Abstract
Mesothelia comprise the epithelial covering of coelomic organs and line the cavities in which they are housed. Mesothelia contribute to the vasculature of the heart and the intestinal tract by developmental processes of epithelial–mesenchymal transition (EMT), migration, and differentiation into endothelial cells, vascular smooth muscle cells, and pericytes. Here, we establish a novel in vitro system to analyze the differentiative potential of mesothelia. Using explants from serosal mesothelium (the mesothelial covering of the gut), we demonstrate that much of the developmental program observed in embryonic mesothelia is retained in the adult structure. Namely, processes of epithelial spreading, EMT, and differentiation into smooth muscle cells from these cells are observed. Interestingly, we were unable to stimulate endothelial cell differentiation using serum or various signaling factors. Taken together, these data reveal that differentiated serosal cells retain vasculogenic potential and provide a generalizable model for future studies on the developmental and differentiative capacity of the mesothelial cell type.
Keywords: mesothelia, coronary and gut vasculogenesis, vascular smooth muscle differentiation, gut development
Introduction
A mesothelium is a simple squamous epithelium of mesodermal origin that comprises the linings of the vertebrate body cavities and the outer coverings of the organs housed within. In general, mesothelia are thought to provide protection and a smooth lubricated surface for movement of organs within coelomic cavities. However, the epicardial mesothelium (epicardium) has been shown to play an important role during organogenesis of the heart (Watt et al., 2004). Specifically, cells of the epicardium give rise to the coronary vasculature (Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996; Dettman et al., 1998; Vrancken Peeters et al., 1999). During embryogenesis, the proepicardium (PE) and, later, the epicardium move over the developing heart, cover the entire organ and produce mesenchyme by epithelial–mesenchymal transition (EMT). The resulting cells migrate freely into the myocardium and differentiate into the coronary vasculature (Mikawa and Gourdie, 1996; Dettman et al., 1998; Vrancken Peeters et al., 1999; Perez-Pomares et al., 2002), presumably induced by local factors released by the primitive vascular plexus (Reese et al., 2002).
We have recently shown that cells of the serosal mesothelium, which covers the intestinal tract, undergo similar differentiative processes of EMT and differentiation into vascular but not visceral smooth muscle (Wilm et al., 2005). Our data show for the first time that mesothelia, other than the epicardium of the heart, have the potential to form vasculogenic cells. This finding suggested that a conserved mechanism accounts for the formation of blood vessels to coelomic organs.
While the differentiative potential of epicardial cells in vivo and in vitro has been established, it is not known whether this is a generalizable trait of mesothelia. Additionally, it is unclear whether adult mesothelia retain the potential to differentiate into elements of the vasculature. We hypothesize that mesothelial cells, independent of their location, have the potential to respond to growth factor signaling, leave the epithelial sheet by EMT, and differentiate into vascular smooth muscle. Thus, we turned to a novel in vitro system using primary culture of isolated serosal mesothelia to test these hypotheses. Here, we established a primary cell/tissue system of mesothelial explants and examine whether cells of these explants can undergo EMT and differentiate into vasculogenic cells. Our data demonstrate that cells of the serosal mesothelium can undergo EMT and differentiate into smooth muscle at high frequency. Interestingly, endothelial cells do not differentiate to any appreciable degree in this system using serum or specific growth factors. Also, we demonstrate that smooth muscle differentiation from serosal mesothelium is strongly promoted by serum or platelet-derived growth factor-BB (PDGF-BB) while only weakly induced by fibroblast growth factor (FGF) or epithelial growth factor (EGF). Taken together, our data demonstrate that serosal mesothelium retains much of its differentiative potential, including the production of smooth muscle. The in vitro system presented within may serve as a generalizable model for the analysis of the mesothelial cell type.
