Abstract
Many associative learning theories assert that the predictive accuracy of events affects the allocation of attention to them. More reliable predictors of future events are usually more likely to control action based on past learning, but less reliable predictors are often more likely to capture attention when new information is acquired. Previous studies showed that a circuit that includes the amygdala central nucleus (CEA) and the cholinergic substantia innominata/nucleus basalis magnocellularis (SI/nBM) is important for both sustained attention guiding action in a five-choice serial reaction time (5CSRT) task, and for enhanced new learning about less predictive cues in a serial conditioning task. In this study, we found that lesions of the cholinergic afferents of the medial prefrontal cortex interfered with 5CSRT performance but not with surprise-induced enhancement of learning, whereas lesions of cholinergic afferents of posterior parietal cortex impaired the latter effects but did not affect 5CSRT performance. CEA lesions impaired performance in both tasks. These results are consistent with the view that CEA affects these distinct aspects of attention by influencing the activity of separate, specialized cortical regions, via its modulation of SI/nBM.
Keywords: amygdala, attention, basal forebrain, medial prefrontal cortex, posterior parietal cortex, prediction error
Attention is a vital component in the organization and control of behavior. However, it is not a monolithic mechanism by which stimulus processing is enhanced or depressed, but rather a collection of processes that interact to guide behavior in a given situation according to current goals (Allport, 1989). Although the term attention typically refers to processes that operate to control current action, attention can also be allocated while learning about stimuli, with that attentionally-guided learning manifesting itself in behavior only at some later time. Indeed, Holland & Gallagher (1999) distinguished between two aspects of attention in associative learning: attention involved in modulating current actions on well-learned tasks, and that affecting the acquisition of new learning. Although enhanced processing of stimuli might be expected to facilitate both new learning and established action, in some circumstances these roles may diverge.
According to many theorists (e.g., Le Pelley, 2004; Mackintosh, 1975; Pearce & Hall, 1980), the predictive validity of a conditioned stimulus (CS) is a crucial factor in determining the allocation of attention to that CS in the context of associative learning. Pearce and Hall (1980) explicitly distinguished between roles for attention in learning, which they described as primarily involving “controlled processing” and in action, which they described as mostly involving “automatic processing”. They asserted that the ease or rate at which a CS may enter into new learning (its “associability”) is proportional to the reinforcement prediction error that previously accompanied that CS. If the outcome of a learning episode is well-predicted (e.g., an expected reinforcer in fact occurs), then the associability of the CS would be set low, and subsequent learning about that CS would be relatively slow. By contrast, if the outcome is surprising (e.g., an expected reinforcer is omitted or an unexpected reinforcer presented) then CS associability would be set high and subsequent learning about that cue would be enhanced. The claim that previously inconsistent predictors of reinforcement acquire new learning more readily than cues with a history of consistent reinforcer prediction has been supported in a variety of experimental contexts, including negative transfer tasks (Hall & Pearce, 1979), partial reinforcement procedures (Kaye & Pearce, 1984), serial conditioning procedures (Wilson, Boumphrey, & Pearce, 1992), and patterning tasks (Holland, Thornton, & Ciali, 2000).
By contrast, substantial evidence shows that when performance, not learning, is guided by the allocation of attention, more consistent predictors of important outcomes are more likely to control action than less consistent predictors (Chiba, Bushnell, Oshiro, & Gallagher, 1999; Posner, 1980). Within Pearce and Hall's (1980) framework, whereas the controlled attentional processing that determines the rate of learning about a CS is enhanced by the surprise or prediction error generated when that CS is an inconsistent predictor of its consequences, the automatic processing that determines responding to a CS could well be greater to CSs that are better predictors. Given such a behavioral dissociation, it is thus reasonable to consider whether different brain systems subserve these two aspects of attentional processing.
An earlier series of experiments (reviewed by Holland & Gallagher, 1999) used a serial conditioning procedure, originally described by Wilson, Boumphrey, & Pearce (1992), to examine the neural circuitry underlying changes in CS associability in appetitive Pavlovian conditioning. These studies showed that a circuit, which included the amygdala central nucleus (CEA), the magnocellular cholinergic neurons of the sublenticular substantia innominata / nucleus basalis (SI/nBM), and the posterior parietal cortex (PPC), was essential for the enhancement of CS associability by reducing the predictive validity of that cue. Intact rats subjected to a reduction in a CS's predictive accuracy later displayed enhanced attention to that CS, as assessed by the rate of learning new associations to it, compared to rats that did not experience a change in the CS's predictive accuracy. By contrast, rats with neurotoxic lesions of the CEA (Holland & Gallagher, 1993), lesions that selectively destroyed the large cholinergic neurons in SI/nBM (Chiba, Bucci, Holland, & Gallagher, 1995), lesions that disconnected CEA from SI/nBM (Han, Holland, & Gallagher, 1999) or lesions that depleted the cholinergic innervation of PPC (Bucci, Holland, & Gallagher, 1998) did not show these enhancements in CS processing.
Components of this circuit have also been identified as critical to sustained automatic attention (Pearce & Hall, 1980) in the performance of a well-learned selective attention task. Holland, Han, and Gallagher (2000) found that CEA lesions impaired performance in an operant multiple choice reaction time paradigm. In the variant of this task used by Holland et al. (2000), rats were required to poke their noses into one of three stimulus-response ports to obtain a food reward. The correct port was signaled by a brief (500 ms) illumination of a lamp within the port. Challenges were introduced to make the task more demanding of attentional resources. In the face of two such attentional challenges (reduced duration of the target stimulus and variability in the duration of the ready signal that signified the beginning of a trial), rats with CEA lesions were impaired in the accuracy and speed of correct responding. Similarly, several studies have examined the role of basal forebrain cholinergic function in the performance of well-established selective visual attention tasks. For example, Muir, Everitt, and Robbins (1994) found that AMPA-induced lesions of the SI/nBM impaired choice accuracy and correct response latency in a 5-choice serial reaction time (5CSRT) task related to the 3-choice task used by Holland et al. (2000) to assess CEA function. Likewise, McGaughy, Kaiser, and Sarter (1996) found impairments in a signal detection task in rats with selective cholinergic basal forebrain lesions made with 192IgG-saporin. These lesions reduced rats' ability to detect brief visual signals, while leaving their ability to correctly reject nonsignals intact. Finally, Holland (in press) found that performance under attentional challenge conditions in a 5CSRT task was disrupted by contralateral lesions that disconnected CEA from SI/nBM. These findings support a role for a circuit including CEA and SI/nBM in allocating attention to guide appropriate action under circumstances requiring sustained task performance.
The SI/nBM is known to provide the major cholinergic innervation to most of the neocortex (e.g., Rye, Wainer, Mesulam, Mufson, & Saper, 1984). Nevertheless, not all cortical regions have been implicated in performance in sustained attention tasks. For example, Muir, Everitt, and Robbins (1996) found no effects of lesions of the parietal cortex (although not necessarily the same region identified as PPC by Bucci et al., 1998, and Reep, Chandler, King, & Corwin, 1994) on speed or accuracy of responding in the 5CSRT task. However, these authors found that another cortical region that receives heavy cholinergic projections from SI/nBM, the medial prefrontal cortex (MFC), has a critical role in performance of the 5CSRT task. Muir et al. (1996) found that excitotoxic lesions of the MFC produced behavioral impairments in 5CSRT task performance similar to those observed after cholinergic lesions of the SI/nBM. More recent studies found that 192IgG-saporin cholinergic-specific lesions of either SI/nBM (McGaughy, Dalley, Morisson, Everitt, & Robbins, 2002) or MFC (Dalley, Theobald, Bouger, Chudasama, Cardinal, & Robbins, 2004) impaired performance in the 5CSRT task. Moreover, McGaughy et al. (2002) found that 192IgG-saporin lesions of the SI/nBM not only produced strong behavioral deficits in the 5CSRT task, but also reduced acetylcholine efflux in the MFC during performance of that task, relative to sham lesioned rats. The results of these studies thus implicate cholinergic projections from SI/nBM to MFC in attentional processes that subserve performance in these tasks.
Taken together, these findings suggest that a circuit that includes CEA and SI/nBM is critical for modulating attention in both learning and action. In the present article, we considered whether these two aspects of attention are subserved by different cortical cholinergic projections of SI/nBM, specifically, projections to PPC for associability changes in learning, and projections to MFC for attentionally-guided action. In Experiment 1, we used a 5CSRT task to assess sustained attention for action, and in Experiment 2 we used the same rats, cues, and apparatus to assess the enhancement of CS associability by prediction error in a new learning task. In both experiments we examined the effects of variations in cue validity on measures of attention. Notably, within the Pearce-Hall (1980) model, whereas less-predictive cues should control more attention for new learning than highly-predictive cues (Experiment 2), the latter cues might capture more automatic attention for action than less-predictive cues in Experiment 1. Separate groups of rats with bilateral lesions of the CEA, the cholinergic innervation of MFC, or the cholinergic innervation of PPC, and rats with corresponding sham surgeries, were tested. We predicted that rats with damage to the CEA, which we assert modulates both attention for action and attention for learning, by broadly influencing SI/nBM, would show altered performance in both Experiment 1 (5CSRT performance) and Experiment 2 (new learning task). By contrast, rats with cholinergic lesions of PPC, hypothesized to modulate only attention for new learning, should show altered new learning in Experiment 2, but normal 5CSRT performance in Experiment 1. Finally, rats with cholinergic lesions of MFC, thought to modulate only attention for action, should show impairments in 5CSRT performance in Experiment 1, but not in new learning in Experiment 2. Figure 1 provides a summary of the designs and methods of both experiments.
Figure 1.
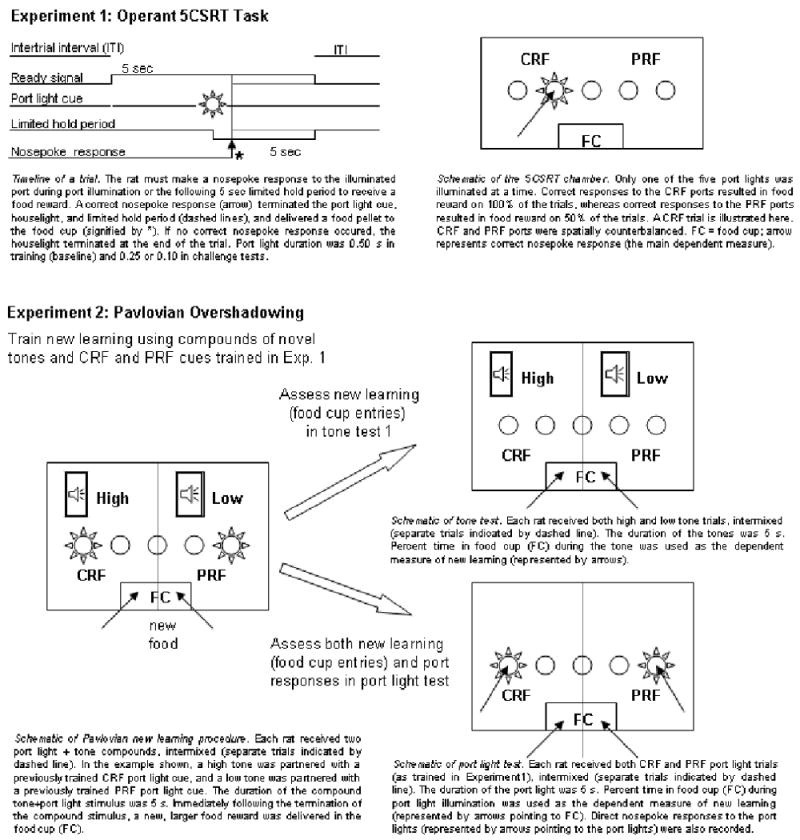
Outline of experimental procedures for Experiments 1 and 2. CRF, consistent reinforcement; PRF, partial reinforcement.
