Abstract
Background
HIV infection, malnutrition and invasive bacterial infections (IBI) are reported among children with severe malaria. However, it is unclear whether their co-occurrence with falciparum parasitization and severe disease is by chance, or by association among children in malaria endemic areas.
Methods
We examined 3,068 consecutive paediatric admissions to a Kenyan district hospital with clinical features of severe malaria, and 592 community controls. We performed multivariable regression analysis with each case weighted for their probability of being due to falciparum malaria using estimates of the fraction of severe disease attributable to malaria at different parasite densities derived from cross sectional parasitological surveys from well children in the same community.
Results
HIV infection was present in 133/1071 (12%, 95%CI 11 to 15%) consecutive parasitemic admissions. Parasite densities were higher in HIV infected children. The odds of admission associated with HIV infection for admission with true severe falciparum malaria were 9.6 (95%CI 4.9 to 19), however this effect was restricted to children age ≥1 year. Malnutrition was present in 507/2,048 (25%, 95%CI 23 to 27%) consecutive parasitemic admissions. The odds associated with malnutrition for admission with true severe falciparum malaria were 4.0 (95%CI 2.9 to 5.5). IBI was detected in 127/2,048 (6.2%, 95%CI 5.2 to 7.3%) of consecutive parasitemic admissions. All three comorbidities were associated with increased case fatality.
Conclusions
HIV, malnutrition and IBI are biologically associated with severe disease due to falciparum malaria rather than being simply alternative diagnoses in co-incidentally parasitized children in an endemic area.
Keywords: Malaria, HIV, Malnutrition, Meningitis, Bacteremia, Comorbidity
INTRODUCTION
Falciparum malaria is a common cause of severe illness among children in sub-Saharan Africa.[1] HIV infection, malnutrition and invasive bacterial infections (IBI) are reported among children with severe malaria.[2-16] However, it is unclear whether they are actually associated with severe malaria among children living in malaria endemic areas.
The clinical signs of severe malaria have been carefully defined, but even when they are supported by finding Plasmodium falciparum parasitemia, not all severe disease is due to malaria. For example, in Malawi, another cause of death was found at post mortem in 23% of children fulfilling WHO criteria for cerebral malaria prior to death.[16] In Kenya, 9% of children fulfilling the same definition had encephalopathic viruses detected in their cerebrospinal fluid,[17] and among all parasitemic children admitted, 26% of inpatient deaths were accompanied by bacteremia.[3] One explanation is that, in endemic areas, asymptomatic malaria parasites are observed among children presenting with non-malarial conditions. The alternative explanation is that severe malaria is associated with other conditions, either biologically or through shared risk factors.
Studies of severe malaria usually exclude parasitemic children with clinical evidence of other illnesses, such as malnutrition, meningitis or pneumonia. However, this approach assumes that parasitemia is co-incidental and excludes the possibility of the actual co-existence of severe malaria with other diseases.
There is no ‘gold standard’ to determine in an individual child that severe disease is truly due to malaria in an endemic area. However, the likelihood of illness being due to malaria is related to parasite density. In studies of uncomplicated malaria, a method for determining the fraction of fevers attributable to malaria using cross-sectional parasite density data from well children in the community was derived by Smith et.al.[18] We recently applied this method to severe malaria for defining endpoints for intervention trials.[19] However, this does not address whether comorbidities may be biologically associated with severe malaria.
Here, we present findings on HIV, malnutrition and invasive bacterial infection (IBI) among 3,068 consecutive pediatric admissions with clinical features compatible with severe malaria and 592 community controls. We included cases with additional evidence of other illnesses. We then estimated the probability that each case was due to true falciparum malaria using estimates of the fraction of severe disease attributable to malaria at different parasite densities derived from multiple cross sectional parasitological surveys among well children in the same community.[18]
METHODS
Location
The Kenya Medical Research Institute (KEMRI) Centre for Geographic Medicine Research (Coast) is located at Kilifi District Hospital, Kenya. The hospital serves ~240,000 people in a malaria-endemic area (<1 to 120 mosquito bites infective for Plasmodium falciparum each year).[20] The prevalence of HIV infection at the hospital antenatal clinic in 2000 was 9.8 %.[21] No dedicated services providing care for HIV were in place at the time of the study. Prophylactic cotrimoxazole was not in use. The study was approved by the Kenyan National Scientific and Ethical Review Committees.