Results and Discussion
Establishing a Model for the Analysis of the Differentiative Potential of the Serosal Mesothelium
Our initial goal was to establish a model to study sheet formation, EMT, migration, and differentiation in an effort to determine the vasculogenic potential of isolated coelomic mesothelium. The differentiated murine serosa is a thin, barely vascularized tissue, consisting essentially of two layers of serosal mesothelium (Wilkosz et al., 2005). Figure 1A demonstrates that our method of isolation results in simple mesothelial epithelia in which greater than 97% of the nuclei are positive for the mesothelial marker Wt1 (Armstrong et al., 1993; Moore et al., 1998; Carmona et al., 2001; Foley-Comer et al., 2002; Wada et al., 2003; Wilm et al., 2005). In addition, cytokeratins, also expressed in mesothelia (Perez-Pomares et al., 1998; Wilm et al., 2005), were present in these explants (Fig. 1B). Bves, an epithelial marker expressed at tight junctional complexes (Osler et al., 2005), was detected at points of cell–cell contact (Fig. 1C). No definitive smooth muscle actin (SMA), smooth muscle myosin (SMM), or platelet endothelial cell adhesion molecule (PECAM) staining was observed, indicating the absence of smooth muscle and endothelial cells (Fig. 1D–F). None of these antibodies produced signal above that observed with secondary antibody alone. Thus, the method of tissue isolation yielded a highly purified population of mesothelial cells for further study.
Fig. 1.
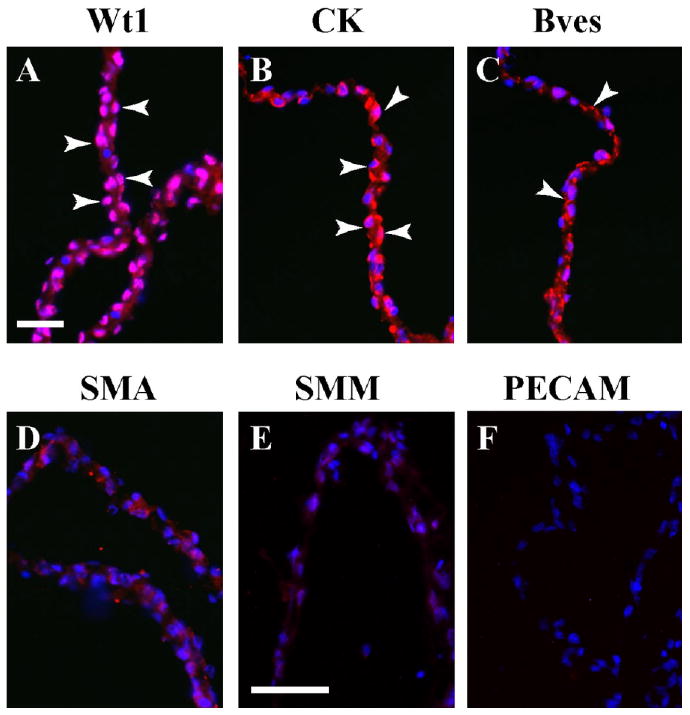
Characterization of serosal mesothelium using marker proteins. Freshly excised serosal mesothelium preparation was sectioned and stained with specific marker antibodies. A,B: This preparation reveals nuclear Wt1 expression (A, arrowheads) and cytokeratin (CK) distribution (B, arrowheads). C–F: Bves is found at cell–cell contacts (C, arrowheads), while SMA (D), SMM (E), and PECAM (F) are not detectable above background. Scale bars = 50 μm in A–F.
Next, the serosa was cultured to determine whether explants would remain viable and may retain specific differentiative properties. After 2 days of culture, explants had attached and established robust epithelial sheets (Fig. 2A). The mesothelial marker Wt1 was found in all nuclei of the forming sheet (Fig. 2B), and expression of cytokeratins was strongest at the periphery of the sheet (Fig. 2C). Cells of the sheet and on the surface of the explant mass were epithelial since Bves was detected at sites of cell–cell contact (Fig. 2D,D′). (Note: Due to the three-dimensional nature of the explant, an edge artifact produces false fluorescence; Fig. 2E.) In cells at the outer periphery of the forming sheet, we observed expression of smooth muscle markers including SMA (Fig. 2E). These cells were also positive for SMM (Fig. 2L) and desmin (data not shown).