Experiment 1
In Experiment 1, intact rats received training with 5CSRT procedures designed to assess sustained attention in current action. In this task, nose pokes to a briefly-illuminated (500 ms) port in an array of 5 ports were initially reinforced with food delivery on every trial (consistent reinforcement, CRF). Then, we introduced partial reinforcement (PRF) contingencies for 2 of the port cues such that correct responses to those ports were reinforced on only half of the trials on which they were presented. The main purpose of introducing PRF contingencies was to prepare the rat for Experiment 2, in which we evaluated the associability of these PRF and CRF cues in new learning. However, as noted in the introduction, comparison of 5CSRT performance to CRF and PRF cues in Experiment 1 might also index the relative ability of such cues to control attentional processes in the performance of action. Next, we assessed the susceptibility of responding to the port cues to attentional challenges in which the duration or timing of those cues was manipulated. Rats then received CEA, MFC, PPC, or sham lesions, followed by repetition of the 5CSRT training and attentional challenge tests, which permitted assessment of the effects of these lesions on the allocation of attention in action. We hypothesized that rats with lesions of either CEA or the cholinergic innervation of MFC would show disruption of performance in the attentional challenges, relative to sham rats, but rats with lesions of the cholinergic innervation of PPC would not.
Method
Subjects
Twenty-eight male Long-Evans rats (Charles River Laboratories, Raleigh, NC), initially weighing 350-400 g, were individually housed in a climate-controlled vivarium on a 14:10-hr light/dark cycle (lights on at 07:00) with free access to water. They were fed ad libitum during acclimation to the vivarium and during postoperative recovery periods but throughout the remainder of the experimental training, they were given limited access to food to maintain their weights at 85% of free-feeding weights.
Surgery
After initial 5CSRT training, rats were anesthetized with isoflurane gas (Abbott Laboratories, North Chicago, IL), and stereotaxic surgery was conducted under aseptic conditions. Seven rats received bilateral ibotenic acid lesions of CEA, using stereotaxic coordinates 2.4 mm posterior to bregma and 4.35 mm from the midline, with infusions at a depth of 7.9 mm from the skull surface. Each CEA lesion was made using 0.25 μl of 10 μg/μl ibotenic acid (Sigma, St. Louis, MO) in PBS solution, infused with a Hamilton 2.0 μl syringe over a 6-min period. Lesions of the cholinergic innervation of PPC and MFC were made by infusing 192IgG-saporin (Chemicon, Temecula, CA) into these target regions. Because few if any neurons in the MFC or PPC express the low affinity nerve growth factor (NGF) receptor to which 192IgG-saporin binds, these lesions produce little cell loss in these cortical regions. Instead, the 192IgG-saporin binds to these receptors found on the cortically-located axon terminals of basal forebrain cholinergic neurons, and is transported back to their cell bodies (Bucci et al., 1998; Holley, Wiley, Lippi, & Sarter, 1994; Ohtake, Heckers, wiley, Lappi, Mesulam, & Geula, 1997). Thus, this procedure selectively removes basal forebrain cholinergic neurons that project to particular cortical target areas. Seven rats received bilateral infusions of 192IgG-saporin (0.25 μg/μl, dissolved in Dulbecco's saline) into the PPC, designed to reduce cholinergic input to that region from SI/nBM. Infusions were made at four sites in each hemisphere, with coordinates 4.0 and 4.7 mm posterior to bregma, 2.5 and 3.7 mm lateral to the midline at each AP coordinate, and 1.5 mm (medial sites) or 1.7 mm (lateral sites) below the skull surface. A volume of 0.25 μl was delivered to each site at a rate of 0.05 μl/min; the injector was left in place for 1 min before and 4 min after each infusion. Likewise, lesions of cholinergic input to medial prefrontal cortex from SI/nBM were made in 6 rats by infusing 192IgG-saporin to six sites in each hemisphere. The coordinates used were 2.9 and 3.4mm anterior to bregma, ± 1.5 mm from the midline, at depths of 2.9, 3.5, and 4.3 mm from the skull surface at each site, with the stereotaxic instrument angled 10 degrees to the midline. The volume of each anterior infusion was 0.15 μl and that of each posterior infusion was 0.10 μl. Finally, 8 rats received bilateral infusions of vehicle (PBS or Dulbecco's saline) into one of the three target regions (2 for CEA, and 3 each for MFC and PPC), conducted in the same manner as the lesions.
Apparatus
The behavioral training apparatus consisted of four individual 5CSRT chambers (25 × 25 × 25 cm; Cambridge Cognition, Cambridge, UK). Each chamber had aluminum front and side walls and a clear acrylic back wall and top. Five 2.5 × 2.5 × 4 cm (deep) stimulus/response ports were spaced 2.5 cm apart, and centered on the front, curved wall of the chamber, 2 cm above a grid floor. A 3w lamp at the back of each port provided port cues; responding in the ports was detected with infrared phototransistors. A recessed food cup was mounted in the center of the back wall of the chamber. It was fitted with a lamp that was illuminated when food pellets were delivered, and a transparent flap to detect food cup entries. A 3w overhead lamp mounted at the center of the chamber ceiling could be illuminated as a ready signal to indicate trials with the port cues. A speaker mounted next to that lamp permitted delivery of auditory signals in Experiment 2. Each chamber was enclosed in a sound-attenuated box where ventilation fans provided masking noise (70 dB). A bank of infrared LEDs provided background illumination for video monitoring and recording, but this illumination was invisible to the rats. A television camera was mounted within each box and images were recorded during behavioral training and testing.
Behavioral training procedures
The rats were first familiarized with the apparatus in four sessions. In the first 30-min session, food pellets (45 mg grain; Research Diets, Lancaster, New Hampshire) were present in the food cup, and the acrylic flap to the food cup was propped open. In the next 30-min session, food pellets were again placed in the food cup, but the flap door was not propped open. In addition, all five port lights were continuously illuminated, and food pellets were placed in each response port as well as in the food cup. In each of the next two 30-min sessions, there were 16 deliveries of a food pellet, accompanied by a 1-s illumination of the food cup light, to train the rats to collect food from the food cup.
In the baseline task used here, the beginning of a trial was signaled at random intervals by the illumination of the overhead lamp ready signal. After a constant 5 s ready period, one of the five target ports was illuminated for 500 ms. Each port was equally likely to be illuminated on any trial. The first response to the correct port within 5 s of port illumination was reinforced with the delivery of a food pellet to the food cup (accompanied by a 1-s illumination of the food cup) and the darkening of both the port (if still illuminated) and the ready signal. If no correct response was made before the end of the 5-s response window, the ready signal was darkened and the trial ended. Responses to the ports that were not illuminated on a trial were recorded as errors, but had no scheduled consequences. Sixty trials were presented in random order at predetermined intervals within each 30-min session; trial delivery was not affected by the rats' behavior. Each rat received two sessions daily, the first around 7:00 a.m. and the second around 3:00 p.m. Notably, in this procedure, comparable to those used by Holland et al. (2000) and Holland (in press), the delivery of all events (except for the food reinforcer) was independent of the rats' behavior. By contrast, in most implementations of the 5CSRT task, the initiation and termination of training and test trials is controlled by individual subjects' behaviors. For example, premature nose-pokes (those occurring prior to cue illumination) typically postpone or cancel trials, and errors (nose-pokes to an unilluminated port) terminate the trial without opportunity to perform the correct response. In an unpublished experiment similar to the present one, but in which errors terminated the trials, the occurrence of a large number of errors destroyed the intended 100% and 50% port stimulus-reinforcer contingencies for CRF and PRF cues. Thus, in Experiment 1, as in Holland et al.'s (2000) and Holland's (in press) studies, we elected not to impose such a time-out contingency.
Rats were shaped to this procedure gradually, but all rats received the same treatment (the shaping was not individualized). Between sessions, the duration of port illumination was reduced and the number of trials increased, from 30 s (6 of each port cue per session) in the first three training sessions to 500 ms (12 of each port cue per session) over the course of 9 sessions. When the target duration was 5 s or more, responses were effective throughout the illumination of the target stimuli. After 5 sessions with 500-ms port cue durations, the final reinforcement contingencies were introduced. For each rat, two of the ports were designated for consistent reinforcement (CRF), two for partial (50%) reinforcement (PRF) and one for no reinforcement (extinction, EXT). Each session included 12 trials with each port cue, as before. CRF trials were identical to those presented previously. Responding on half of the trials with the PRF port cues was reinforced as before, but on the other half of those trials, correct responses terminated the ready signal but did not produce food delivery or food cup illumination. On trials with the EXT port cues, “correct” responses had the same consequences as errors.
After 8-10 CRF/PRF training sessions, all rats received a series of attentional challenge sessions, to determine the effects of the partial reinforcement contingencies on the allocation of attention prior to surgery. In the first two challenge sessions, the port cue duration was reduced to 250 ms and 100 ms, respectively; otherwise these sessions were identical to the training sessions. Next, the rats were returned to the original 500-ms target condition for 2 sessions that were identical to the final training sessions. In the second two challenge sessions, the duration of the ready signal for each trial type was made variable, with equally-probable values of 1, 5, or 9 s. Each session included trials with one CRF port and one PRF port, as well as the EXT port. Otherwise, these sessions were similar to the training sessions. Finally, all rats received five additional sessions with the original CRF/PRF training conditions, as a preparation for Experiment 2.
Behavioral data analysis
Behavioral performance was assessed with several measures. The primary measures (reported here) were the percentage of trials on which at least one correct response occurred during the 5-sec interval after port light onset, and the percentage of trials on which at least one error occurred. In addition, we recorded several measures of port entry response latency (time since onset of the port light), premature responses (port entries that occurred during the ready signal but before onset of a port stimulus), and perseverative responses (port entries that occurred after food delivery). Although Holland et al. (2000) used a composite latency measure as their primary index of performance, in the current study, we elected to instead use the percentage of trials with a correct response as our primary measure. First, the composite measure is seldom reported in other studies that use the 5CSRT procedure, and in both the present study and that of Holland et al. (2000), statistical analyses showed similar results for both measures. Second and more important, the percentage of trials on which a correct response occurred was more informative for a key experimental variable in this study, reinforcement probability, because it also specifies the actual reinforcement probabilities received in different experimental conditions. Thus, rats that responded correctly on 90% of PRF trials actually received food on 45% (rather than the scheduled 50%) of those trials.