Clinical and laboratory methods
Research clinicians managed patients and collected standardized clinical and laboratory data on all pediatric admissions between August 1998 and July 2002.[3] We defined clinical features compatible with severe malaria as a history of fever or axillary temperature ≥ 37.5°C plus one or more of: impaired consciousness (inability to localise a painful stimulus if aged >8 months, or without directed eye movements if age ≤8 months), respiratory distress (deep breathing) or severe anemia (hemoglobin <50g/l).[22]
Weight for age z-scores (WAZ) were calculated using NCHS reference data (EpiInfo 6.04b. CDC, Atlanta, USA). Kwashiorkor was defined by the presence of bipedal edema and characteristic skin and hair changes. Malnutrition was defined as severe underweight (WAZ<−3) or kwashiorkor.
Thick and thin blood smears were stained with Giemsa and examined at x1000 for asexual forms of Plasmodium falciparum. Results expressed per μl using individual red and white blood cell counts (Beckman/Coulter, UK). Two further slides were performed at 4-6 hourly intervals if the first slide was negative.
Blood was aerobically cultured for pathogenic bacteria (BACTEC, Becton-Dickinson, New Jersey, USA).[3] Lumbar punctures were conducted according to a clinical protocol.[23] Bacterial meningitis was defined as a positive cerebrospinal fluid (CSF) culture, positive CSF latex agglutination test, bacteria seen on CSF Gram stain or CSF leukocyte count >50 cells/μl.[23] The leukocyte criterion was included because we gave intravenous antibiotics and delayed lumbar puncture among children with deep coma or focal neurological signs. IBI was defined as bacteremia or bacterial meningitis.
HIV infection status was determined for all admissions from October 1999 by ELISA using anonymized samples at the end of the study. PCR was done for children age <18 months (Ampliclor®, Roche). Results that include HIV are limited to this systematically collected subset.
Clinical management
Children with impaired consciousness or deep breathing were normally admitted to the high dependency unit (HDU) and treated with intravenous quinine until three blood slides were confirmed negative or, for a positive slide, until clinical and parasitological recovery, when a single dose of oral sulphadoxine-pyrimethamine (recommended first line treatment at that time) was given. Children in the HDU were treated with intravenous penicillin and chloramphenicol until CSF and/or blood culture results were known.
Children with severe anemia, without impaired consciousness or respiratory distress, were normally treated on the paediatric ward. Blood transfusion, antibiotics, management of severe malnutrition and other treatments were given according to current WHO recommendations.[24] Malaria was treated with sulphadoxine-pyrimethamine.
Community data
Between 1997 and 2004, seven cross sectional surveys were conducted in the wet and dry seasons at three locations in the catchment area of the hospital representing low, medium and high malaria transmission.[25] In these surveys a total of 11,823 parasite density measurements were obtained from 2,397 afebrile, well children.[19] Data on HIV and malnutrition in the community were from 592 healthy children age ≥60 days old, age, sex, season and location matched to hospital admissions for a concurrent case-control study of childhood bacteremia.
Analysis
We included all children age ≥60 days with signs compatible with severe malaria at admission to hospital, except those due to accidents. We excluded data from children with missing or contaminated blood cultures. Two-sided Fisher's exact test, Chi squared test, Chi-squared test for trend and multivariable logistic regression were used to examine categorical data. Multivariable linear regression was used to examine parasite density. The Kruskall-Wallis test was used to compare distributions of age, since these were skewed.
To estimate the probability that each case was due to falciparum malaria, we used estimates of the fraction of severe disease attributable to malaria calculated using a logistic method[18] from parasite density data among admissions with severe disease and community-based surveys of afebrile children, adjusted for age, season and location as previously described.[19] Malaria slide negative admissions were assumed to have zero probability of being due to malaria. We estimated the odds of admission with true severe malaria versus community controls using multiple logistic regression models where each case was weighted for their probability of being true severe malaria and adjusted for age by specifying ‘probability weights’ in STATA 9.0 (Stata Corp., Texas, USA), which has robust variance estimation for this regression technique.
RESULTS
Clinical signs compatible with severe malaria were present in 3,362/17,301 (19%) consecutive admissions. We excluded 294 (9%) admissions with contaminated (n=278) or missing (n=16) blood cultures, and analyzed 3,068 admissions. Parasitemia was detected in 2,048 (67%): 1,986 on the first slide, 35 on the second and 27 on the third. The age of parasitemic admissions (median 22 months (IQR 11 to 40)) was similar to non-parasitemic admissions (median 19 months (IQR 9 to 41)) and 592 community controls (median 23 months (IQR 12 to 41)).