Fig. 2.
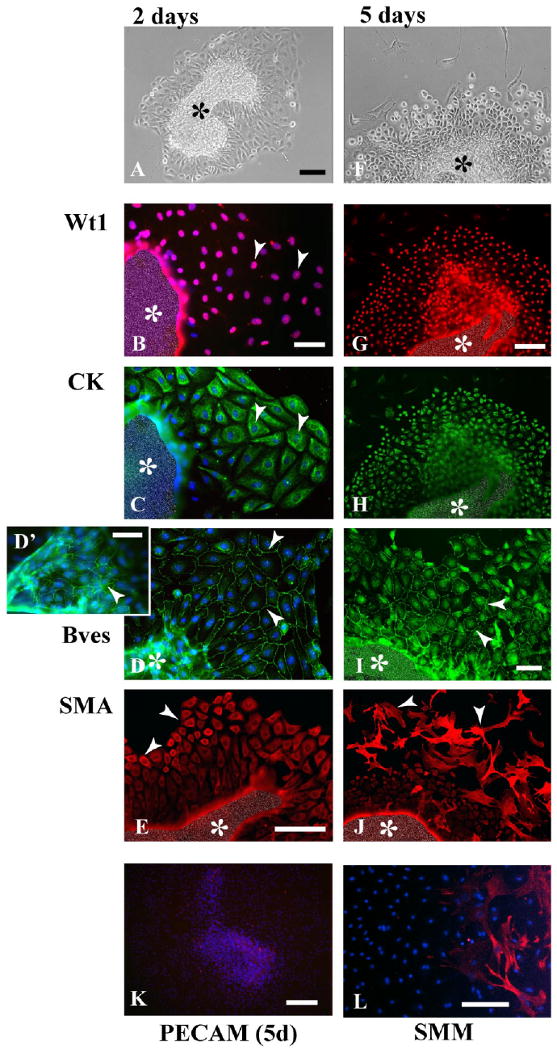
Marker expression in cultured serosal mesothelial explants. A–C: At day 2, an epithelial sheet has formed around the central explant piece (A), and most of its cells express Wt1 (arrowhead, B), and cytokeratin (CK, arrowhead, C). D: Bves is found in the epithelial sheet (arrowheads, D), and on the serosal mass surface (arrowhead, D′). E: Smooth muscle actin (SMA) is not detectable in cells close to the omentum mass, but in cells at the epithelial sheet periphery (arrowheads). F–H: After 5 days of culture, cells at the epithelial sheet periphery have started to differentiate (arrowheads, F), but still express Wt1 (G) and cytokeratin (H). I: Bves is found at cell–cell contacts in the proximal epithelial sheet (arrowheads). J–L: At the periphery, many SMA-expressing (arrowheads, J), and smooth muscle myosin (SMM; L) -expressing cells have formed. Platelet endothelial cell adhesion molecule (PECAM) staining is not detectable in explants at this stage (K). The central mass (asterisks) is artificially obscured in (B,C,E,G–J) to reduce strong fluorescence resulting from the additive effect of multilayered signals. Scale bars = 100 μm in A,F, 50 μm in B,C,D, 50 μm in E, 100 μm in G,H,J, 50 μm in I, 200 μm in K, 100 μm in L.