Separate analyses of variance (ANOVAs) were conducted for each of the response measures, followed by planned individual comparisons and post hoc tests using the Tukey honestly significant difference (HSD) procedure. The hypothesis that drove this research was that MFC is specialized for attention for action and PPC for attention for learning, with CEA modulating both functions, via its projections to SI/nBM. In Experiment 1, we evaluated the hypothesis that PPC-lesioned and VEH control rats would show similar behavioral profiles, whereas CEA- and MFC-lesioned rats should exhibit deficits relative to VEH and PPC-lesioned rats. Thus, in our evaluation of lesion effects in Experiment 1, we performed planned comparisons that contrasted performances of VEH and PPC rats with that of CEA and MFC rats. The level of significance adopted was p < 0.050.
Histology
After completion of behavioral testing in Experiment 2, the rats were anaesthetized and perfused with 0.9 % saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed, post-fixed and cryoprotected overnight in 4% paraformaldehyde in 0.1 M PB containing 12% sucrose, frozen with powdered dry ice, and stored at −80°C. Sections (30-μm) were taken from each brain on a freezing microtome and alternate sections were mounted on slides. One series was Nissl-stained and the other was stained for acetylcholinesterase (AChE), using a standard protocol (Bucci et al., 1998) adapted from Karnovsky and Root (1964). Lesions of CEA were evaluated by inspection of the Nissl-stained sections. Cholinergic lesions targeting cholinergic innervation of MFC and PPC were evaluated by examining relative AChE depletion in the AChE-stained sections. For each cortical region, 4 coronal sections were imaged with an Olympus BH-2 microscope and Micropublisher 3.3 camera (Qimaging, Burnaby, BC, Canada). The digitized images were then analyzed with ScionImage software. For each section, MFC or PPC was outlined on the image, and the optical density of that region (as calculated by the software) recorded. To compensate for differences in levels of staining, for each section, the optical density of a similarly-sized area in the striatum or hippocampus was also recorded. Adjusted optical density scores were then formed by taking the ratio of the optical densities of the target and comparison regions in each section. The adjusted scores were then averaged across sections for each brain hemisphere.
Results
Histology
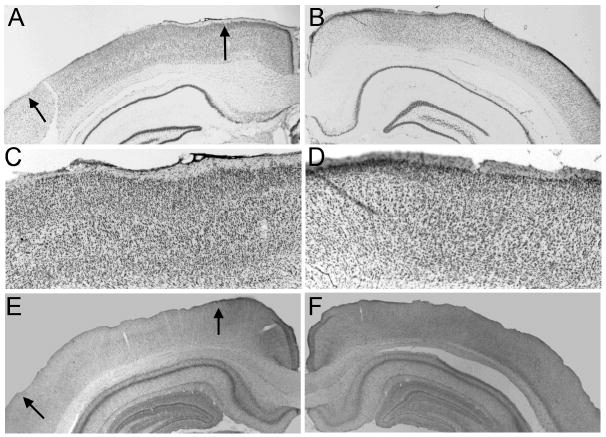
Five rats were judged as having acceptable bilateral lesions of CEA, averaging 60% damage to medial CEA and 45% damage to CEA overall (Figure 2). CEA lesions were rejected (n = 2) if there was less than 40% damage to the medial portion of CEA in either hemisphere, or if there was substantial bilateral damage to regions adjoining CEA. Although overall the extent of cholinergic depletion observed here was less than we have reported previously (Bucci et al., 1998; Chiba et al., 1995), 5 rats were judged as having acceptable lesions of MFC and 6 as having acceptable lesions of PPC, based on the distribution of the rats' adjusted optical density scores. Five of the 6 MFC-lesioned brains had adjusted MFC optical density scores (0.83 ± 0.02) lower than all of the comparable scores for the VEH control brains (1.12 ± 0.02); the remaining brain was rejected. Among the accepted brains, cholinergic depletion was most obvious in the infralimbic and prelimbic regions, but extended to the cingulate cortex dorsally or dorsal peduncular cortex ventrally in some cases. Likewise, 6 of the 7 PPC-lesioned brains had adjusted PPC optical density scores (0.82 ± 0.01) that were lower than all of the comparable scores for the VEH control brains (1.08 ± 0.02) in both hemispheres; the remaining brain was rejected. Thus, for both regions, lesioned and VEH rats had non-overlapping distributions of AChE staining. Notably, the optical density scores of the comparison (non-target) regions were similar in lesioned and VEH rats (133.79 ± 5.09 and 134.51 ± 5.72 for PPC comparisons, and 139.42 ± 5.78 and 136.62 ± 8.52 for MFC comparisons), justifying the use of the ratio measure. Finally, inspection of Nissl-stained sections of MFC-lesioned and PPC-lesioned brains showed no evidence for neuronal damage in those regions beyond mechanical damage around the injectors, which was found in both lesioned and sham-lesioned brains. Figure 3 (MFC) and Figure 4 (PPC) show Nissl-stained (Figures 2A, 2B, 2E, 2F, and 3A-D) and AChE-stained (Figures 2C, 2D, 2G, 2H and 3E-F) sections from the lesioned and sham-lesioned brains that displayed the median optical density score for each of the groups.
Figure 2.
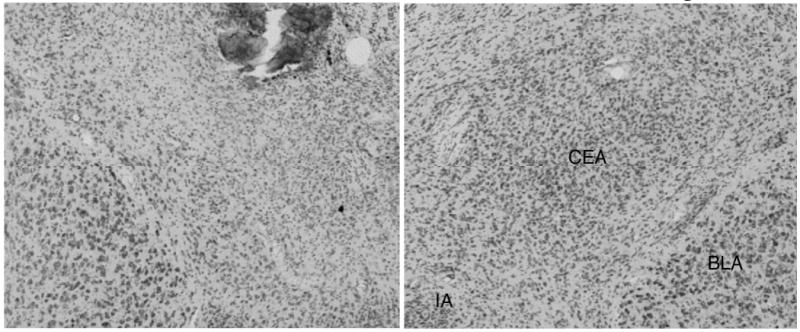
Photomicrographs of representative ibotenic acid (A) and sham (B) lesions of amygdala central nucleus (CEA). Substantial neuron loss is visible throughout the medial CEA, with some sparing in the lateral and capsular areas. Basolateral amygdala (BLA) and intercalated amygdalar (IA) are intact. Damage at the location of the injector tip was confined to immediately adjoining sections.
Figure 3.
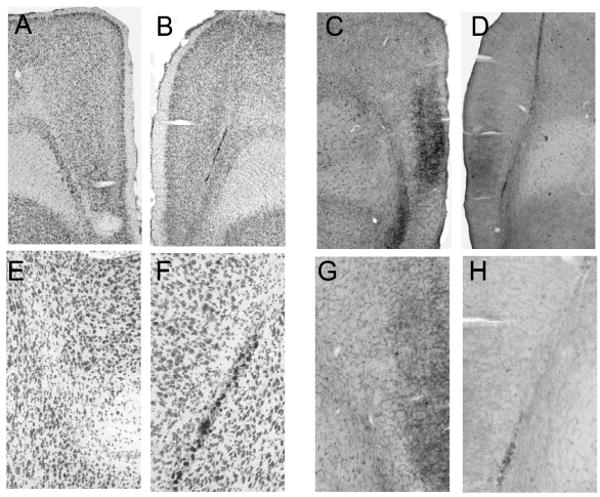
Photomicrographs of sections showing 192IgG-saporin (B, D, F, and H) and sham (A, C, E, G) lesions of the medial prefrontal cortex (MFC). Panels A-B and E-F show low- and high-power images (respectively) of Nissl-stained sections, and panels C-D and G-H show corresponding images of AChE-stained sections. The sections shown are from the brain with the median optical density ratio in each lesion condition. Although the Nissl-stained sections show no evidence for more than minimal neuronal damage around the injector in either lesioned or sham-lesioned brains, AChE staining in MFC is darker in the sham-lesioned rats.
Figure 4.
Photomicrographs of sections showing 192IgG-saporin (A, C, E) and sham (B, D, F) lesions of the posterior parietal cortex (PPC). Panels A-B and C-D show low- and high-power images (respectively) of Nissl-stained sections, and panels E-F show the corresponding low-power images of AChE-stained sections. Arrows mark boundaries of PPC. The sections shown are from the brain with the median optical density ratio in each lesion condition. Although the Nissl-stained sections show no evidence for more than minimal neuronal damage around the injector in either lesioned or sham-lesioned brains, AChE staining in PPC is darker in the sham-lesioned rats.
Behavior
Presurgical training
Over the course of the initial 16 training sessions, all rats adapted to the decreasing duration port cues; by the end of those sessions, they were responding correctly on 80-85% of the trials.
Presurgical cue duration challenge
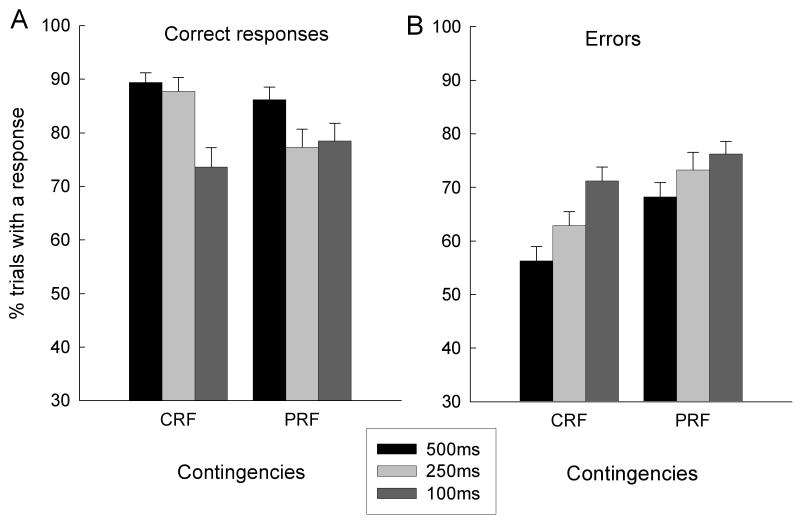
Figure 5 shows performance in the presurgical cue duration challenge test sessions. Port light duration was kept at the baseline value of 500 ms in the first and fourth of these sessions, and reduced to 250 ms and 100 ms in the second and third sessions, respectively. Overall, the rats displayed more correct responses and fewer errors to the CRF cues than to the PRF cues. More important, the rats made fewer correct responses (Figure 5A) and more errors (Figure 5B) in the challenge sessions, supporting the use of this manipulation as a means to provide an added demand on attentional processing.
Figure 5.
Mean (sem) percentage of trials with correct (A) and error (B) responses in the baseline (500 ms) and cue duration challenge sessions (250 and 100 ms) given prior to surgery in Experiment 1. CRF, consistently reinforced cues; PRF, partially reinforced cues.
