICC scores for both overall damage [1.50 (1.79) vs 0.80 (1.20), P = 0.01] and renal-specific damage [0.67 (0.18) vs 0.12 (0.55), P = 0.0001] than those without renal disease activity (renal BILAG scores of C–E). A higher percentage of these patients had proteinuria on dipstick (61.1 vs 23.3%, P < 0.0001), as well as lower glomerular filtration rate (GFR)s [69.2 (36.2) vs 89.0 (21.5), P = 0.0001] (Table 5).
Table 5.
Clinical characteristics of patients in the London cohort
| Total SLE patients | SLE patients |
||
|---|---|---|---|
| Renal disease | No renal disease | ||
| Renal BILAG | 0.64 (1.19) | 3.50 (1.69)**** | 0.31 (0.46) |
| Non-renal BILAG | 3.48 (2.95) | 3.88 (3.58) | 3.44 (2.88) |
| Total BILAG | 4.12 (3.30) | 7.38 (4.11)**** | 3.75 (2.99) |
| Renal SLICC damage index | 0.18 (0.66) | 0.67 (0.18)*** | 0.12 (0.55) |
| Total SLICC damage index | 0.87 (1.29) | 1.50 (1.79)** | 0.80 (1.20) |
| Proteinuria percentagea, % | 38.6 | 61.1**** | 23.3 |
| Proteinuria (urine protein/creatinine ratio)b | 82.25 (88.98) | 100.59 (89.78) | 75.48 (88.71) |
| GFR | 86.9 (24.1) | 69.2 (36.2)*** | 89.0 (21.5) |
| C3a | 1.34 (4.74) | 1.02 (0.34) | 1.38 (5.02) |
| Anti-dsDNA antibody titres | 156.06 (359.85) | 78.83 (116.04) | 165.33 (377.82) |
| uNGAL (concurrent), ng/ml | 16.36 (25.08) | 22.40 (32.74) | 15.68 (24.08) |
Clinical and laboratory markers of lupus and LN for SLE patients during visits concurrent with and without renal disease for the cross-sectional London cohort (n = 212). BILAG scores were calculated with the formula A = 9, B = 3, C = 1, D/E = 0. BILAG parameters include indices for the following organ systems: neurological, musculoskeletal, cardio-respiratory, renal, vasculitis and haematological. Data were analysed using Student’s t-tests. All values reported are means with s.d. in parentheses, unless otherwise noted. aUrine dipstick ≥1 on a scale of 0–4, where trace or no protein was 0; baverage urine protein/creatinine ratio for those subjects who had positive dipstick protein (≥1). *P = 0.02; **P = 0.01; ***P = 0.0001; ****P < 0.0001.
For the panel of patients used in the validation study, significantly more patients with renal disease activity were found to have proteinuria by dipstick, but had lower anti-dsDNA antibody titres, than those without significant renal disease activity (Table 6). There was a non-significant trend in lower concurrent uNGAL levels in patients with renal disease activity, yet significantly higher levels of uNGAL in the preceding visit (Table 6). In a cross-sectional analysis, renal-specific SLICC damage index scores demonstrated a significant correlation with uNGAL levels (r = 0.40, P = 0.0003), whereas global SLICC scores showed no significant correlation with uNGAL (r = 0.12, P = 0.2). In a subgroup analysis of those 15 patients in the London longitudinal cohort with biopsy-proven nephritis, although concurrent uNGAL levels were similar in patients with and without renal disease activity, once again we found that the previous visit’s uNGAL levels were significantly higher (Table 6).
Table 6.