After 5 days of culture, the appearance of explants had changed considerably. Although the epithelial sheet was still prominent, many cells at the periphery had a stellate-like shape (Fig. 2F). Both Wt1 and cytokeratin expression was detected in cells of the sheet, whereas stellate-like cells at the periphery did not express either marker (Fig. 2G,H). Also, the presence of Bves at cell–cell contacts revealed that most cells of the sheet retained epithelial characteristics (Fig. 2I) and ZO-1 (not shown). Additionally, smooth muscle markers were expressed in stellate cells at the periphery of the sheet, but not in epithelial cells close to the tissue mass (Fig. 2J,L). Of interest, endothelial cells were not detected in serosal mesothelial cultures using markers for PECAM (Fig. 2K) and Flk-1 (not shown).
Thus, this model provides a system where relatively pure populations of mesothelial cells can be isolated and studied in the controlled environment of cell culture. Our data demonstrate that predicted epithelial/mesothelial proteins mark these cells and that after a culture period of 2 days, cells form an epithelial sheet, while after 5 days of culture, differentiation of cells into smooth muscle cells can be observed. There are two plausible explanations for the lack of endothelial cell differentiation in this model: (1) these explants lack factors that could induce the differentiation of serosa into endothelial cells or (2) cultured serosal mesothelial cells do not possess the potential to differentiate into endothelial cells.
Serosal Mesothelial Cells Respond Differentially to Growth Factors
To determine whether serosal mesothelium can respond to specific signaling factors by undergoing EMT and cellular differentiation, we cultured explants in the presence of basic fibroblast growth factor (bFGF), EGF, and PDGF-BB, and subsequently analyzed for marker expression.
As a positive control, explants were cultured in standard medium containing 10% serum and assayed for their differentiative response. Only cells at the periphery of the graft were scored as the three-dimensional nature of the graft center did not allow scoring of differentiation. An average of 115 ± 28 SMA-positive cells was seen at the periphery of these explants at 5 days of culture (Figs. 3A,B, 4; Table 1). Of these SMA-positive stellate cells, approximately 80% were negative for Wt1 in agreement with data from Figure 2, whereas all cells of the explant and in the epithelial sheet were positive for this mesothelial marker (Figs. 3A,C, 4; Table 2).