Reinforcement contingency (CRF or PRF) × cue duration (500, 250, or 100 ms) × subsequent lesion condition ANOVAs, conducted separately for correct and error responses, revealed a main effect of cue duration for both correct responses, F(2,40) = 13.63, p < 0.001, and errors, F(2, 40) = 16.62, p < 0.001. For correct responses, the reinforcement contingency × cue duration interaction was significant, F(2,40) = 7.21, p < 0.003; PRF responding was more impaired by the 250-ms challenge than CRF responding, F(1, 20) = 7.95, p = 0.011. For errors, although there were significantly more errors to PRF cues than to CRF cues, F(1, 20) = 23.83, p < 0.001, the reinforcement contingency × cue duration interaction was not significant, F < 1. Finally, there were no significant main effects or interactions that involved the subsequent lesion condition, ps > 0.140.
Presurgical ready signal duration challenge
All rats also received a second challenge, in which the duration of the ready signal (usually 5s) was varied unpredictably across the values of 1, 5, or 9s. However, this challenge reduced the frequency of both correct and incorrect responding on trials with the 1-s ready stimulus, and had no significant effect on target responding on trials with the usual 5-s ready stimulus or the 9-s ready stimulus. Thus, we do not consider this challenge a reasonable assessment of the effects of attentional overload in this experiment, and we do not comment further on the results of these tests. Otherwise, the pattern of responding in these sessions was similar to that found in the baseline sessions described previously; the rats made more correct responses to the CRF cues than to the PRF cues.
Lesion effects on 5CSRT retraining
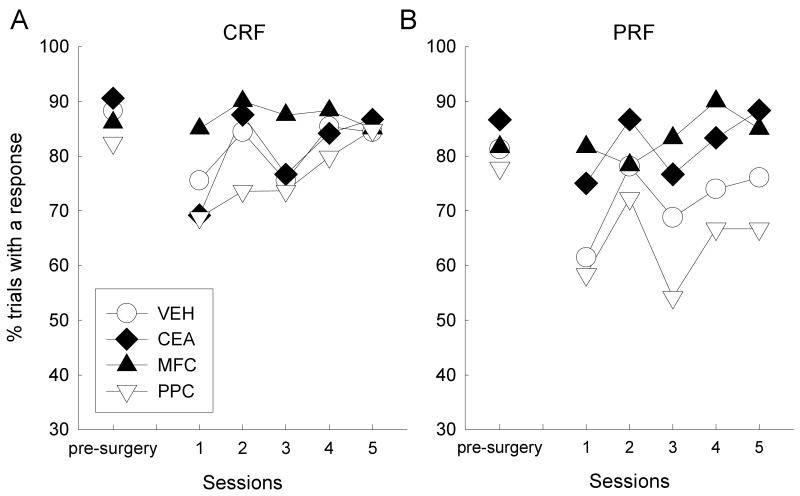
Figure 6 shows correct response performance averaged over the last three baseline sessions before surgery, as well as performance in each of the five baseline retraining sessions after surgery, in each lesion condition. Behavior in the presurgical baseline sessions (leftmost points in each of the panels of Figure 6) was similar to that in the challenge baseline sessions described earlier. The patterns of behavior observed post-surgically differed across lesion and reinforcement contingency conditions. Responding to the CRF cues (Figure 6a) was initially suppressed in all groups except the MFC-lesioned group, but performance of all groups rapidly returned to high levels over the course of retraining. By contrast, responding to the PRF cues (Figure 6b) was initially suppressed in the VEH and PPC groups, but not in the CEA or MFC groups; furthermore, although responding to the PRF cues increased over the course of retraining, these differences were maintained.
Figure 6.
Percentage of trials with a correct response during the last 3 baseline training sessions (combined) prior to surgery, and the first 5 post-surgical baseline recovery sessions. CRF, consistently reinforced cues; PRF, partially reinforced cues; VEH, vehicle (sham) lesion controls; CEA, lesions of amygdala central nucleus; MFC, lesions of medial prefrontal cortex; PPC, lesions of posterior parietal cortex.
Thus, a major enduring effect of the surgical procedures and consequent passage of time was to reduce the apparent sensitivity of the rats in the MFC and CEA lesion groups to the PRF contingencies; those rats responded similarly to CRF and PRF cues. By contrast, rats in the VEH and PPC conditions responded more to CRF than to PRF cues, as they did before surgery, although those differences were greater after surgery. A lesion × reinforcement contingency × session ANOVA showed significant effects of contingency, F(1, 20) = 10.87, p = 0.004, and session, F(4, 80) = 4.31, p = 0.003. The planned contrast of performance of rats in Groups VEH and PPC with that of rats in Groups CEA and MFC was significant for responding to the PRF cues, F(1, 20) = 6.31, p = 0.021, and for the differences between CRF and PRF responding, F(1, 20) = 7.06, p = 0.015, but not for CRF responding, F(1, 20) = 1.69, p = 0.209.
Unlike with correct responses, the surgery and intervening time period had no effect on the frequency of errors (not shown). ANOVA of errors post-surgery revealed no significant effects or interactions involving lesion, Fs (3, 20) < 1.71, ps > 0.157. As with the analysis of errors prior to surgery, there was a significant effect of contingency, F(1, 20) = 10.84, p = 0.004, (more errors with PRF cues).
Post-surgical cue duration challenge
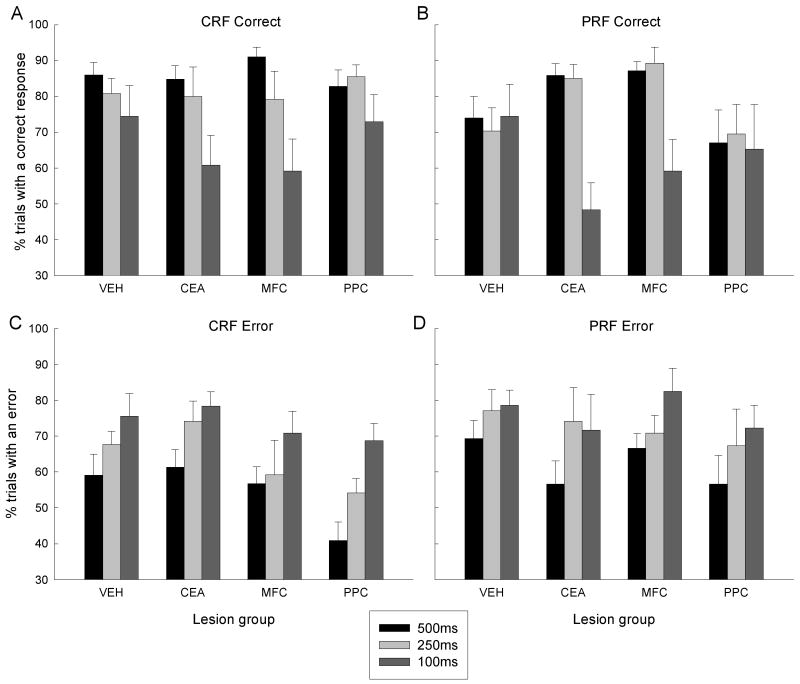
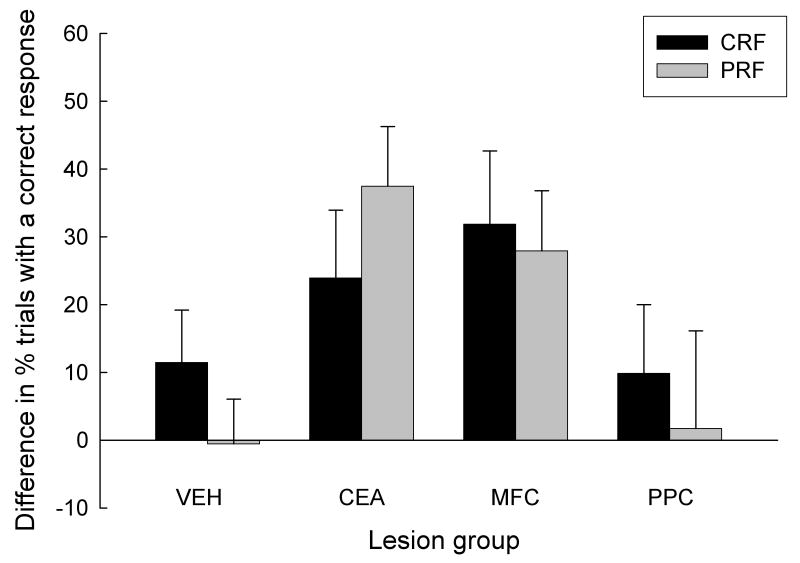
After post-surgical recovery of baseline performance, the rats received cue duration challenge tests identical to that given prior to surgery. In the first and last test sessions, which served as a baseline, cue durations were 500 ms, and in the second and third session, the cues were 250 ms and 100 ms, respectively. As in the presurgical challenges (Figure 5), relative to baseline responding, the rats made fewer correct responses and more errors when the cue durations were reduced (Figures 7-8). Additionally, across the board, the rats displayed more correct responses and fewer errors to consistently reinforced cues than to partially reinforced cues. Most important, the effects of cue duration and reinforcement contingency differed among the different lesion groups. Consistent with our proposal that a system including CEA and MFC mediates attention for action, the disruptive effect of reducing cue duration was greater in the MFC and CEA groups than in the VEH and PPC groups. Furthermore, this greater susceptibility of CEA and MFC rats to the reduction of cue duration to 100 ms extended to responding to the PRF cues, even though those rats showed greater responding to those cues under baseline and 250-ms cue conditions. Thus, the effects of reinforcement contingency (CRF or PRF) depended on cue duration and lesion condition. Figure 8 summarizes the effects of reduction in cue duration on CRF and PRF cues in each of the lesion conditions, by presenting 500-ms cue minus 100-ms cue response difference scores.
Figure 7.
Mean (sem) percentage of trials with a correct (A and B) or error (C and D) response in the baseline (500 ms) and cue duration challenge sessions (250 and 100 ms) given after surgery in Experiment 1. CRF, consistently reinforced cues; PRF, partially reinforced cues; VEH, vehicle (sham) lesion controls; CEA, lesions of amygdala central nucleus; MFC, lesions of medial prefrontal cortex; PPC, lesions of posterior parietal cortex.
Figure 8.
Mean (sem) difference in percent correct responses between the 500-ms (baseline) and 100-ms cue duration challenge sessions that were given after surgery in Experiment 1. CRF, consistently reinforced cues; PRF, partially reinforced cues; VEH, vehicle (sham) lesion controls; CEA, lesions of amygdala central nucleus; MFC, lesions of medial prefrontal cortex; PPC, lesions of posterior parietal cortex.