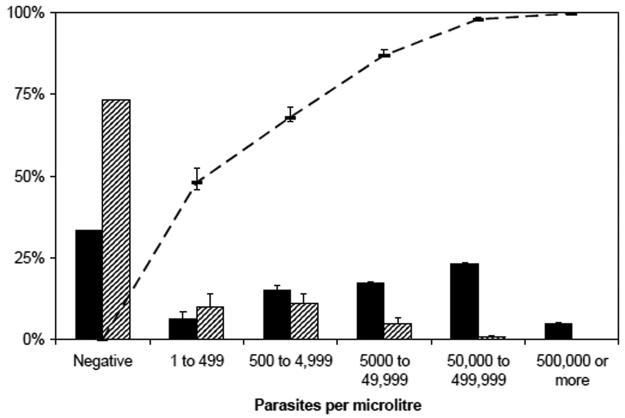
In community surveys, 3,142/11,823 (26%) malaria slides were positive. A parasite density of ≥50,000/μl occurred in 100 (<1%) community slides and in 711 (23%) admissions. The overall fractions of severe disease and death attributable to malaria were 85% (95%CI 84 to 86%) and 76% (95%CI 71 to 80%) respectively among admissions with a positive malaria slide. The fractions of severe disease attributable to malaria are shown in figure 1. At parasite densities of ≥50,000/μl, 98% (96% CI 97 to 99%) of severe disease and 95% (95%CI 92 to 97%) of deaths were attributable to malaria. Parasite densities of ≥500,000/μl did not occur in the community and therefore all disease was considered attributable to malaria.
Figure 1. Distribution of Plasmodium falciparum parasite density among 3,068 admissions with signs of severe malaria and 11,823 cross sectional community samples, and the calculated fraction of severe disease attributable to malaria.
Solid bars: admissions. Striped bars: community surveys. Line: calculated fraction of severe disease attributable to malaria (weighted by age and location) with 95%CI.
HIV infection
HIV status was determined in 1,755/1,914 (92%) consecutive admissions from October 1999. Those not tested during this period were more likely to have died: 19% vs. 12% (P=0.01). HIV infection was present in 133/1,071 (12%, 95%CI 10 to 15%) parasitemic vs. 119/684 (17%, 95%CI 15 to 21%) non-parasitemic admissions (P=0.004), and in 10/592 (1.7%, 95%CI 0.8% to 3.1%) community controls.
Among non-parasitemic admissions with HIV infection, there was a bimodal age distribution with peaks at 6 and 24 months. Among parasitemic admissions, the distribution was unimodal and those with HIV infection were older (median 38 months (IQR 26 to 63)) than HIV-uninfected admissions (median 19 months (IQR 10 to 35), P<0.001). HIV prevalence varied with age among parasitemic admissions: below 1 year of age, 1.8% (95%CI 0.02 to 3.2%), above one year of age, 16% (95%CI 14 to 19%).
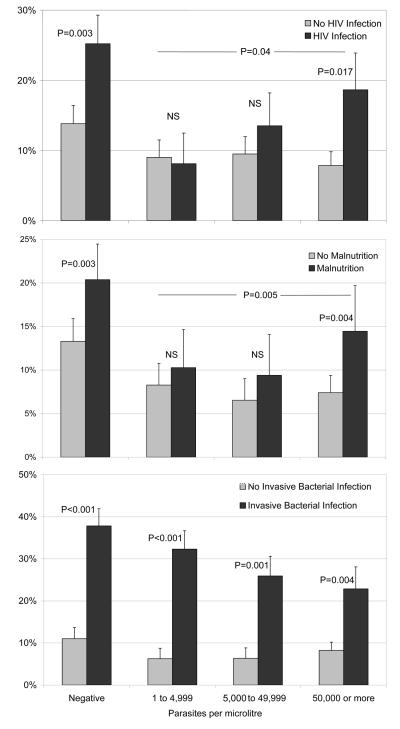
Parasite density was higher among HIV infected (median 37,500/μl (IQR 2,680 to 172,150) than HIV uninfected admissions (median 22,352/μl (IQR 2,213 to 142,590), P=0.02 by age-adjusted linear regression). HIV infected children were more likely to die (figure 2).