London longitudinal cohort
| Total SLE patients (n = 35) | All SLE patients (n = 35) |
SLE patients with biopsy-proven nephritis (n = 15) |
|||
|---|---|---|---|---|---|
| Visits with renal disease (13 visits; n = 11) | Visits with no renal disease (68 visits; n = 33) | Visits with renal disease (8 visits; n = 7) | Visits with no renal disease (23 visits; n = 11) | ||
| Renal BILAG | 1.00 (1.44) | 3.46 (1.66)**** | 0.44 (0.50) | 3.75 (2.12)**** | 0.739 (0.449) |
| Non-renal BILAG | 4.99 (3.81) | 4.85 (4.56) | 5.02 (3.67) | 5.75 (5.39) | 5.57 (4.85) |
| Total BILAG | 4.33 (4.31) | 8.31 (4.97)* | 5.46 (3.71) | 9.50 (5.83) | 6.30 (4.84) |
| Renal SLICC damage index | 0.19 (0.71) | 0.23 (0.83) | 0.18 (0.68) | 0.38 (1.06) | 0.48 (1.04) |
| Total SLICC damage index | 0.74 (1.30) | 0.77 (1.48) | 0.74 (1.28) | 1.25 (1.75) | 1.30 (1.55) |
| Proteinuria percentagea, % | 59.7 | 100*** | 50.9 | 100 | 91.3 |
| Proteinuria (urine protein/creatinine ratio)b | 95.61 (84.47) | 113.18 (87.02) | 85.95 (83.69) | 94.63 (59.56) | 104.81 (83.48) |
| GFR | 86.6 (25.1) | 84.6 (28.5) | 87.1 (24.5) | 77.9 (34.6) | 77.0 (34.0) |
| C3a | 0.947 (0.315) | 0.953 (0.282) | 0.945 (0.325) | 0.844 (0.252) | 0.840 (0.239) |
| Anti-dsDNA antibody titres | 192.26 (396.12) | 104.38 (146.54)**** | 213.04 (433.14) | 64.13 (59.83) | 258.82 (459.42) |
| uNGAL, ng/ml | |||||
| Concurrent | 16.92 (22.08) | 15.76 (19.97) | 17.20 (22.72) | 22.21 (23.37) | 25.06 (29.07) |
| Previous visit | 25.46 (25.30) | 46.55 (43.30)* | 21.09 (18.09) | 54.39 (43.41)**** | 25.81 (18.65) |
Clinical and laboratory markers of lupus and LN for SLE patients during visits concurrent with and without renal disease. Columns 3 and 4 indicate visits for the subgroup of patients with biopsy-proven LN (these patients are included in calculations for columns 1 and 2). BILAG scores were calculated with the formula A = 9, B = 3, C = 1, D/E = 0. BILAG parameters include indices for the following organ systems: neurological, musculoskeletal, cardio-respiratory, renal, vasculitis and haematological. Data were analysed using Student’s t-test. All values reported are means with s.d. in parentheses, unless otherwise noted. aUrine dipstick ≥1 on scale of 0–4, where trace or no protein was 0; baverage urine protein/creatinine ratio for those subjects who had positive dipstick protein (≥1). *P = 0.02; **P = 0.01; ***P = 0.001, ****P < 0.0001.
In a random effects model with the outcome of renal disease activity (BILAG score of A or B) and the predictor as the previous visit’s uNGAL level, uNGAL was found to be a significant predictor (P < 0.05) with an OR of 1.55 (1.01, 2.38) per s.d. increase in uNGAL (using the s.d. determined by the Bronx cohort data of 12.6 ng/ml) (Table 7). uNGAL was a significant predictor in a model for renal disease activity in LN patients (P = 0.006) with an OR of 9.84 (1.92, 50.34) per s.d. increase in uNGAL. In a multivariate model including the GFR, uNGAL continued to be a strong predictor of renal disease activity in LN patients with an OR of 9.22 (1.51, 56.20) (Table 7).
Table 7.
uNGAL as a predictor of renal disease activity in the following visit for SLE patients in the London validation cohort
| OR (95% CI) by s.d. (12.6 ng/ml) increase in uNGAL for SLE patients | OR (95% CI) by s.d. increase in uNGAL for patients with biopsy-proven nephritis | |
|---|---|---|
| Crude OR | 1.55 (1.01, 2.38) | 9.84 (1.92, 50.34) |
| Adjusted OR | 1.43 (0.62, 3.29)a | 9.22 (1.51, 56.20)b |
aAdjusted for previous visit’s GFR, history of biopsy-proven nephritis; badjusted for previous visit’s GFR.