An ANOVA of correct responses, with variables of lesion, reinforcement contingency, and cue duration, revealed significant main effects of reinforcement contingency, F(1, 20) = 4.61, p = 0.044, and cue duration, F(2, 40) = 12.72, p < 0.001. Analysis of responding in the baseline sessions alone showed a pattern similar to that reported earlier for the surgical recovery sessions: the difference between responding on CRF and PRF trials was greater in VEH and PPC rats than in CEA and MFC rats, F(1,20) = 8.10, p = 0.010. Performance in the 250-ms cue condition was similar to baseline responding; an ANOVA that contrasted performance in the baseline and 250-ms cue conditions showed no significant main effects or interactions with cue duration, Fs < 1, ps > 0.400. Furthermore, as in the baseline sessions, the difference between responding on 250-ms CRF and PRF trials was greater in VEH and PPC rats than in CEA and MFC rats, F(1,20) = 8.90, p = 0.007. Nevertheless, performance was substantially degraded in the 100-ms cue test. An ANOVA that contrasted the 100-ms cue duration condition with the baseline showed a significant cue duration effect, F(1,20) = 17.33, p < 0.001. Most important, as predicted, the performance decrement observed with the 100-ms cue was greater in the MPC and CEA rats than in the VEH and PPC rats, F(1,20) = 8.02, p < 0.020. These lesion-dependent effects of cue duration were noted for both CRF and PRF responding; a similar comparison that contrasted the magnitude of disruption for CRF and PRF cues was not significant, F < 1, p > 0.394.
Although errors were affected by both reinforcement contingency, F(1,20) = 7.00, p = 0.016, and cue duration, F(2,40) = 11.56, p < 0.001, they were unaffected by the lesions, F(1, 20) = 1.01, p = 0.406. The planned CEA + MFC vs. PPC + VEH contrast described for correct responding was not significant for errors, F < 1, p = 0.787.
Other response measures
As in Holland et al.'s (2000) study, the pattern of deficits observed in lesioned rats with response latency measures (not shown) were comparable to those observed with the accuracy measures presented here. However, other measures of 5CSRT responding, including omissions (trials on which no response occurred), premature responses (responses made prior to port cue onset), and perseverative responses (responses to port cues made after delivery of the food reinforcers), were unaffected by the lesions, in either baseline or challenge sessions. Separate lesion × contingency × cue duration ANOVAs for premature and perseverative responses showed no significant effects or interactions involving lesion or cue duration, Fs < 1, but a significant effect of reinforcement contingency for the relatively uncommon perseverative responses, F(1, 24) = 9.49, p < 0.001. Mean ± sem perseverative responses on CRF trials were 0.13 ± 0.04, and 0.04 ± 0.01 on PRF trials.
Discussion
As in previous experiments, reductions in cue light duration reduced the frequency of correct responses and increased the frequency of errors. Thus, deficits produced by the cue duration challenge provided a measure of attention in responding to the cue lights. By this measure, lesions of the CEA or of the cholinergic innervation of MFC, but not lesions of the cholinergic innervation of PPC, impaired attention in this task: the deficits produced by the cue duration challenge were significantly greater in the rats with CEA or MFC lesions than in those with PPC or sham lesions. Thus, these results are consistent with our assertion that attention for action is modulated by SI/nBM's cholinergic projections to MFC, but not those to PPC.
In a previous study that used 5CSRT training procedures identical to those used here, Holland (in press) found that in intact rats, responding to PRF cues was more susceptible to attentional challenges than responding to CRF cues. That observation was consistent with the notion that less predictive cues should command less attention for purposes of controlling action than cues that are highly predictive of important events such as food. The less attention paid to an event, the more that attention would be susceptible to challenge. Unfortunately, the evidence that CRF cues capture more attention for action than PRF cues was less convincing in the present study. Although intact rats (presurgically) and sham-lesioned rats responded significantly less to the PRF cues than to the CRF cues, the evidence that PRF responding was especially disrupted by the cue duration challenge was weaker: the contingency × cue duration interaction was significant only in the presurgical challenges. Nevertheless, as in Holland's (in press) study, rats with lesions that were expected to disrupt attention for action showed comparable impairments of both CRF and PRF responding in the cue duration challenges.
Despite these disruptive effects of CEA or MFC lesions on responding under conditions of attentional challenge (reduced cue duration), under the training conditions (500-ms cues), rats with those lesions showed more responding to the PRF cues than the other two groups. Indeed, that difference, which we have also observed in rats with lesions that disconnect CEA and SI/nBM (Holland, in press) and in rats with bilateral lesions of MFC itself (Maddux & Holland, 2004), was evident immediately after recovery from surgery. The basis of this apparently reduced sensitivity to PRF contingencies is unclear. Although it could be argued that damage to the innervation of MFC produced greater “impulsiveness” or interfered with extinction learning on nonreinforced PRF trials, or with the suppresion of action of excitatory learning (Quirk, Russo, Barron, & Lebron, 2000), CEA has not been implicated in such learning or response inhibition (Falls & Davis, 1995). Furthermore, three phenomena that potentially could contribute to port light responding, autoshaping (Collins & Pearce, 1985; Parkinson, Robbins, & Everitt, 2000), conditioned orienting (Gallagher, Graham, & Holland, 1990; Kaye & Pearce, 1984), and frustrative nonreward (Henke, 1977) are all disrupted, rather than facilitated, by CEA lesions.
Our general claim that MFC cholinergic lesions produced attention deficits in this task is consistent with the conclusions of other investigators. However, it is notable that the nature of the deficits observed varied among these studies. We found that these lesions had no effect on baseline (0.50-s cue duration) 5CSRT accuracy but reduced accuracy when the cue duration was reduced to 0.10 s. By contrast, although Dalley et al. (2004) found that rats with 192IgG-saporin lesions of MFC showed reduced accuracy with another attentional challenge, increased event presentation rate, they found no effect of those lesions on performance when cue duration was shortened to 0.125 s. Similarly, Chudasama, Dalley, Nathwani, Bouger, and Robbins (2004) found that reductions in cue duration (to 0.25 sec) differentially affected response accuracy of control rats and rats with 192IgG-saporin lesions of MFC only if the 5CSRT task also included a significant working memory component. Furthermore, Muir et al. (1996) and Passetti, Chudasama, and Robbins (2002) found that non-selective (quinolinic acid) lesions of MFC reduced 5CSRT accuracy even under baseline training conditions. Moreover, recent studies show that variations in lesion placement within MFC can produce substantial variations in the pattern of deficits observed (Passetti, Chudasama, & Robbins, 2002). At the same time, in each of the studies just described, those investigators found lesion-induced deficits in one or more other response measures, such as premature or perseverative responses, or response omission, which we did not observe. Of course, unlike in those experiments, in which errors, premature, or perseverative responses resulted in delay or cancellation of rewarded trial opportunities, in Experiment 1 these responses had no scheduled consequences. Thus, it is not surprising that we did not find these measures to be lesion-sensitive.
Experiment 2
Experiment 2 examined the effects of CEA, MFC and PPC lesions on attention in new learning, using cues trained in Experiment 1. Immediately after the final retraining sessions of Experiment 1, all rats received Pavlovian conditioning sessions in which two auditory-visual compound stimuli were consistently paired with the delivery of a new, larger and more palatable reinforcer. One compound included a port cue that had been trained with CRF contingencies in Experiment 1, and the other included a port cue that had been trained with PRF contingencies. If training with the PRF contingency in Experiment 1 enhances the associability of that port cue relative to the CRF cue, as predicted by the Pearce-Hall (1980) model, then the PRF cue should especially overshadow new Pavlovian learning of food cup entry CRs to the auditory cue it accompanied. Thus, in normal rats, the tone accompanied by the PRF cue should show less acquisition of conditioned responding than the tone partnered with the CRF cue. We chose to assess the associability of the port cues indirectly, by measuring their ability to overshadow conditioning to a tone rather than their ability to acquire new CRs, to avoid confounding the effects of new learning about the port cues with the performance of port-directed CRs already established to those cues in Experiment 1.
According to our view of the role of CEA and SI/nBM's cholinergic projections to cortex in controlled attention for new learning, in rats with lesions of CEA or of PPC's cholinergic afferents, training with PRF contingencies in Experiment 1 should not enhance port light associability for new learning in Experiment 2, relative to that of port lights trained with CRF contingencies. Thus, rats with these lesions should fail to show differential overshadowing of the two tones. By contrast, rats with lesions of the cholinergic projections to MFC should be unimpaired in this task, despite their impairments in performance to the same cues in the attentional challenge of Experiment 1.
Method
The subjects and apparatus were those used in Experiment 1.
Each of four 32-min Pavlovian conditioning sessions included 4 of each of two trial types. Each trial began with a 5-s illumination of the overhead light, as in 5CSRT training. Then, on CRF trials, a compound of one of the previously-established CRF port cues and either an 80-db, 1000-hz (low) tone or an 80-db 4000-hz (high) tone (counterbalanced), was presented for 5 s, and followed by termination of the overhead light and the delivery of 6 45-mg fruit-punch pellets (Research Diets) over a 3-s period. PRF trials were similar, except that the 5-s compound CS included one of the PRF port cues and the other tone. For each rat, only one of the previously-trained CRF cues and one of the PRF cues were used, and every trial with each compound was reinforced with food. Thus, the CRF and PRF designations referred to the past 5CSRT training history of the port lights, and not the Pavlovian contingencies present in Experiment 2, which were the same for both compounds. Finally, the rats received two 32-min test sessions to assess responding to the 5-s tones and 5-s port lights, separately. Test 1 included 4 presentations of each 5-s tone, randomly intermixed, and test 2 included 4 presentations of each of the two port lights, randomly intermixed. In both tests, each cue was preceded and accompanied by the overhead light, as in Pavlovian training.
The measure of food cup responding was the amount of time spent in the food cup (as recorded by a switch activated by the clear acrylic flap over the cup), expressed as a percentage of the 5-s CS duration.
Results
Pavlovian compound training
Acquisition of food cup responding over the course of the four Pavlovian training sessions was rapid, reaching 41.0 ± 4.8% during the compound that included the CRF cue and 38.46 ± 4.8% during the compound that included the PRF cue. A lesion × port light contingency × tone (high or low) × session ANOVA showed only significant effects of session, F(3, 48) = 26.63, p < 0.001, and of tone, F(1, 16) = 7.10, p = 0.017; compounds that included the high tone (42.9 ± 4.9%) generated more responding than those that included the low tone (36.4 ± 4.5%).