Figure 2. Case fatality with and without HIV infection, malnutrition and invasive bacterial infection among admissions with signs of severe malaria, by Plasmodium falciparum parasite density.
P values refer to age-adjusted odds ratios for death (see text).
The overall age adjusted odds ratio for admission with severe disease associated with HIV infection, weighted for the probability of being a true malaria case, was 9.6 (95%CI 4.9 to 19). An interaction term for age below 1 year was statistically significant (P=0.021). The stratified odds ratios were 1.4 (95%CI 0.3 to 7.4) under 1 year of age and 12 (95%CI 5.7 to 25) above 1 year. The estimated weighted odds of fatal true malaria admission associated with HIV infection were 15 (95%CI 6.5 to 33).
Malnutrition
Malnutrition was present in 507/2,048 (25%, 95%CI 23 to 27%) parasitemic, 373/1,020 (37%, 95%CI 34 to 40%) non-parasitemic admissions (P<0.001) and 44/592 (7.4%, 95%CI 5.4 to 9.8%) community controls. Among parasitemic admissions, those with malnutrition were slightly older (median 24 months (IQR 13 to 38)) than those without (median 21 months (IQR 10 to 40), P=0.004).
Parasite density was lower among malnourished admissions (median 13,619/μl (IQR 1,603 to 98,650) than non-malnourished admissions (median 27,330/μl (IQR 2,570 to 176,685), P<0.001 on age-adjusted linear regression). However, among admissions with ≥50,000 parasites/μl, 20% (95%CI 17 to 23%) were malnourished. Malnutrition was associated with increased case fatality at all parasite densities (figure 2).
The age adjusted odds ratio for malnutrition of admission with severe disease, weighted for the probability of being a true malaria case, was 4.0 (95%CI 2.9 to 5.5). There was no evidence that this relationship was influenced by age. When tested together, there was no significant interaction (P=0.77) between the effects of HIV infection and malnutrition on the odds of admission with severe malaria. The estimated weighted odds of fatal true malaria admission associated with malnutrition were 6.5 (95%CI 4.2 to 10).
Invasive Bacterial Infection
IBI was detected in 127/2,048 (6.2%, 95%CI 5.2 to 7.3%) parasitemic and 185/1,020 (18%, 95%CI 16 to 21%) non-parasitemic admissions (P<.001). IBI was not determined among community controls. Among admissions with ≥50,000 and ≥500,000 parasites/μl, IBI was detected in 4.0% (95%CI 2.7 to 5.3%) and 4.7% (95%CI 1.2 to 8.0%) respectively.
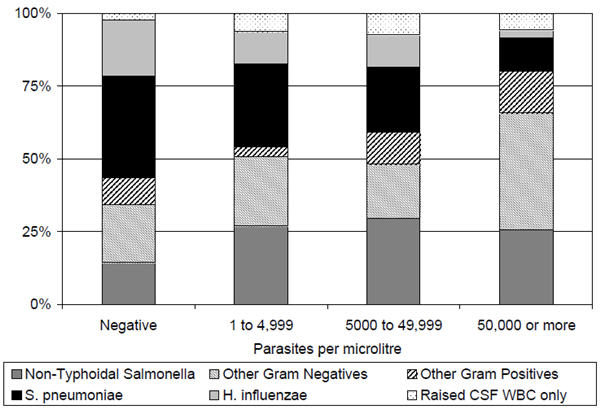
Streptococcus pneumoniae and Haemophilus influenzae comprised the majority of isolates in non-parasitemic admissions (figure 3), but lower proportions among those with malaria parasitemia (both P=0.02). Non-Typhoidal Salmonellae comprised a greater proportion (P=0.005) of isolates among parasitemic admissions. At ≥50,000 parasites/μl, Gram negative isolates other than Non-Typhoidal Salmonella or H. influenzae (principally Acinetobacter sp., E. coli and P. aeruginosa) were common.
Figure 3. Bacterial isolates among 312 admissions with signs of severe malaria and invasive bacterial infection, by Plasmodium falciparum parasite density.
There was no evidence of an association between IBI and age. Sixty two percent of parasitaemic admissions with bacteremia had neither HIV nor malnutrition; 22% had malnutrition only, 4% had HIV only; and 12% had both HIV and malnutrition.