The original cut-off point for uNGAL of 11.7 ng/ml in all lupus patients had a sensitivity of 67% in the London cohort (compared with 75% in the Bronx cohort) and a specificity of 38% (compared with 40%) in predicting renal disease activity. The cut-off point of 13.6 ng/ml determined from the Bronx data pertaining to patients with known LN was found to have a sensitivity of 80% in the London cohort (compared with 78% in the Bronx cohort) and a specificity of 44% (compared with 69%).
Discussion
We show in this study that uNGAL was a significant predictor of renal disease activity in all SLE patients, and of renal flares in patients with a history of biopsy-proven nephritis. These findings persisted when accounting for important confounders and were reproducible in a second patient population using a different disease activity index. uNGAL had high sensitivity and moderate specificity for predicting future renal flare in patients with a history of LN, outperforming anti-dsDNA antibody titres in our study. An important clinical conclusion is that adding measurement of uNGAL to the routine follow-up of LN patients, particularly those with biopsy-proven disease, may result in earlier diagnosis of a renal flare, and therefore less delay in institution of appropriate treatment. Thus, our results are exciting and important as they suggest a novel approach to the clinical management of lupus patients.
We could not demonstrate a significant difference in concurrent uNGAL levels between patients exhibiting active renal disease and those who did not. One possible explanation is that uNGAL levels peak before flares are manifest, and then are already receding by the time the flare can be detected. Alternatively, it is possible that higher levels of uNGAL may exist in patients who have more accumulated renal damage, explaining why patients with a history of biopsy-proven nephritis, regardless of disease activity, have higher uNGAL levels than other SLE patients [18]. Therefore, as a marker of concurrent flare in cross-sectional data, uNGAL may be confounded by an association with accumulated damage. This hypothesis is further supported by our findings that uNGAL levels correlated significantly with SLICC renal damage indices.
Our finding that preceding levels of uNGAL are higher in patients before a flare is at odds with a recent report by Suzuki et al. [19] in a cohort of paediatric SLE patients with overall low levels of fixed renal damage. In this study, patients with childhood-onset SLE had major increases in the uNGAL levels during a visit at the time of a flare relative to uNGAL levels 3 months earlier. One possible explanation for this difference is that in our study with a relatively long follow-up time in between visits, we are detecting flares by increasing SLEDAI scores after the peak of disease activity. However, it must be noted that in the London cohort we had similar findings with a shorter follow-up time. Another possibility is that there are differences in the behaviour of uNGAL between paediatric and adult SLE.
One of the strengths of our study was the examination of uNGAL’s ability to predict renal disease activity using two widely used scoring systems, SLEDAI and BILAG. Notably, using the BILAG scoring system, the effect of higher uNGAL levels on renal disease activity was found to be even greater than in the original study (OR 9.8 vs 4.5 for patients with LN). One may assume that the SLEDAI score may not be sensitive to every renal flare in patients who already manifest baseline indicators of nephritis. For instance, patients who have a renal flare manifested by an increase in urinary protein excretion from already significant baseline levels of proteinuria, will not be picked up by SLEDAI, which has already awarded the maximal score possible for proteinuria. In contrast, BILAG has the advantage of reflecting increases in disease activity based on a clinician’s intention to treat. Thus BILAG is more specific, but does have the potential to be more variable between clinicians. Nevertheless, the BILAG has been validated as an accurate and reliable marker of lupus disease activity, specifically of lupus flares [23].