Fig. 3.
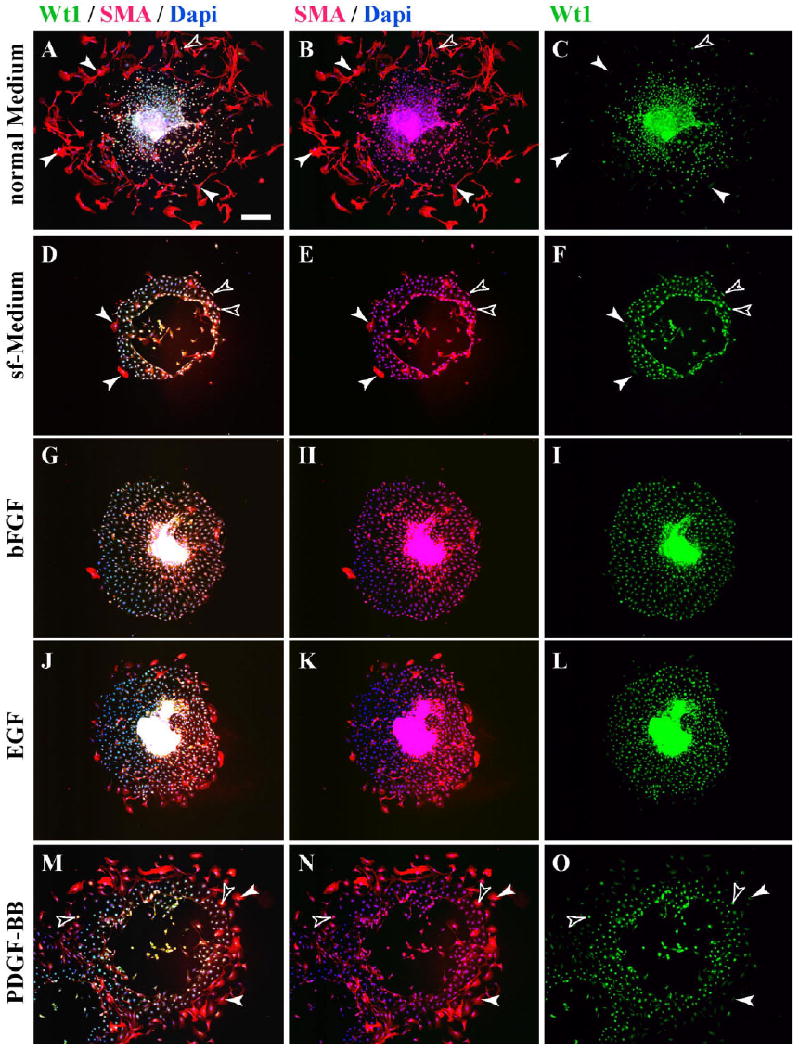
Growth factors induce epithelial–mesenchymal transition (EMT) and smooth muscle actin (SMA) expression in serosal mesothelial cultures. Explants were cultured for 5 days under various conditions and analyzed for expression of Wt1 and SMA. A–C: After culture in normal medium, many peripheral cells of explants are fibroblastic and express SMA but not Wt1 (arrowheads), while Wt1-positive cells are negative for SMA (yellow open arrowhead). D–F: Under serum-free (sf) conditions, few of the epithelial sheet cells of cultured explants express SMA, whereas its majority express Wt1. SMA-positive cells can be Wt1-positive (open arrowheads) and Wt1-negative (arrowheads). G–L: Similar results were obtained for explants grown in sf-medium supplemented with basic fibroblast growth factor (bFGF) or epithelial growth factor (EGF), with few SMA-positive cells in the epithelial sheets. M–O: In explants grown in sf-medium supplemented with platelet-derived growth factor-BB (PDGF-BB), many fibroblastic peripheral cells in the epithelial sheet express SMA (arrowheads), many of which coexpress Wt1 (open arrowheads). Scale bar = 200 μm.
Fig. 4.
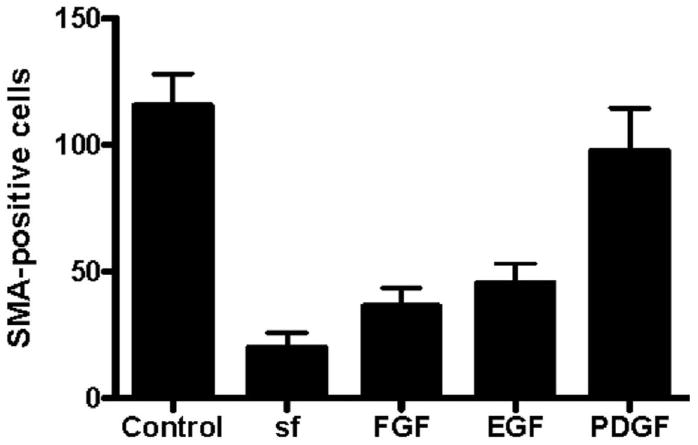
Production of smooth muscle actin (SMA) -positive cells in cultures of serosal mesothelium in the presence of specific growth factors. Cultures were analyzed at 5 days of culture with anti-SMA and anti-Wt1 antibodies. The total number of SMA-positive cells at the periphery was determined for each group indicated. One-way analysis of variance analysis determined that the number of SMA-positive cells in control serum-containing cultures was not significantly different from cultures with serum-free (sf) medium supplemented with platelet-derived growth factor-BB (PDGF-BB). In contrast to differentiation in serum-containing conditions, the number of SMA-positive cells in sf-medium or sf-medium supplemented with epithelial growth factor (EGF) or basic fibroblast growth factor (bFGF) was significantly (P < 0.001) reduced.