Tone test
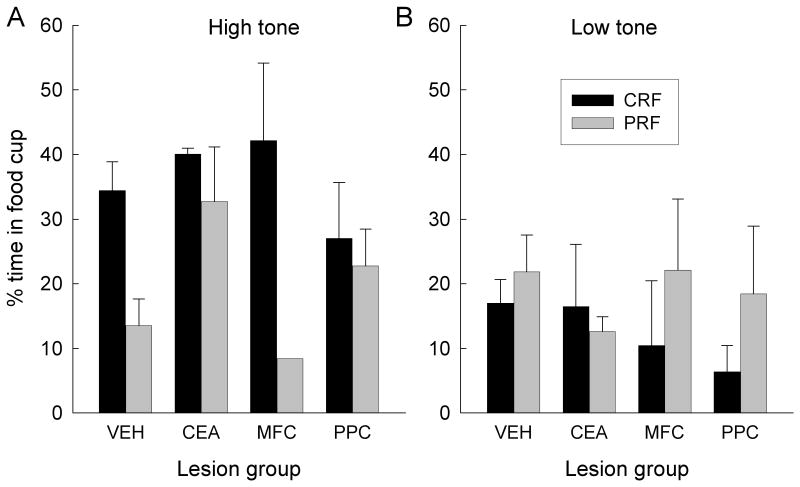
The primary data of Experiment 2 are the results of the test of food-cup responding acquired to the two tone CSs, which were paired with food in compound with either CRF or PRF port lights. We used acquisition of responding to the tones as an indirect measure of attention to the port lights: the more attention was directed to a port light, the less attention, and hence less conditioning, would accrue to the tone trained in compound with that light.
Unexpectedly, very low levels of food cup CRs were displayed to the low tone in the test session, and these low levels were unaffected by contingency or lesion condition (Figure 9B). Nevertheless, substantial food cup responding was acquired to the high tone; moreover, this responding depended on both reinforcement contingency and lesion condition (Figure 9A.). Thus, we restricted our subsequent analyses to responding of each rat to the high tone. Consistent with the hypothesis driving this experiment, in the VEH and MFC groups, the PRF cue overshadowed conditioning of the high tone more than the CRF cue. That is, less food cup responding was acquired to the high tone if it had been trained in compound with the PRF cue than if it had been trained in compound with the CRF cue. By contrast, in the CEA and PPC groups, the PRF cue did not overshadow conditioning to the high tone any more than the CRF cue; high levels of responding were acquired to both tones.
Figure 9.
Mean (sem) percentage time in the food cup during the test of responding to the high and low frequency tones in Experiment 2. The bars labeled CRF show responding to tones that were trained in compound with a port cue that had been consistently-reinforced in Experiment 1, and the bars labeled PRF show responding to tones that had been trained in compound with a port cue that had been partially-reinforced in Experiment 1. VEH, vehicle (sham) lesion controls; CEA, lesions of amygdala central nucleus; MFC, lesions of medial prefrontal cortex; PPC, lesions of posterior parietal cortex.
An initial lesion × contingency × tone identity ANOVA revealed a significant interaction of contingency and tone identity, F(1, 16) = 22.79, p < 0.001. Thus, we then conducted separate ANOVAs for the low tone and the high tone, each with the variables of lesion and contingency. The low tone ANOVA showed no significant effects, Fs < 1, whereas the high tone ANOVA showed a significant main effect of contingency, F(1, 16) = 6.52, p = 0.021. More important, a planned comparison, which evaluated our hypothesis that the PRF cue would overshadow tone conditioning more than the CRF cue in VEH and MFC-lesioned rats, but not in CEA- or PPC-lesioned rats, was significant, F(1, 16) = 7.44, p = 0.015.
Port light test
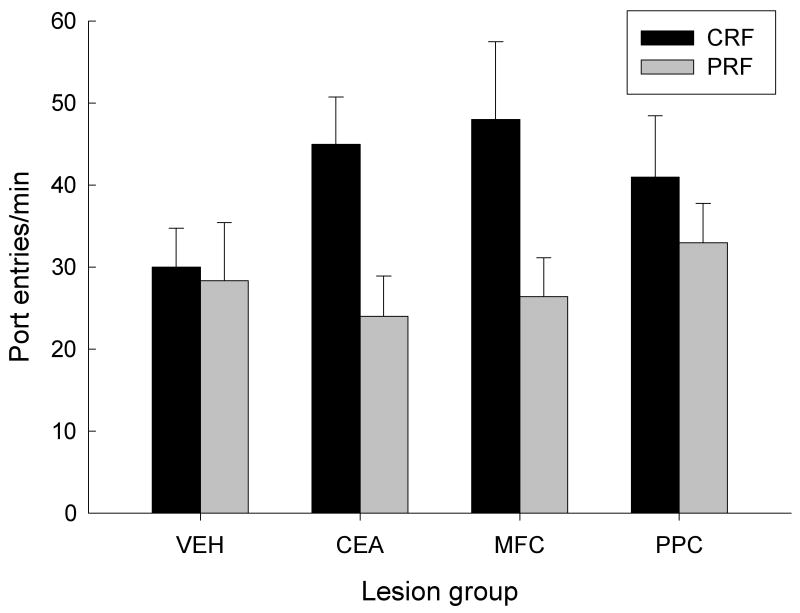
After the test of responding to the two tones, we examined food cup and port entry responding to the CRF and PRF port cues that were used in Experiment 2. Although it would be reasonable to expect that the display of food cup responding to the port lights in this test would provide a direct measure of new Pavlovian learning to the port lights, there was little evidence for the acquisition of food cup CRs in any contingency or lesion condition; these CRs ranged from 7.5 ± 1.7% to 12.4 ± 5.3%, and did not differ from baseline (pre-CS) levels of responding, nor among lesion or contingency conditions, Fs < 1.28, ps > 0.312. At the same time, we expected that port entry responses might also reflect the learning acquired in Experiment 1's 5CSRT task. Figure 10 shows the test rates of port entry during the 5-sec port light cues. PRF port responding was depressed relative to CRF responding in the CEA- and MFC-lesioned groups, but not in the VEH or PPC groups. This observation is consistent with our conclusion in Experiment 1, that attention for action for PRF cues was especially vulnerable to challenge in rats with CEA or MFC lesions.
Figure 10.
Mean port entry rates during the final test of responding to the port lights in Experiment 2. The bars labeled CRF show responding to the port cue that had been consistently-reinforced in Experiment 1, and the bars labeled PRF show responding to the port cue that had been partially-reinforced in Experiment 1. VEH, vehicle (sham) lesion controls; CEA, lesions of amygdala central nucleus; MFC, lesions of medial prefrontal cortex; PPC, lesions of posterior parietal cortex.
A lesion × contingency × prior tone partner (high or low) ANOVA revealed a main effect of contingency, F(1, 16) = 8.30, p < 0.011. Notably, neither prior tone partner nor any of that variable's interactions with other variables was significant, Fs < 1, ps > 0.619, in this test. Most important, a planned contrast (as in Experiment 1) of VEH and PPC responding with CEA and MFC responding for the superiority of CRF over PRF responding was significant, F(1, 16) = 5.62, p = 0.031.
Discussion
As predicted, in sham-lesioned rats, port light cues previously trained with PRF contingencies overshadowed new conditioning of food cup responding to the high tone more than did port light cues trained with CRF contingencies. By contrast, no such differential overshadowing was observed in rats with lesions of CEA or cholinergic afferents of PPC. This result is consistent with our previous observations using a serial conditioning task in which the associability of a light cue was assessed by the rate of acquisition of new Pavlovian light-food learning, after prior arrangement of various light-tone relations (Wilson et al., 1992). In that task, the associability of the light was higher after training in which it was followed by a tone on 50% of trials and by nothing on 50% of the trials (PRF) than after training in which it was consistently followed by the tone (CRF). As noted in the introduction, this enhanced associability after PRF training was absent in rats with lesions of CEA (Holland & Gallagher, 1993), the SI/nBM (Chiba et al., 1995), or SI/nBM's cholinergic efferents to PPC (Bucci et al., 1998). Thus, the results of Experiment 2 are consistent with our previous claims (Holland & Gallagher, 1999) that a circuit that includes those brain regions is critical for maintaining or enhancing attention under conditions of prediction error (surprise).
Notably, rats with lesions of MFC's cholinergic afferents performed normally in Experiment 2, with the PRF-trained port light overshadowing new learning to the high tone more than the CRF-trained port. Recall that in Experiment 1, responding of these rats to this same PRF cue was disrupted by the attentional challenge, relative to responding in Groups VEH and PPC. Thus, cholinergic deafferentation of MFC impaired automatic attention for action but not controlled attention for new learning. Thus, although important for aspects of attention for action, cholinergic afferents to MFC are apparently not a critical part of circuitry used in the surprised-induced enhancement of CS associability in new learning.
An unexpected outcome of Experiment 2 was the rats' failure to acquire much food cup responding to the low tone, as revealed in the tone-alone tests. One account for this observation is that this tone was of very low salience and hence was especially overshadowed by conditioning to the port lights. However, there was no evidence for greater food cup or port responding to the port lights that accompanied the low tone, rather than the high tone. At the same time, acquisition responding to the compounds that included the high and low tones differed only minimally, suggesting either that the rats formed a light + low tone configural cue in which the identity of the constituent elements was lost (Pearce, 1987), or that responding to the port light cues in the final test did not adequately reflect new learning to those cues. Regardless of the source for this lack of conditioning to the elements of the compounds that included the low tone, it is worth pointing out that it was unaffected by the lesion condition.
Finally, port entry responding to the port cues themselves reflected the lesion grouping found in Experiment 1, with a greater difference in the frequency of port entries during the CRF and PRF cues in rats with CEA and MFC lesions than in rats with sham (VEH) or PPC lesions. This outcome would be expected if responding to the ports reflected action based on learning acquired in Experiment 1, rather than new Pavlovian learning in Experiment 2, for example conditioned orienting (Holland, 1977) or autoshaping (Parkinson et al., 2000) to the port lights paired with food. Although we can not rule out the possibility that port entry responding in this test primarily reflected new learning, several features of these data are inconsistent with that possibility. First, previous data would suggest that CEA lesions but not PPC lesions (Bucci & Chess, 2005; Gallagher et al., 1990) would prevent the acquisition of new ORs or autoshaped responses regardless of the prior CRF/PRF history of the cues. Second, PPC lesions would be expected to prevent any enhanced new learning based on prior PRF contingencies (Bucci et al., 1998). Neither of these outcomes was observed here with port cue responses. Nevertheless, if as we suspect, port cue responding primarily reflected learning established in Experiment 1, a puzzling feature of that responding remains. In the port cue test of Experiment 2, the difference between CRF and PRF response rates was exaggerated in Groups CEA and MFC relative to Groups VEH and PPC. By contrast, in Experiment 1, the difference in CRF and PRF response probabilities under baseline conditions was smaller in Groups CEA and MFC than in the other two groups; only in the challenge tests did the CEA- and MFC-lesioned rats respond less than VEH and PPC rats. Of course, it is difficult to compare port cue responding in the two experiments, which differed in their response contingency (operant reward vs response-independent Pavlovian), response measure (probability of at least one response vs response rate), and cue durations (500 ms vs 5000 ms).
General Discussion
The results of these experiments supported a distinction between two aspects of attention in associative learning, one related to the control of action by previously-trained cues under conditions of enhanced attentional demand (Experiment 1), and one concerned with acquisition of new learning (Experiment 2). In Experiment 1, we examined rats' ability to respond accurately in a 5CSRT cued response task when the duration of the cue was reduced. All rats showed reduced correct responding and increased errors in the face of this attentional challenge, but rats with lesions of the CEA or the cholinergic innervation of the MFC were especially impaired, relative to sham-lesioned control rats and rats with lesions of the cholinergic innervation of the PPC (which showed no such impairments relative to sham-lesioned controls).