SLEDAI and BILAG aim to capture current levels of disease activity, but are not intended to predict flares. While these measures of disease activity are in widespread use and have surely improved the follow-up of lupus patients, current monitoring programmes are far from optimal. Indeed, this is the justification for the continued search for non-invasive biomarkers and for the current study. While urinalysis is easily performed and may very well be the best concurrent non-invasive indicator of a renal flare, patients with active nephritis on biopsy may have normal urinalyses or the abnormalities may appear relatively late [24, 25]. Therefore, an important goal would be to demonstrate a change in biomarker level before the diagnosis of flare by urinalysis (or repeat kidney biopsy), as we have shown here for uNGAL.
The increase in effect size of uNGAL seen in the validation cohort may be a function of the higher sensitivity of the BILAG scoring system, but may also be due to the shorter follow-up time in the validation cohort. Detectable increases in uNGAL may not occur as early as 6 months before a flare, and thus we would expect uNGAL to be a more informative biomarker in the shorter follow-up time of the validation cohort. Nevertheless, we were able to conclusively demonstrate that high uNGAL levels were present in LN patients even months before a flare of renal, but not non-renal, lupus activity. In fact, if confirmed this may make the use of NGAL as a biomarker for LN particularly valuable, since early treatment may prevent irreversible renal damage. At the least, even if physicians do not elect to start treatment prophylactically based upon a single laboratory value, knowing which patients need to be followed more closely, since they are likely to flare, would clinically be very beneficial [26].
How does the performance of uNGAL compare with that of other recently described urinary biomarker candidates [27]? In investigating a candidate lupus biomarker for predicting renal flares, the first crucial step we focused on was to compare uNGAL performance with accepted measurements in common use such as anti-DNA titres—which it significantly outperformed. The question of the relative performance of uNGAL as compared with other new biomarkers has to be addressed in a side-to-side comparison. Indeed, we will also need to determine if any given combination of standard and novel urinary biomarkers (of which there are several possible candidates) would have enhanced diagnostic or predictive capability. These questions are important and not trivial, and therefore we plan to address them separately in the near future.
Although we did not find a difference in uNGAL levels between different types of LN, this study was not powered to answer this question conclusively. Since different classes of LN, most notably WHO Class V LN, respond differently to treatment and may diverge in their pathogenesis, it will be interesting to see whether uNGAL is more or less predictive of disease in different classes of nephritis in a larger cohort of biopsied patients. Interestingly, in paediatric lupus higher levels of uNGAL were seen in patients with Class IV nephritis relative to patients with Class V nephritis [19].
Is uNGAL abnormally elevated in LN patients with a combination of membranous and proliferative disease (i.e. III/IV and IV/V)? Although we would have liked to have analysed patients with such mixed histopathological patterns, despite including all eligible patients, unfortunately none of the patients undergoing a biopsy displayed these particular nephritis classes in combination. However, the fact that we did not find significant differences in uNGAL levels between patients with membranous vs proliferative disease, and the similar uNGAL levels present in the Bronx and London cohorts despite a higher percentage of patients with Class V in the latter patient group, suggest that uNGAL is similarly elevated in both proliferative and membranous subtypes of LN (and likely in their combination as well). Based upon the available data, the practical recommendation at this time would therefore be not to limit uNGAL monitoring to patients with pure proliferative renal disease.
In addition to the relatively small number of biopsy-proven LN patients included in this study, particularly in the validation cohort, there are some other limitations to this study. One was the lack of uniformity in patient follow-up, with a relatively wide range of follow-up times in our original patient population (2 weeks to 2 years). Although it is difficult to predict how this may have affected our results, it is probable that more active patients had shorter follow-up times and more visits. The random effects model that we constructed took into account multiple observations per subject; however, our ROC curves did not. Therefore, our ROC analysis should be viewed as a descriptive analysis. The specific cut-offs have meaning only in that we were able to use them to show that our second cohort validated our findings that uNGAL is a sensitive marker for predicting flare in patients with biopsy-proven LN.