IBI was strongly associated with death (Figure 2). However, case fatality with IBI declined with increasing parasite density (Chi squared for trend; P=0.05). Among those with ≥50,000 parasites/μl, the case fatality of admissions with Gram negative organisms other than Non-Typhoidal Salmonella or H. influenzae was 4/14 (29%) compared to 72/847 (8.5%) among those without IBI (P=0.05), suggesting that these were not simply contaminants.
DISCUSSION
Among children with severe malaria, comorbidities were common, frequently fatal and appeared to be biologically associated with true severe malaria disease, rather than alternative causes of disease in asymptomatically parasitized children.
An association between HIV infection and malaria is established in adults [13, 26] but not among children in malaria endemic areas. Cohort studies in Zaire during the 1980's found no association between HIV infection and malaria in young children.[27, 28] In South Africa, in an area of unstable transmission and consequently a high fraction of parasitemic disease attributable to malaria, HIV was associated with severe malaria, but not with parasite density.[12] The study was too small to identify an association with mortality. More recently in Malawi, HIV infection was reported in 16% of children admitted with clinically defined severe malaria and there was no association with death.[5] This prevalence appears higher than expected among children in the community, but no formal comparison was made.
In a malaria endemic area, we found that HIV infection was associated with admission with true severe malaria among children age above one year. This, and higher parasite density suggest a failure of acquired immunity, as has been proposed among adults.[26] We found no evidence that HIV was associated with increased risk of malaria in infants, who have not yet acquired natural immunity to severe malaria. We may have underestimated the effects of HIV since deaths were over-represented among those not tested. An odds ratio of nearly 10 represents a profound effect of untreated HIV on severe malaria. HIV diagnosis is important in this context, because of the effectiveness of cotrimoxazole prophylaxis in preventing malaria.[29]
Protein-energy malnutrition is associated with many life-threatening infections. However, early reports of effects on malaria suggested it might be protective.[10, 30, 31] More recent studies suggest that malnutrition is a susceptibility factor.[6, 7] We found malnutrition was far commoner among true severe malaria cases than in the community. Some weight loss may occur with acute illness, but we believe this is unlikely to account for our observations.[32] An odds of 6.5 for fatal admission, concords with estimates modelled from pooled data by Caulfield et. al.[6] Our findings strongly support the view that improving nutrition is likely to reduce malaria deaths.
There are several reports of IBI among children with severe malaria, especially due to Salmonella species.[2, 8, 9, 11, 15, 33] Some report no association between invasive IBI and mortality among children with severe malaria, suggesting IBI may be benign in this context. [5, 8] We found no evidence that severe malaria was associated with Streptococcus pneumoniae and Haemophilus influenzae. Rather, non-Typhoidal Salmonellae and other Gram negative organisms occurred more commonly than expected, and were associated with fatality. This is in contrast to the findings relating to mortality from Malawi and Gambia, [5, 8] and is likely to reflect our different approach of not excluding children with evidence of other infections from being classified as cases of severe malaria.
We did not collect data on bacteremia among healthy children in the community. However, among children attending the outpatient department at our hospital, we previously found a prevalence of bacteremia of 2%,[34] and less than 1% amongst those without fever (A Brent, personal communication). We therefore expect the prevalence of bacteremia among healthy children in the community to be <1%, considerably lower than we found amongst children with severe malaria and high parasite density, where almost all disease if due to malaria.
Most IBI among children with severe malaria was not in the context of malnutrition or HIV. It is conceivable that IBI might result in loss of immunological control of an asymptomatic parasitemia. However, we believe that IBI is more likely to have occurred as a direct consequence of severe malaria. As well as immunoparesis,[35, 36] sequestration in the microvasculature of the gut[37, 38] and at other barriers may permit bacterial invasion. If so, some IBIs might be prevented by interventions that prevent severe malaria.
Could these apparent associations be simply reflect bias towards admission to hospital of children who have both parasitemia and co-morbidity? At health centers in our area, blood film microscopy was rarely available. Furthermore, evidence from East Africa suggests that microscopy has limited influence on practice.[39, 40] Once at hospital, we believe that parasitemia was unlikely to influence admission because the children were severely ill, clearly requiring admission. There was no HIV testing of children and little voluntary counseling and testing of adults at the time of the study. The presence of IBI would not have been known to parents or referring health workers. We therefore think that for HIV infection and IBI, bias is unlikely. Malnutrition is visible to parents and health workers, and could therefore have influenced presentation to hospital in either direction. Our experience is that parents seek care because their malnourished children are sick, rather than for malnutrition per se. However, it is impossible to exclude significant bias in relation to malnutrition.