TABLE 1. Differentiation of SMA-Positive Cells in Serosal Mesothelial Culturesa.
| Control medium | EGF | FGF | PDGF | sf-medium | |
|---|---|---|---|---|---|
| 120 | 45 | 6 | 79 | 12 | |
| 101 | 7 | 7 | 90 | 9 | |
| 87 | 47 | 11 | 147 | 62 | |
| 109 | 38 | 30 | 191 | 21 | |
| 161 | 43 | 84 | 102 | 43 | |
| 122 | 31 | 66 | 14 | ||
| 58 | 60 | 52 | |||
| 26 | 53 | 53 | |||
| 74 | 54 | ||||
| 28 | 28 | ||||
| 30 | 35 | ||||
| 43 | |||||
| Average | 115.6 | 45.5 | 36.3 | 97.5 | 19.8 |
| SD | 36.7 | 28.2 | 24.5 | 48.7 | 13.7 |
The total number of SMA-positive cells at the periphery of cultures was determined by each group. SMA, smooth muscle actin; EGF, epithelial growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; sf, serum-free.
TABLE 2. Ratio of SMA/Wt1-Positive Cells in Serosal Mesothelial Culturesa.
| Control medium | EGF | FGF | PDGF | sf-medium | |
|---|---|---|---|---|---|
| 0.333 | 0.933 | 0.833 | 0.975 | 0.916 | |
| 0.198 | 1 | 0.714 | 0.966 | 0.952 | |
| 0.184 | 0.893 | 1 | 0.932 | 1 | |
| 0.083 | 1 | 0.933 | 0.937 | 1 | |
| 0.136 | 0.93 | 0.928 | 0.931 | 0.928 | |
| 0.918 | 1 | 0.954 | |||
| 0.948 | 0.916 | 1 | |||
| 0.923 | 0.943 | 0.943 | |||
| 0.973 | 0.907 | ||||
| 0.928 | 0.893 | ||||
| 0.933 | 0.8 | ||||
| 0.884 | |||||
| Average | 0.18+/−0.09 | 0.93+/−0.04 | 0.89+/−0.09 | 0.95+/−0.08 | 0.96+/−0.04 |
All SMA-positive cells were identified at the explant periphery and the number of cells that were also positive for anti-Wt was determined for each group. SMA, smooth muscle actin; EGF, epithelial growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; sf, serum-free.
When explants were cultured in serum-free (sf) medium, the integrity of the epithelial sheet was maintained with little production of stellate cells. Of interest, the number of SMA-expressing cells was greatly depressed in these cultures with an average of 19.8 ± 13.7 SMA-positive cells/explant (Figs. 3D,E, 4; Table 1). Of the small number of SMA-positive cells produced, the majority (approximately 95%) remained positive for Wt1 (Fig. 3D,F; Table 2). Similarly, serosal mesothelium cultured in sf-medium supplemented with bFGF or EGF (Figs. 3G–L, 4; Table 1) resulted in a significant reduction in SMA-positive cells at the periphery of the explant. In contrast, when explants were cultured in sf-medium containing PDGF-BB, an average of 97.5 ± 48.7 cells at the periphery of the explant expressed SMA (Figs. 3M,N, 4; Table 1). This number was not statistically different from the number of SMA-producing cells observed in control serum-containing conditions (Figs. 3A,B, 4; Table 1). However, in contrast to smooth muscle cells differentiated in serum, >97% of SMA-positive cells differentiated in response to PDFG-BB retained Wt1 expression (Figs. 3N,O, 4; Table 2).
Cultures were stained with PECAM and Flk-1 to determine whether these specific growth factors could induce endothelial cell differentiation. Of interest, we were unable to detect any endothelial cell differentiation after addition of these growth factors at varying concentrations at any time point in culture.