Another potential limitation to our study was the lack of medication data for the Bronx cohort. However, while in the original study of NGAL and childhood lupus by Brunner et al. [4], a correlation between immunosuppressant medication and higher uNGAL was observed, no such correlation was seen in a more recent study [19]. Furthermore, there are no data at this time to suggest that particular medications specifically modulate uNGAL production directly. Although we did find in the current study that higher uNGAL levels correlated with the use of rituximab, AZA and mycophenolate mofetil, we believe that this correlation is most likely confounded by higher levels of renal disease activity in patients on these medications rather than a direct effect on uNGAL levels. In patients followed prospectively, a decrease in uNGAL levels in LN patients responding to treatment with improved renal function while continuing treatment with these drugs, would support this particular interpretation.
An interesting issue not addressed here directly is: what is the cellular source(s) of NGAL appearing in the urine in LN? Although for uNGAL to be useful as a biomarker it is not essential for it to be produced de novo by renal cells; which particular cell type is responsible for the production of uNGAL as a consequence of renal injury is an important question, and in fact differs based upon the aetiology of kidney damage [28, 29]. Additional basic laboratory studies to further define the kidney component responsible for uNGAL production in LN may provide further mechanistic insight, but are likely to be complex and beyond the scope of the present study. Nevertheless, our previous observations that: (i) pathogenic anti-DNA antibodies up-regulate NGAL expression and secretion in mesangial cells in vitro [30]; and (ii) there was no correlation between serum and urine NGAL in adult LN [18], strongly support our contention that the kidney itself is an important source of uNGAL in LN.
While uNGAL performed similarly to C3 and C4 in predicting flares and renal disease activity in LN patients, it outperforms a commonly used biomarker, anti-dsDNA antibody titres. In particular, our data suggest that regularly following uNGAL levels can be valuable to monitor for the upcoming flares of renal disease, particularly in patients with known LN. Based on our significant results reported above, it is clearly important to further study the possible clinical use of uNGAL in adult lupus patients in a prospective format. While no results are yet available, we have embarked on such a prospective trial. Moreover, in the future, it is likely that to best follow patients with lupus and LN, a panel of biomarkers (including uNGAL and others yet to be defined) may have the best diagnostic accuracy. Such a panel of diagnostic markers that can reliably predict LN flares or precede the development of renal disease in SLE patients may lead to earlier and more aggressive treatment of patients, in the hope of preventing or at least postponing renal damage. Rigorously executed clinical trials of the use of uNGAL and other putative biomarkers and any possible effect on patient outcomes are needed, and may help us address this clinically important question.
Acknowledgements
We acknowledge the excellent help of Emma Ross in the UCL tissue repository centre and Sharon Chambers in collection of damage scores, and thank our patients for their participation.
Funding: This publication was made possible by the CTSA Grants UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Centre for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its content is solely the responsibility of the authors and does not necessarily represent the official view of the NCRR or NIH. This research was also supported by an NIH grant AR48692 (to C.P.). T.R. and N.S. were supported by Medical Student Research Preceptorship Awards from the American College of Rheumatology Research and Education Foundation and grants from the New York Chapter of the Arthritis Foundation. J.M.P.-R. was supported by grants from the Instituto de Salud Carlos III (Spanish Ministry of Health and Consume) and the Fundación Española de Reumatología (Spanish Society of Rheumatology).
Disclosure statement: D.I. has consulted for Roche, Merck-Serono, Teva, Bristol Myers Squibb and Celltech. He does not accept personal remuneration from these companies and asks that the honoraria are paid to a local arthritis charity. All other authors have declared no conflicts of interest.
