Abstract
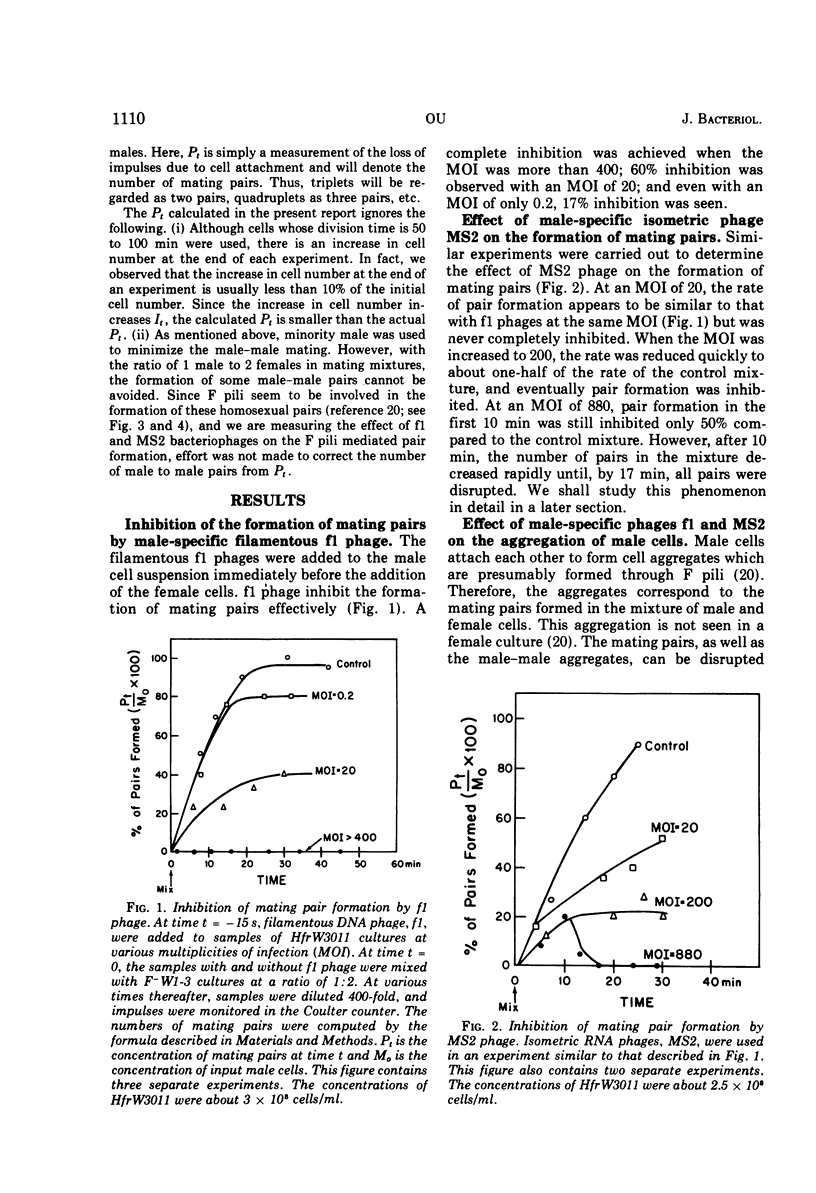
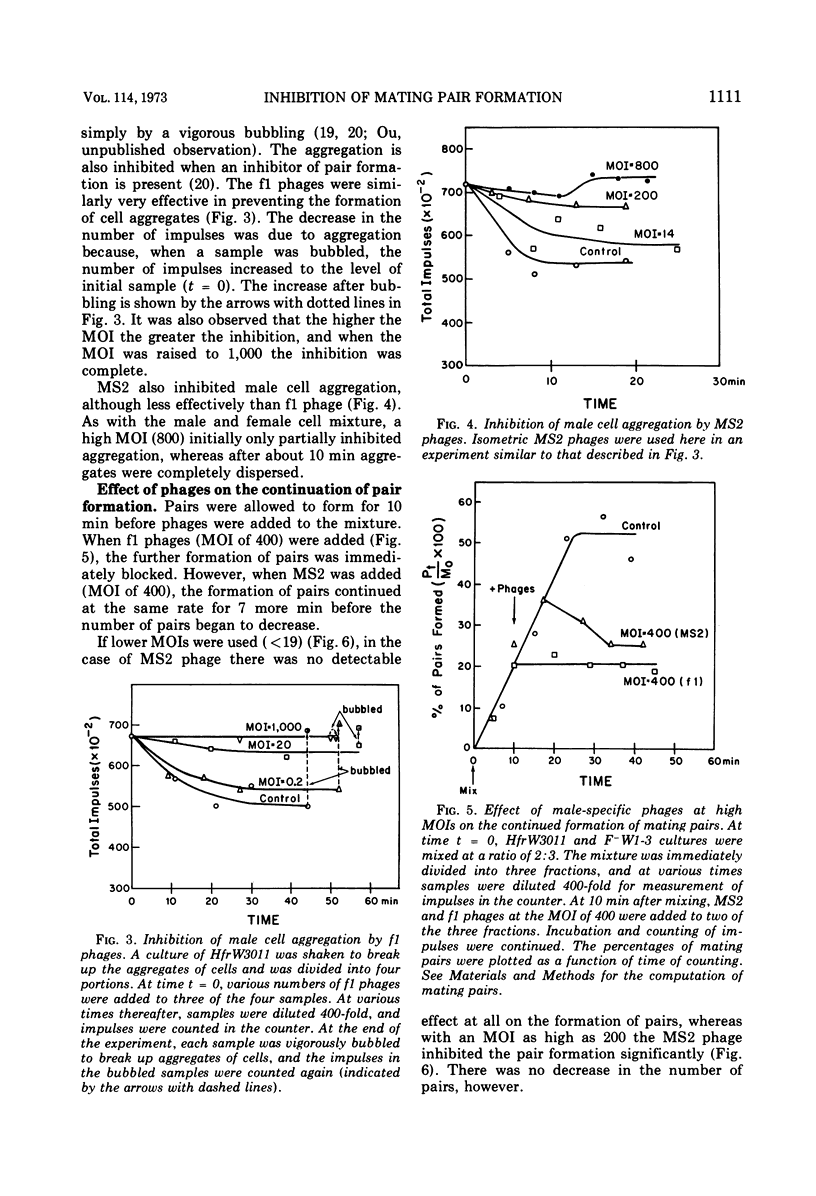
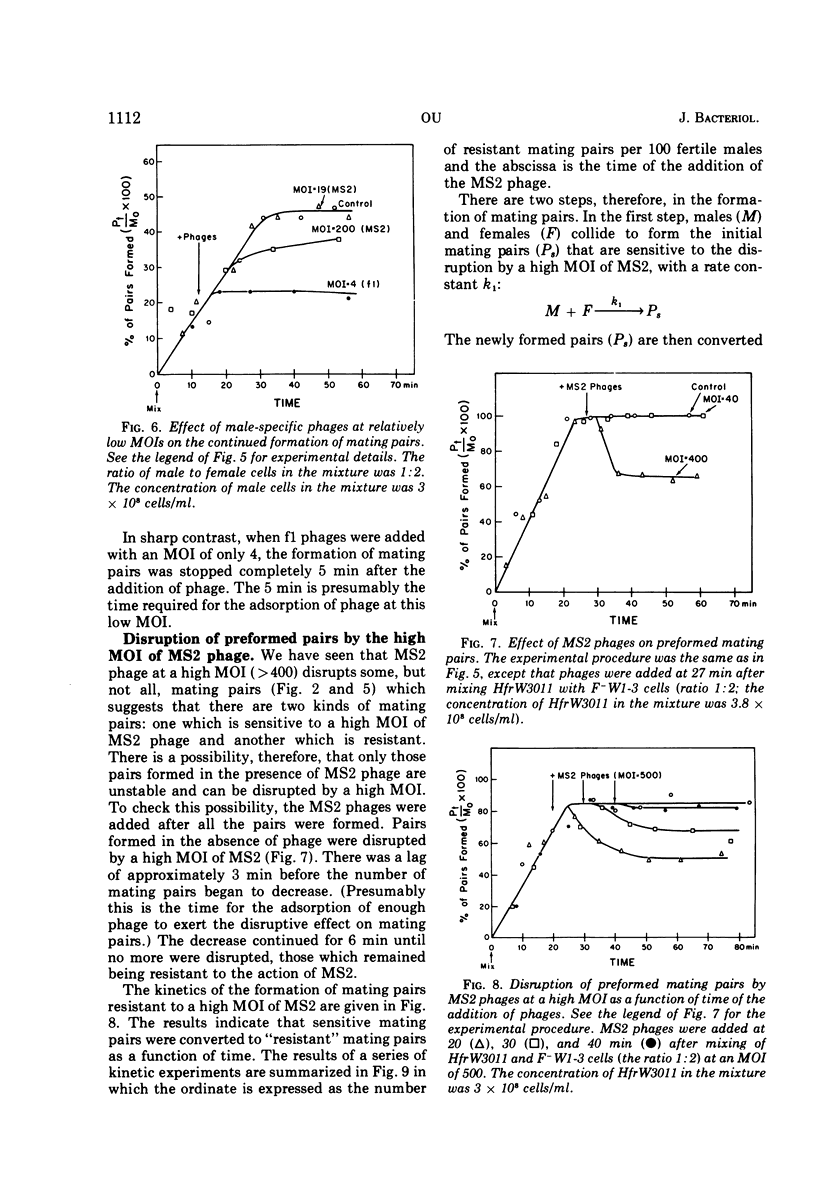
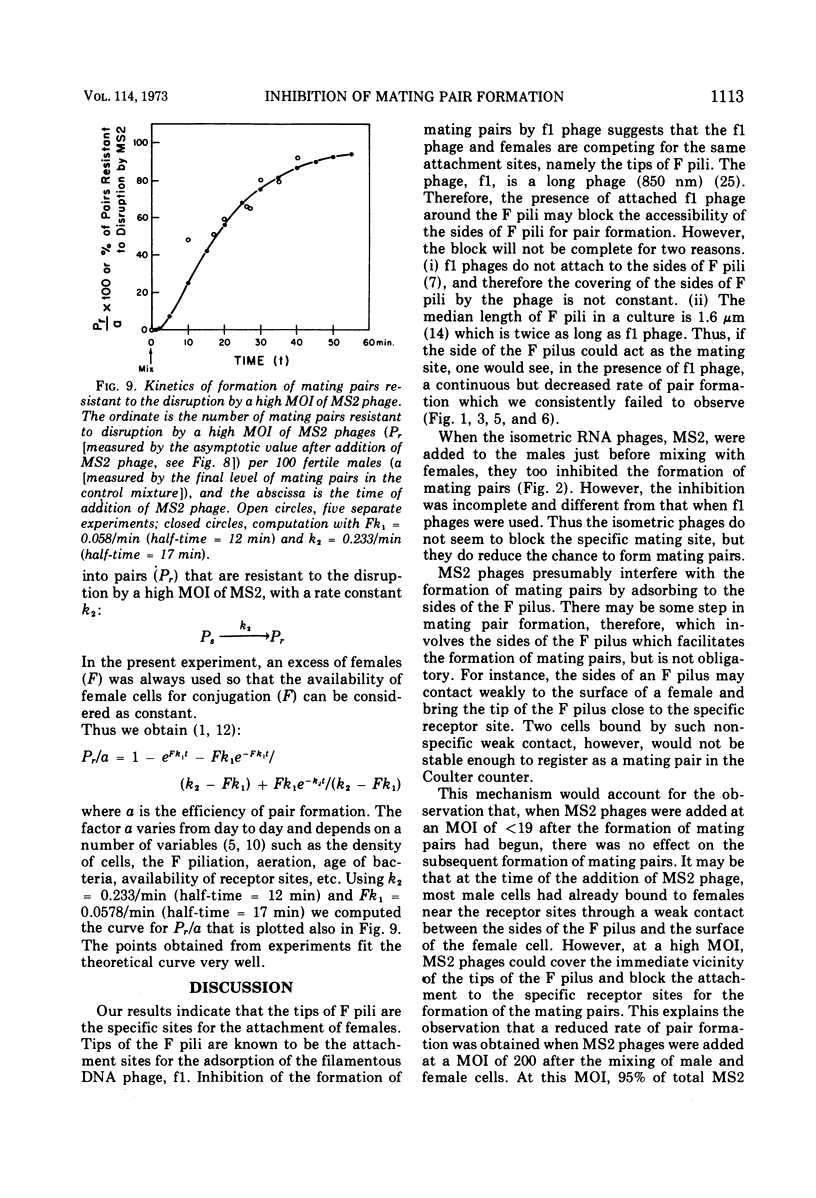
The effect of male-specific filamentous deoxyribonucleic acid (f1) and isometric ribonucleic acid (MS2) bacteriophages on the formation of mating pairs in Escherichia coli conjugation was examined directly in the Coulter counter. When a sufficient multiplicity of infection (MOI) was used, the f1 phage immediately and completely inhibited the formation of mating pairs. On the other hand, the MS2 phage at a relatively high MOI also inhibited the formation of mating pairs significantly although not completey. The inhibitory effect of MS2 phage was dependent on the time of addition and the MOI used. At relatively low MOI (<20), the MS2 phage showed some inhibitory effect when added to a male culture prior to mixing with females, whereas no effect was observed when phages were added after mating pair formation had already commenced. At a high MOI (>400) MS2 phage disrupted the mating pairs already formed. Some preformed mating pairs were resistant to the high MOI of MS2 phages, however, and the “sensitive” (to high MOI) mating pairs