Summary
Our data demonstrate that differentiated serosal mesothelial cells retain their vasculogenic potential to produce smooth muscle cells. This model reflects or possibly recapitulates the developmental process observed in developing mesothelia of the heart and gut (Mikawa and Gourdie, 1996; Dettman et al., 1998; Manner, 1999; Vrancken Peeters et al., 1999; Morabito et al., 2001; Wilm et al., 2005) with the important exception that endothelial cell differentiation is not observed. At present, we cannot resolve whether the absence of cells expressing endothelial markers is a failure to identify the proper conditions for their emergence or that differentiated serosal mesothelium truly lacks the potential to produce this cell type. The native ability of this easily isolated tissue to produce vasculogenic cells may be exploited in the future. Indeed, vascular surgeons have known for many years that grafted omentum enhances revascularization after surgery (Adams et al., 1992). Of interest, all work on this topic thus far has focused on potential growth factor production from grafts and not on the possibility that grafted cells actually participate in repair. Our data further suggest that PDGF signaling, which plays an important role in vasculogenesis (Leveen et al., 1994; Soriano, 1994; Lindahl et al., 1997; Hellstrom et al., 1999) is a powerful inducer of smooth muscle differentiation of serosal mesothelium over other signaling factors tested in this study. Indeed, PDGF-B has been described to induce EMT in proepicardial explants (Lu et al., 2001) and is expressed in coronary arterial endothelial cells, while its receptor PDGFR-β was found in the epicardium, subepicardium, and later in smooth muscle cells (Van Den Akker et al., 2005). Thus, it is possible that the PDGF signaling is natively used in this developmental system even while Wt-1 expression is not down-regulated during in vitro differentiation presented here. Finally, this model provides a simple system to isolate, culture, and assay differentiated mesothelial cells from the normal or genetically altered mouse and may serve as a platform for additional work on this interesting cell type.
Experimental Procedures
Explants From the Serosa
Serosal explants were excised from 2- to 5-week-old mice by opening the peritoneal cavity and isolating the organ complex consisting of stomach, spleen, and pancreas with its associated serosa. While submerged in DMEM with 10% fetal bovine serum (FBS), the epithelial serosa (serosal mesothelium) was carefully separated from the organs using scissors, and further dissected into small pieces. Serosal pieces were slightly compacted using fine forceps and transferred into fresh DMEM supplemented with 10% FBS and 1:1,000 Penicillin/Streptomycin; three to four pieces were placed into each well of eight- or four-well chamberslides using a pipet. Approximately 20 to 30 serosal pieces were isolated from one isolated serosal preparation, and roughly 80% of these had attached and started to spread after overnight culture. Explants were cultured for up to 6 days, and medium was changed daily by carefully pipetting.
Growth Factors
Growth factors used were recombinant human EGF (40 ng/ml; R&D systems, 236-EG), recombinant human bFGF (40 ng/ml; R&D systems, 234-FSE/CF), and recombinant human homodimer PDGF-BB (40 ng/ml; Chemicon, GF018). Medium containing growth factors was replaced daily.
Immunofluorescence
Antibodies against Bves (B846, polyclonal rabbit; Wada et al., 2001), cytokeratin (polyclonal rabbit, DAKO, Z0622), SMA (Sigma, A 2574), SMM (Sigma, M 7786), Desmin (monoclonal mouse, BD Pharmingen, 550626), PECAM (BD Pharmingen, 550274), Flk-1(BD Pharmingen, 550549), ZO-1 (Zymed, 33-9100), and Wt1 (DAKO M3561) were used according to cited literature. Secondary antibodies were Alexa-coupled anti-rabbit and anti-mouse (Molecular Probes). It should be noted that anti-SMA (Sigma) produced slightly higher background than other antibodies used here. This finding was determined to be background by comparing images obtained from cell lines known not to express this protein with images from representative control and experimental samples from the current study. All control and experimental images were exposed and processed similarly.