It is possible that the age and location weighted attributable fraction calculation did not accurately represent admissions. However, associations were strong at parasite densities ≥50,000/μl where almost all severe disease was attributable to malaria, reflecting the fact that these parasite densities very rarely occurred at any age or location in the community surveys, but were common among admissions.
By considering the fraction of severe disease attributable to malaria, we found that HIV infection (above one year of age), malnutrition and IBI occur more commonly among hospitalized children with true severe malaria than expected by chance. We conclude that these important comorbidities are true biological associations of severe malaria, rather than simply alternative reasons for admission in asymptomatically parasitized children.
Acknowledgments
We thank the District Medical Officer of Health, the Director of the Centre and the staff of Kilifi District Hospital, and for their support. We are grateful to all the KEMRI/Wellcome Trust clinical, laboratory and computing staff for assistance with collecting the data. JAB, PB, CN, TW, KeM, FO and ME are financially supported by the Wellcome Trust (UK). This paper is published with the permission of the director of the Kenya Medical Research Institute.
Footnotes
Conflict of interest statement
All authors declare no conflict of interest. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Berkley J, Mwarumba S, Bramham K, et al. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93(3):283–6. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- 3.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352(1):39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 4.Berkley JA, Mwangi I, Mellington F, et al. Cerebral malaria versus bacterial meningitis in children with impaired consciousness. Qjm. 1999;92(3):151–7. doi: 10.1093/qjmed/92.3.151. [DOI] [PubMed] [Google Scholar]
- 5.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian Children with Severe Malaria: Prevalence, Etiology, HIV Coinfection, and Outcome. J Infect Dis. 2007;195(6):895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 6.Caulfield LE, Richard SA, Black RE. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. Am J Trop Med Hyg. 2004;71(2 Suppl):55–63. [PubMed] [Google Scholar]
- 7.Deen JL, Walraven GE, von Seidlein L. Increased risk for malaria in chronically malnourished children under 5 years of age in rural Gambia. J Trop Pediatr. 2002;48(2):78–83. doi: 10.1093/tropej/48.2.78. [DOI] [PubMed] [Google Scholar]
- 8.Enwere G, Van Hensbroek MB, Adegbola R, et al. Bacteraemia in cerebral malaria. Ann Trop Paediatr. 1998;18(4):275–8. doi: 10.1080/02724936.1998.11747959. [DOI] [PubMed] [Google Scholar]
- 9.Evans JA, Adusei A, Timmann C, et al. High mortality of infant bacteraemia clinically indistinguishable from severe malaria. Qjm. 2004;97(9):591–7. doi: 10.1093/qjmed/hch093. [DOI] [PubMed] [Google Scholar]
- 10.Genton B, Al-Yaman F, Ginny M, et al. Relation of anthropometry to malaria morbidity and immunity in Papua New Guinean children. Am J Clin Nutr. 1998;68(3):734–41. doi: 10.1093/ajcn/68.3.734. [DOI] [PubMed] [Google Scholar]
- 11.Graham SM, Walsh AL, Molyneux EM, et al. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94(3):310–4. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 12.Grimwade K, French N, Mbatha DD, et al. Childhood malaria in a region of unstable transmission and high human immunodeficiency virus prevalence. Pediatr Infect Dis J. 2003;22(12):1057–63. doi: 10.1097/01.inf.0000101188.95433.60. [DOI] [PubMed] [Google Scholar]
- 13.Korenromp EL. Malaria attributable to the HIV-1 epidemic, sub-Saharan Africa. Emerging Infectious Diseases. 2005;11(9):1410–19. doi: 10.3201/eid1109.050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olumese PE, Sodeinde O, Ademowo OG, et al. Protein energy malnutrition and cerebral malaria in Nigerian children. J Trop Pediatr. 1997;43(4):217–9. doi: 10.1093/tropej/43.4.217. [DOI] [PubMed] [Google Scholar]
- 15.Prada J, Alabi SA, Bienzle U. Bacterial strains isolated from blood cultures of Nigerian children with cerebral malaria. Lancet. 1993;342(8879):1114. doi: 10.1016/0140-6736(93)92096-c. [DOI] [PubMed] [Google Scholar]
- 16.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10(2):143–5. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 17.Schubart CD, Mturi N, Beld MG, et al. Role of viruses in Kenyan children presenting with acute encephalopathy in a malaria-endemic area. Am J Trop Med Hyg. 2006;75(6):1148–50. [PubMed] [Google Scholar]