seem to mature into “resistant” mating pairs as a function of time. We conclude that the tip of an F pilus is the specific attachment site for mating. The following process of mating pair formation has been formulated by deduction. (i) The sides of F pili weakly contact female cells, (ii) then the tips of F pili attach to the specific receptor sites to form initial mating pairs, and (iii) those pairs mature into mating pairs that are resistant to the high MOI of MS2 phages. The high MOI of MS2 prevents the first step, whereas f1 phages affect the second step—the binding between the tips of F pili and the receptor sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. Recombination and segregation in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1958;23:47–58. doi: 10.1101/sqb.1958.023.01.007. [DOI] [PubMed] [Google Scholar]
- ANDERSON T. F., WOLLMAN E. L., JACOB F. Sur les processus de conjugaison et de recombinaison chez Escherichia coli. III. Aspects morphologiques en microscopie électronique. Ann Inst Pasteur (Paris) 1957 Oct;93(4):450–455. [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. W. Mechanically caused damage to Hfr cells of Escherichia coli K12. Genet Res. 1966 Apr;7(2):267–271. doi: 10.1017/s0016672300009678. [DOI] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. The sex hair of E. coli as sensory fiber, conjugation tube, or mating arm? Biochem Biophys Res Commun. 1967 Jun 23;27(6):674–680. doi: 10.1016/s0006-291x(67)80088-3. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Role of F pili in the penetration of bacteriophage fl. J Virol. 1972 Oct;10(4):835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle P. Evidence for the identity of the mating-specific site of male cells of Escherichia coli with the receptor site of an RNA phage. Biochem Biophys Res Commun. 1967 Apr 7;27(1):81–87. doi: 10.1016/s0006-291x(67)80043-3. [DOI] [PubMed] [Google Scholar]
- MARVIN D. A., HOFFMANN-BERLING H. A FIBROUS DNA PHAGE (FD) AND A SPHERICAL RNA PHAGE (FR) SPECIFIC FOR MALE STRAINS OF E COLI. II. PHYSICAL CHARACTERISTICS. Z Naturforsch B. 1963 Nov;18:884–893. doi: 10.1515/znb-1963-1106. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Knight W. S., Brinton C. C., Jr Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968 Feb;95(2):314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. T., Anderson T. F. Effect of Zn 2+ on the adsorption of male-specific filamentous deoxyribonucleic acid and isometric ribonucleic acid bacteriophages. J Virol. 1972 Oct;10(4):869–871. doi: 10.1128/jvi.10.4.869-871.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. T., Anderson T. F. Effect of Zn2+ on bacterial conjugation: inhibition of mating pair formation. J Bacteriol. 1972 Jul;111(1):177–185. doi: 10.1128/jb.111.1.177-185.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. T., Anderson T. F. Role of pili in bacterial conjugation. J Bacteriol. 1970 Jun;102(3):648–654. doi: 10.1128/jb.102.3.648-654.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARANCHYCH W., GRAHAM A. F. Isolation and properties of an RNA-containing bacteriophage. J Cell Comp Physiol. 1962 Dec;60:199–208. doi: 10.1002/jcp.1030600303. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- TZAGOLOFF H., PRATT D. THE INITIAL STEPS IN INFECTION WITH COLIPHAGE M13. Virology. 1964 Nov;24:372–380. doi: 10.1016/0042-6822(64)90174-6. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D., VALENTINE R. C., ROGER M., STOECKENIUS W. F1, A ROD-SHAPED MALE-SPECIFIC BACTERIOPHAGE THAT CONTAINS DNA. Virology. 1963 Aug;20:638–640. doi: 10.1016/0042-6822(63)90290-3. [DOI] [PubMed] [Google Scholar]