Quantification of Cell Differentiation in Serosal Explants
Antibodies to Wt1 and SMA were used to quantify the number of mesothelial and smooth muscle cells after 5 days of culture. All cells at the periphery of standard explants were counted (at least five samples were collected for each experimental group). Only explants of similar size (epithelial explant of 700–900 μm in diameter at day 5) were used in these measurements. It should be noted that “edge artifact” was detected at the junction between the three-dimensional center of the graft and the advancing epithelial growth (for example, Fig. 2B,C,I,J regardless of the primary antibody used). Cells in this region were never scored as positive or negative in any of the tests to assay cell differentiation. The total number of positive cells was determined for each phenotype. Analysis of each group determined statistical significance using one-way analysis of variance with Bonferroni's post-test (alpha < 0.05).
Acknowledgments
We thank Sam Reddy, R. Pierre Hunt, Rebecca Coyle, and Dr. Charles Lin for technical assistance and help with the experiments. We also acknowledge Sarah Nordstrom for help with the statistical analysis. We thank present and former members of the Bader lab for fruitful discussions.
References
- Adams W, Ctercteko G, Bilous M. Effect of an omental wrap on the healing and vascularity of compromised intestinal anastomoses. Dis Colon Rectum. 1992;35:731–738. doi: 10.1007/BF02050320. [DOI] [PubMed] [Google Scholar]
- Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech Dev. 1993;40:85–97. doi: 10.1016/0925-4773(93)90090-k. [DOI] [PubMed] [Google Scholar]
- Carmona R, Gonzalez-Iriarte M, Perez-Pomares JM, Munoz-Chapuli R. Localization of the Wilm's tumour protein WT1 in avian embryos. Cell Tissue Res. 2001;303:173–186. doi: 10.1007/s004410000307. [DOI] [PubMed] [Google Scholar]
- Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- Foley-Comer AJ, Herrick SE, Al-Mishlab T, Prele CM, Laurent GJ, Mutsaers SE. Evidence for incorporation of free-floating mesothelial cells as a mechanism of serosal healing. J Cell Sci. 2002;115:1383–1389. doi: 10.1242/jcs.115.7.1383. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, Nagata K, Inagaki M, Majesky MW. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol. 2001;240:404–418. doi: 10.1006/dbio.2001.0403. [DOI] [PubMed] [Google Scholar]
- Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Moore AW, Schedl A, McInnes L, Doyle M, Hecksher-Sorensen J, Hastie ND. YAC transgenic analysis reveals Wilms' tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev. 1998;79:169–184. doi: 10.1016/s0925-4773(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- Osler ME, Chang MS, Bader DM. Bves modulates epithelial integrity through an interaction at the tight junction. J Cell Sci. 2005;118:4667–4678. doi: 10.1242/jcs.02588. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev Biol. 1998;200:57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev Biol. 2002;247:307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- Reese DE, Mikawa T, Bader DM. Development of the coronary vessel system. Circ Res. 2002;91:761–768. doi: 10.1161/01.res.0000038961.53759.3c. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Van Den Akker NM, Lie-Venema H, Maas S, Eralp I, DeRuiter MC, Poelmann RE, Gittenberger-De Groot AC. Platelet-derived growth factors in the developing avian heart and maturating coronary vasculature. Dev Dyn. 2005;233:1579–1588. doi: 10.1002/dvdy.20476. [DOI] [PubMed] [Google Scholar]
- Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199:367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- Wada AM, Reese DE, Bader DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128:2085–2093. doi: 10.1242/dev.128.11.2085. [DOI] [PubMed] [Google Scholar]
- Wada AM, Smith TK, Osler ME, Reese DE, Bader DM. Epicardial/mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circ Res. 2003;92:525–531. doi: 10.1161/01.RES.0000060484.11032.0B. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkosz S, Ireland G, Khwaja N, Walker M, Butt R, de Giorgio-Miller A, Herrick SE. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berl) 2005;209:251–261. doi: 10.1007/s00429-004-0446-6. [DOI] [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]