- 18.Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13(22):2345–58. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- 19.Bejon P, Berkley JA, Mwangi T, et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med. 2007;4(8):e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keating J, Mbogo CM, Mwangangi J, et al. Anopheles gambiae s.l. and Anopheles funestus mosquito distributions at 30 villages along the Kenyan coast. J Med Entomol. 2005;42(3):241–6. doi: 10.1093/jmedent/42.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ministry of Health, GoK . AIDS in Kenya. Sixth ed. Nairobi: 2001. [Google Scholar]
- 22.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332(21):1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 23.Berkley JA, Mwangi I, Ngetsa CJ, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. Lancet. 2001;357(9270):1753–7. doi: 10.1016/S0140-6736(00)04897-2. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Pocket book of hospital care for children - guidelines for the management of common illnesses with limited resources. WHO; Geneva: 2005. [PubMed] [Google Scholar]
- 25.Mwangi TW, Ross A, Snow RW, et al. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191(11):1932–9. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356(9235):1051–6. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg AE, Nsa W, Ryder RW, et al. Plasmodium Falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med. 1991;325(2):105–9. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen-Dinh P, Greenberg AE, Mann JM, et al. Absence of association between Plasmodium falciparum malaria and human immunodeficiency virus infection in children in Kinshasa, Zaire. Bull World Health Organ. 1987;65(5):607–13. [PMC free article] [PubMed] [Google Scholar]
- 29.Thera MA, Sehdev PS, Coulibaly D, et al. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192(10):1823–9. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendrickse RG, Hasan AH, Olumide LO, et al. Malaria in early childhood. An investigation of five hundred seriously ill children in whom a "clinical" diagnosis of malaria was made on admission to the children's emergency room at University College Hospital, Ibadan. Ann Trop Med Parasitol. 1971;65(1):1–20. [PubMed] [Google Scholar]
- 31.Murray MJ. Diet and cerebral malaria : The effect of Famine and Refeeding. American Journal of Clinical Nutrition. 1978;37:57–61. doi: 10.1093/ajcn/31.1.57. [DOI] [PubMed] [Google Scholar]
- 32.Berkley J, Mwangi I, Griffiths K, et al. Assessment of severe malnutrition among hospitalized children in rural Kenya: comparison of weight for height and mid upper arm circumference. Jama. 2005;294(5):591–7. doi: 10.1001/jama.294.5.591. [DOI] [PubMed] [Google Scholar]
- 33.Brent AJ, Oundo JO, Mwangi I, et al. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J. 2006;25(3):230–6. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 34.Brent AJ, Ahmed I, Ndiritu M, et al. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. Lancet. 2006;367(9509):482–8. doi: 10.1016/S0140-6736(06)68180-4. [DOI] [PubMed] [Google Scholar]
- 35.Greenwood BM, Bradley-Moore AM, Bryceson AD, et al. Immunosuppression in children with malaria. Lancet. 1972;1(7743):169–72. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- 36.Hviid L, Theander TG, Abu-Zeid YA, et al. Loss of cellular immune reactivity during acute Plasmodium falciparum malaria. FEMS Microbiol Immunol. 1991;3(4):219–27. doi: 10.1111/j.1574-6968.1991.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 37.Seydel KB, Milner DA, Jr., Kamiza SB, et al. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis. 2006;194(2):208–5. doi: 10.1086/505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilairatana P, Meddings JB, Ho M, et al. Increased gastrointestinal permeability in patients with Plasmodium falciparum malaria. Clin Infect Dis. 1997;24(3):430–5. doi: 10.1093/clinids/24.3.430. [DOI] [PubMed] [Google Scholar]
- 39.Reyburn H, Mbatia R, Drakeley C, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. Bmj. 2004;329(7476):1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zurovac D, Midia B, Ochola SA, et al. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop Med Int Health. 2006;11(4):432–40. doi: 10.1111/j.1365-3156.2006.01587.x. [DOI] [PubMed] [Google Scholar]