References
- 1.Wallace D. The clinical presentation of systemic lupus erythematosus. In: Wallace DJ, Hahn BH, editors. Dubois lupus erythematosus. Baltimore: Williams and Wilkins; 1996. p. 627. [Google Scholar]
- 2.Fiehn C. Early diagnosis and treatment in lupus nephritis: how we can influence the risk for terminal renal failure. J Rheumatol. 2006;33:1464–6. [PubMed] [Google Scholar]
- 3.Fiehn C, Hajjar Y, Mueller K, Waldherr R, Ho AD, Andrassy K. Improved clinical outcome of lupus nephritis during the past decade: importance of early diagnosis and treatment. Ann Rheum Dis. 2003;62:435–9. doi: 10.1136/ard.62.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner HI, Mueller M, Rutherford C, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54:2577–84. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 5.Liu CC, Manzi S, Ahearn JM. Biomarkers for systemic lupus erythematosus: a review and perspective. Curr Opin Rheumatol. 2005;17:543–9. doi: 10.1097/01.bor.0000174182.70159.22. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein T, Pitashny M, Putterman C. The novel role of neutrophil gelatinase-B associated lipocalin (NGAL)/lipocalin-2 as a biomarker for lupus nephritis. Autoimmun Rev. 2008;7:229–34. doi: 10.1016/j.autrev.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Moroni G, Pasquali S, Quaglini S, et al. Clinical and prognostic value of serial renal biopsies in lupus nephritis. Am J Kidney Dis. 1999;34:530–9. doi: 10.1016/s0272-6386(99)70082-x. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj S, Albert L, Gladman DD, Urowitz MB, Hallett DC, Ritchie S. Serial renal biopsy in systemic lupus erythematosus. J Rheumatol. 2000;27:2822–6. [PubMed] [Google Scholar]
- 9.Schwartz N, Michaelson JS, Putterman C. Lipocalin-2, TWEAK, and other cytokines as urinary biomarkers for lupus nephritis. Ann NY Acad Sci. 2007;1109:265–74. doi: 10.1196/annals.1398.032. [DOI] [PubMed] [Google Scholar]
- 10.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–24. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 11.Trachtman H, Christen E, Cnaan A, et al. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol. 2006;21:989–94. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 12.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–15. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 13.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–73. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malyszko J, Malyszko JS, Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S, Mysliwiec M. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc. 2009;41:158–61. doi: 10.1016/j.transproceed.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 15.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL) J Biol Chem. 2001;276:37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 16.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Berger T, Togawa A, Duncan GS, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–9. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitashny M, Schwartz N, Qing X, et al. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum. 2007;56:1894–903. doi: 10.1002/art.22594. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Wiers KM, Klein-Gitelman MS, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol. 2008;23:403–12. doi: 10.1007/s00467-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 20.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 21.Yee CS, Farewell V, Isenberg DA, et al. British Isles Lupus Assessment Group 2004 index is valid for assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 2007;56:4113–9. doi: 10.1002/art.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoll T, Stucki G, Malik J, Pyke S, Isenberg DA. Further validation of the BILAG disease activity index in patients with systemic lupus erythematosus. Ann Rheum Dis. 1996;55:756–60. doi: 10.1136/ard.55.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon C, Sutcliffe N, Skan J, Stoll T, Isenberg DA. Definition and treatment of lupus flares measured by the BILAG index. Rheumatology. 2003;42:1372–9. doi: 10.1093/rheumatology/keg382. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Crespo MR, Lopez-Fernandez JI, Usera G, Poveda MJ, Gomez-Reino JJ. Outcome of silent lupus nephritis. Semin Arthritis Rheum. 1996;26:468–76. doi: 10.1016/s0049-0172(96)80027-8. [DOI] [PubMed] [Google Scholar]
- 25.Zabaleta-Lanz ME, Munoz LE, Tapanes FJ, et al. Further description of early clinically silent lupus nephritis. Lupus. 2006;15:845–51. doi: 10.1177/0961203306070002. [DOI] [PubMed] [Google Scholar]
- 26.Hinze CH, Suzuki M, Klein-Gitelman M, et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 2009;60:2772–81. doi: 10.1002/art.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovin BH, Zhang X. Biomarkers for lupus nephritis: the quest continues. Clin J Am Soc Nephrol. 2009;4:1858–65. doi: 10.2215/CJN.03530509. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Amer Soc Nephrol. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 29.Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–94. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 30.Qing X, Zavadil J, Crosby MB, et al. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006;54:2198–210. doi: 10.1002/art.21934. [DOI] [PubMed] [Google Scholar]