In Experiment 2, we examined the effects of Experiment 1's training with CRF or PRF contingencies on the ability of the port cues to overshadow new Pavlovian tone-food learning. In sham-lesioned rats, cues that were trained with PRF contingencies in Experiment 1 overshadowed tone learning more than cues that had been trained with CRF contingencies in Experiment 1. Thus, consistent with previous studies with other conditioning procedures (e.g., Kaye & Pearce, 1984; Wilson et al., 1992), for purposes of new learning, the PRF cues commanded more attention than the CRF cues. Notably, this enhanced attention to PRF cues was also observed for rats with lesions of the cholinergic innervation of MFC, rats that were impaired in their ability to maintain attention to the port cues in the face of a cue duration challenge. By contrast, rats with lesions of CEA or the cholinergic innervation of PPC failed to show such differential overshadowing. Thus, in those rats, PRF contingencies failed to enhance attention to those cues for purposes of new learning. Notably, the rats with PPC lesions were unimpaired in their ability to maintain attention to the port cues in the cue duration challenge of Experiment 1.
It is important to note that the dissociation of action and learning effects reflects changes in attention that occurred contemporaneously in the same subjects, with the same cues and training procedures. Although the ability of the CRF and PRF port cues to command attention in new learning was assessed in Experiment 2, the training that established the different associabilities of the CRF and PRF cues occurred in Experiment 1. Thus, in MFC-lesioned rats, the same Experiment 1 training that produced normal enhancement of the associability of the PRF cues in Experiment 2 left those rats deficient in their ability to respond to PRF cues in the attentional challenge of Experiment 1. Likewise, in PPC-lesioned rats, the same training that produced a normal response to the attentional challenge of Experiment 1failed to produce enhancements of the associability of the PRF cues in Experiment 2.
Thus, our findings provide strong support for a role of CEA in both attention for action (Holland et al., 2000) and attention for new learning (Holland & Gallagher, 1993), and a double dissociation at the cortical level. The cholinergic innervation of MFC was important for the maintenance of action, but not for the modulation of attention for new learning, whereas the cholinergic innervation of PPC was critical to the modulation of attention for new learning but not to the maintenance of attention for action.
Considerable evidence links magnocellular cholinergic neurons in SI/nBM (lesioned in this study) to widespread modulation of cortical activity (e.g. Everitt & Robbins, 1997; Sarter & Bruno, 1997; Sarter, Hasselmo, Bruno, & Givens, 2005; Whalen, Kapp, & Pascoe, 1984), including the activity of a number of regions implicated in various human attention networks (e.g., Posner & Petersen, 1990). For example, as noted earlier, cholinergic-specific lesions of either SI/nBM (McGaughy et al., 2002) or MFC (Dalley et al., 2004) produce deficits in 5CSRT task performance. Moreover, a number of investigators have observed increased acetylcholine efflux in cortical regions (including frontal and parietal areas, specifically) during performance of visual attention tasks (Arnold, Burk, Hodgson, Sarter, & Bruno, 2002; Himmelheber, Sarter, & Bruno, 2000; Himmelheber, Sarter, & Bruno, 2001; Kozak, Bruno, & Sarter, 2006; Passetti, Dalley, O'Connell, Everitt, & Robbins, 2000). Similarly, Apparsundaram, Martinez, Parikh, Kozak, & Sarter (2005), found increased capacity and density of choline transporters on the synaptic membranes of right medial prefrontal cortical neurons in rats performing a sustained attention task. Many of these task-induced changes in cortical cholinergic activity have also been shown to depend on input from SI/nBM. For example, McGaughy et al. (2002) found that cholinergic-specific SI/nBM lesions produced both deficits in behavior and reduced acetylcholine efflux in the mPFC during performance of a 5CSRT task. In a related fashion, Gill, Sarter, & Givens (2000), using single unit recording during a sustained attention task in rats, found that unilateral cholinergic deafferentation produced a decrease in the overall firing rate of medial prefrontal neurons and a reduced correlation between firing patterns and behavioral performance. Taken together, these findings all support a role of basal forebrain modulation of cortical cholinergic function, specifically in regard to attentional processes.
The data presented in this article complement and extend these findings by indicating that modulation of the activity of frontal and parietal regions by cholinergic SI/nBM neurons may serve distinct attentional functions in the context of simple associative learning. The double-dissociation in the effects of 192IgG-saporin infusions into PPC and MFC further indicates that separate subpopulations of SI/nBM neurons within SI/nBM are responsible for these complementary aspects of attention. Notably, if the same SI/nBM neurons served both functions, projecting to both PPC and MFC, infusions of 192IgG-saporin into either of those cortical regions would destroy the same SI/nBM neurons, and hence would have the same behavioral effects. The dissociation in lesion effects we observed is consistent with the results of anatomical studies (Gritti, Mainville, Mancia, & Jones, 1997; Saper, 1984), including double-labeling retrograde tracer studies (Bigl, Woolf, & Butcher, 1982; Price & Stern, 1983), which show little evidence for collateralization of axons of SI/nBM neurons, and reveal very limited cortical projection fields of individual SI/nBM neurons. At the same time, these subpopulations of neurons, which project to vastly different neocortical regions, are largely intermingled throughout SI/nBM, showing little evidence of topographical organization. Finally, the observations that both “action” and “learning” aspects of attention in associative learning were altered in the present experiments by CEA lesions, and by disconnection of CEA from SI/nBM cholinergic neurons in previous studies (Han et al., 1999; Holland, in press) indicate that CEA modulates the activity of both of these subpopulations.
Most views of attention in human information processing describe it as a collection of interrelated processes that converge to produce task-relevant behaviors. These processes may be event-driven (bottom-up) or goal-driven (top down), and include event detection, orienting, perceptual analysis, selection of items to be further processed or ignored, aspects of working memory, vigilance, decision and response selection, response production and others (e.g., Allport, 1989; Awh & Jonides, 2001; Egeth & Yantis, 1997; Jonides, Lacey, & Nee, 2005, Pashler, 1998). Anatomically, these processes are often thought to be served by networks of brain regions, each of which may implement different aspects of attentional processing (e.g., Posner & Dahaene, 1994; Posner & Petersen, 1990). Our observation that selection of attention for new learning and for directed action in associative learning are mediated by different cortical regions is consistent with this general statement. Whether the parietal and frontal (respectively) systems we examined in this study relate to the parietal and frontal attention networks described in humans remains to be seen. Many findings suggest caution. For example, most tasks used to study attention in humans can be characterized as assessing attention in the selection of action, rather than the selective acquisition of new information. Nevertheless, performance in many of these tasks produces parietal activation in humans, and is impaired in patients with parietal damage. Indeed, performance in response selection tasks in which cue validity is manipulated (as in Experiment 1) is typically attributed to the activity of parietal systems (e.g., Posner & Petersen, 1990; Posner, Walker, Friedrich, & Rafal, 1984).
Similarly, in humans, MFC has been reported to be sensitive to reward prediction error, which we relate to attention in new learning. Potts, Martin, Burton, and Montague (2006) found that that the event-related potential (ERP) recorded from medial frontal locations was most positive when an unpredicted reward was delivered and most negative when a predicted reward was omitted. Potts et al. (2006) placed these results in the framework of a gating hypothesis (Cohen, Braver, & Brown, 2002) whereby midbrain dopamine neurons, which respond to prediction error, modulate activity of MFC to allow enhanced processing of incoming information when reward expectation is violated. Notably, this suggestion is comparable to the function that we ascribed to PPC, rather than to MFC, in Experiment 2, in which we found no effects of MFC lesions on the rats' ability to use prediction error information to enhance overshadowing in new learning. Of course, our lesions, which were intended to selectively reduce cholinergic innervation of MFC, likely spared MFC functions that might be modulated by dopaminergic input to MFC. It is tempting to speculate that dopaminergic and cholinergic modulation of cortical subregions subserve different functions, and that lesions of the dopaminergic input to MFC might have had very different effects in our current studies.
In this regard it is informative to place the present data within other theoretical perspectives of cholinergic action in attention. In particular, Sarter et al. (2005) distinguished between signal-driven cholinergic modulation of detection processes and top-down or cognitive modulation of detection and other aspects of information processing. In the present context, the cue duration-dependent response deficits observed with MFC cholinergic deafferentation would reflect a role for MFC in the former, and a role for PPC in the latter. Notably, within Sarter et al's (2005) framework, a major function of prefrontal cortex in attention is to provide top-down modulation of cholinergic innervation of other cortical areas, especially the posterior attention system that includes PPC. Thus, the SI/nBM-PPC projections critical to surprise-induced enhancement of learning might themselves be modulated by projections from MFC to PPC (both direct and indirect, via SI/nBM), which could convey such top-down prediction error information (Nelson, Sarter, & Bruno, 2005; Reep et al., 1994). Although the results of Experiment 2 show that any such modulation of PPC by MFC does not depend on intact cholinergic input to MFC, they do not preclude the possibility that MFC serves this critical role in top-down information processing. Thus, it would be valuable to determine the effects on surprise-induced learning enhancements of destruction of MFC neurons themselves, and of the various noncholinergic afferents of MFC.
Considerations of how MFC and PPC may interact in these tasks aside, it is important to address how the functions of these regions and networks may be modulated by CEA. Previously, we suggested that CEA's ability to modulate cortical attentional processing of CSs is mediated by its direct projections to magnocellular cholinergic neurons in SI/nBM (Gallagher & Holland, 1992; Holland & Gallagher, 1999). In support of this claim, we found that communication between these two regions is critical for both attention in action, as assessed in the task used in Experiment 1 (Holland, in press), and with the enhancement of CS associability that is observed when the CS is made an inconsistent predictor of subsequent events (Han et al., 1999). In those studies, performance was abolished by contralateral lesions of CEA and SI/nBM, which severely limited communication between these two regions but spared functions of each region that did not require communication between them.
However, the logic of the disconnection lesion procedure does not distinguish between the roles of direct and indirect connections between the regions of interest. Recent evidence raises the possibility that indirect connections between CEA and SI/nBM are critical to the enhanced associability of CSs found after the omission of expected outcomes. Using transient inactivation procedures, Holland and Gallagher (2006) found that normal CEA function was critical only at the time when the associability enhancements are thought to be acquired (analogous to Experiment 1's port cue PRF training), and not at the time when they are expressed in more rapid learning (analogous to Experiment 2's assessment of the overshadowing abilities of the port cues). By contrast, normal SI/nBM function was only required at the time of the expression of the enhanced CS associability, and not at the time of acquisition of that higher associability.
The observation that there was no time at which both structures needed to be functional simultaneously suggests an intermediate structure that receives information from CEA at the time of acquisition and influences SI/nBM function at the time of expression. A recent study identified the substantia nigra pars compacta (SNc), a major innervator of SI/nBM (Hasue & Shammah-Lagnado, 2002), as a potential intermediate structure in that circuit. Lee, Youn, Gallagher, and Holland (2006) found that disconnection of CEA from the SNc prior to all training also prevented these enhancements of CS associability. Thus, information about enhanced CS associability, processed by CEA when expectancies are disconfirmed (as in PRF contingencies), might be maintained in SNc, which influences SI/nBM at the time of expression of that information in new learning.
The potential role of CEA→SNc projections in the modulation of CS associability is especially interesting given the role of these projections in another aspect of attention in associative learning, the acquisition of CS-specific conditioned orienting responses (ORs), which serve to redirect animals' attention to predictors of significant events, such as food delivery. Although unconditioned ORs to salient visual and auditory stimuli generally habituate rapidly, those ORs often reemerge gradually when the stimulus is paired with a reinforcer in Pavlovian conditioning procedures (Holland, 1977; Maltzman, 1977). Evidence from disconnection lesion studies shows that a circuit that includes the CEA, the SNc, and the dorsolateral striatum (DLS) is critical to the acquisition and expression of these conditioned ORs (Han, McMahan, Holland, & Gallagher, 1997; Lee, Groshek, Petrovich, Cantalini, Gallagher, & Holland, 2005). Within this circuit each structure has distinguishable roles, with CEA function critical for acquisition but not expression of these responses (Groshek, Kerfoot, McKenna, Polackwich, Gallagher, & Holland, 2005; McDannald, Kerfoot, Gallagher, & Holland, 2004), DLS function important for expression but not acquisition (Han et al., 1997), and SNc function required at both times (El-Amamy & Holland, 2006). Notably, lesions of DLS also have substantial effects on 5CSRT accuracy (Rogers, Baunez, Everitt, & Robbins, 2001). It is interesting to speculate that CEA→SNc projections play an important role in the modulation of both basal forebrain cholinergic systems and striatal dopaminergic systems involved in a variety of attentional functions. In addition, it is notable that CEA is reciprocally connected to another population of midbrain dopamine neurons, those of the VTA (Krettek & Price, 1978), which are known to directly influence cortical activity (Pirot, Godbout, Mantz, Tassin, Glowinsky, & Thierry, 1992), and which have been implicated in the gating of MFC attentional function (Cohen et al., 2002; Potts et al., 2006). Thus, CEA may be a critical component involved in a range of attention networks.
Attention remains a broadly defined and hence often poorly specified construct. The distinction between attention in new learning and attention in action, loosely based on Pearce and Hall's (1980) distinction between controlled and automatic attention, is useful in understanding brain-behavior relations that are important in associative learning. Ecological demands on the allocation of attention for purposes of learning and action may often be quite different. Whereas for purposes of selecting for action it is likely to be adaptive to direct one's attention to the most reliable predictors of future significant events, a different strategy may be more useful when one is attempting to learn about causal relations among events in the world. In that case, focusing attention on events whose consequences are already well-known would waste resources better allocated to cues whose consequences are currently less well understood. As formalized in the Pearce-Hall (1980) model, for purposes of new learning, attention to CSs should be adaptively related to the prediction error (surprise) consequent to those events. Thus, although the direction of attention to a particular stimulus may often enhance early-stage sensory processing that could augment all functions of that stimulus, attention may also enhance stimulus processing selectively within more functionally specialized systems, including those linked to changes in learning rate, selection of action, and facilitation of sensory-motor response patterns. Within this framework, attention might be directed to different events for different purposes, even at the same time, within the same stimulus array.
Footnotes
Notes: Portions of these data were reported at the Society for Neuroscience 2005 meeting (Maddux et al., 2005). Erin Kerfoot is now at the University of Virginia and Souvik Chatterjee is now at the University of North Carolina School of Medicine.
References
- Allport A. Visual attention. In: Posner M, editor. Foundations of cognitive science. Cambridge, MA: MIT Press; 1989. pp. 631–682. [Google Scholar]
- Apparsundaram S, Martinez V, Parikh V, Kozak R, Sarter M. Increased capacity and density of choline transporters situated in synaptic membranes of the right medial prefrontal cortex of attentional task-performing rats. Journal of Neuroscience. 2005;25:3851–3856. doi: 10.1523/JNEUROSCI.0205-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8:727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Chess AC. Specific changes in conditioned responding following neurotoxic damage to the posterior parietal cortex. Behavioral Neuroscience. 2005;119:1580–1587. doi: 10.1037/0735-7044.119.6.1580. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. Journal of Neuroscience. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. Journal of Neuroscience. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bushnell PJ, Oshiro WM, Gallagher M. Selective removal of cholinergic neurons in the basal forebrain alters cued target detection in rats. NeuroReport. 1999;10:3119–3123. doi: 10.1097/00001756-199909290-00044. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW. Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learning & Memory. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Collins L, Pearce JM. Predictive accuracy and the effects of partial reinforcement on serial autoshaping. Journal of Experimental Psychology. Animal Behavior Processes. 1985;11:548–564. [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cerebral Cortex. 2004;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: Control, representation, and time course. Annual Review of Psychology. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Substantia nigra pars compacta is critical to both the acquisition and expression of learned orienting of rats. European Journal of Neuroscience. 2006;24:270–276. doi: 10.1111/j.1460-9568.2006.04896.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Falls WA, Davis M. Lesions of the central nucleus of the amygdala block conditioned excitation, but not conditioned inhibition of fear as measured with the fear-potentiated startle effect. Behavioral Neuroscience. 1995;109:379–387. doi: 10.1037//0735-7044.109.3.379. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. Understanding the function of the central nucleus: Is simple conditioning enough? In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 307–321. [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. Journal of Neuroscience. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. Journal of Comparative Neurology. 1997;383:163–177. [PubMed] [Google Scholar]
- Groshek F, Kerfoot E, McKenna V, Polackwich AS, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning, but not expression, of conditioned auditory orienting. Behavioral Neuroscience. 2005;119:202–212. doi: 10.1037/0735-7044.119.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G, Pearce JM. Latent inhibition of a CS during CS-US pairings. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:31–42. [PubMed] [Google Scholar]
- Han JS, McMahan RW, Holland PC, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. Journal of Neuroscience. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behavioral Neuroscience. 1999;113:143–151. doi: 10.1037//0735-7044.113.1.143. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: A combined retrograde tracing and immunohistochemical study in the rat. Journal of Comparative Neurology. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Henke PG. Dissociation of the frustration effect and the partial reinforcement extinction effect after limbic lesions in rats. Journal of Comparative and Physiological Psychology. 1977;91:1032–1038. [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Cognitive Brain Research. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. The effects of manipulations of attentional demand on cortical acetylcholine release. Cognitive Brain Research. 2001;12:353–370. doi: 10.1016/s0926-6410(01)00064-7. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Disconnection of the amygdala central nucleus and the substantia innominata/nucleus basalis magnocellularis disrupts performance in a sustained attention task. Behavioral Neuroscience. doi: 10.1037/0735-7044.121.1.80. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behavioral Neuroscience. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. Journal of Neuroscience. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Han JS, Gallagher M. Lesions of the amygdala central nucleus alter performance in a selective attention task. Journal of Neuroscience. 2000;20:6701–6706. doi: 10.1523/JNEUROSCI.20-17-06701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Thornton JA, Ciali L. The influence of associability changes in negative patterning and other discriminations. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:462–476. doi: 10.1037//0097-7403.26.4.462. [DOI] [PubMed] [Google Scholar]
- Holley LA, Wiley RG, Lappi DA, Sarter M. Cortical cholinergic deafferentation following the intracortical infusion of 192IgG-saporin: A quantitative histochemical study. Brain Research. 1994;663:277–286. doi: 10.1016/0006-8993(94)91274-2. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lacey SC, Nee DE. Processes of working memory in mind and brain. Current Directions in Psychological Science. 2005;14:2–5. [Google Scholar]
- Karnovsky MJ, Root L. A “direct-colouring” thiocholine method for cholinesterases. Journal of Histochemistry and Cytochemistry. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kaye H, Pearce JM. The strength of the orienting response during Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:90–109. [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cerebral Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. Journal of Comparative Neurology. 1978;178:225–253. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Le Pelley ME. The role of associative history in models of associative learning: A selective review and a hybrid model. Quarterly Journal of Experimental Psychology. 2004;57B:193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. Journal of Neuroscience. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. Journal of Neuroscience. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Maddux JM, Chatterjee S, Kerfoot EC, Holland PC. Society for Neuroscience. 2005. Distinct roles for amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex in attention for learning and action. Abstract 411.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JM, Holland PC. Society for Neuroscience. 2004. Lesions of medial prefrontal cortex interfere with rats' sensitivity to the predictive value of cues in a 5-choice serial reaction time task. Abstract 332.10. [Google Scholar]
- Maltzman I. Orienting in classical conditioning and generalization to the galvanic skin response to words: An overview. Journal of Experimental Psychology: General. 1977;106:111–119. [PubMed] [Google Scholar]
- McDannald M, Kerfoot E, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting. European Journal of Neuroscience. 2004;20:240–248. doi: 10.1111/j.0953-816X.2004.03458.x. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192IgG-saporin on attentional performance in a five-choice serial reaction time task. Journal of Neuroscience. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192IgG-saporin into the basal forebrain: Selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behavioral Neuroscience. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. AMPA-induced excitotoxic lesions of the basal forebrain: A significant role for the cortical cholinergic system in attentional function. Journal of Neuroscience. 1994;14:2313–2326. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: Dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruono JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ohtake T, Heckers S, Wiley RG, Lappi DA, Mesulam MM, Geula C. Retrograde degeneration and colchicine protection of basal forebrain cholinergic neurons following hippocampal injections of an immunotoxin against the p75 nerve growth factor receptor. Neuroscience. 1997;78:123–133. doi: 10.1016/s0306-4522(96)00520-9. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. European Journal of Neuroscience. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Pashler H. The Psychology of Attention. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attention performance: Dissociable functions of distinct medial prefrontal subregions. Cerebral Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O'Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. European Journal of Neuroscience. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Pirot S, Godbout R, Mantz J, Tassin JP, Glowinsky J, Thierry AM. Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: Evidence for the involvement of both dopaminergic and GABAergic components. Neuroscience. 1992;49:857–865. doi: 10.1016/0306-4522(92)90362-6. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends in Neurosciences. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: The medial prefrontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18:1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Price JL, Stern R. Individual cells in the nucleus basalis-diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Research. 1983;269:352–356. doi: 10.1016/0006-8993(83)90145-2. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortico and thalamic connections. Experimental Brain Research. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behavioral Neuroscience. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: A study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. I. Magnocellular basal nucleus. Journal of Comparative Neurology. 1984;222:313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Research Reviews. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive nodulation of signal detection. Brain Research Reviews. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS, Pascoe JP. Neuronal activity within the nucleus basalis and conditioned neocortical electroencephalographic activation. Journal of Neuroscience. 1994;14:1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PN, Boumphrey P, Pearce JM. Restoration of the orienting response to a light by a change in its predictive accuracy. Quarterly Journal of Experimental Psychology. 1992;44B:17–36. [Google Scholar]