Abstract
Resveratrol is a naturally occurring polyphenol that exhibits pleiotropic health beneficial effects including anti-inflammatory, cardio- and cancer-protective activities. It is recognized as one of the more promising natural molecules in the prevention and treatment of chronic inflammatory and autoimmune disorders. Ulcerative Colitis (UC) is an idiopathic, chronic inflammatory disease of the colon associated with a high colon cancer risk. Here, we used a Dextran Sulfate Sodium (DSS) mouse model of colitis, which resembles human UC pathology. Resveratrol mixed in food ameliorates DSS-induced colitis in mice in a dose-dependent manner. Resveratrol significantly improves inflammation score, down regulates the percentage of neutrophils in the mesenteric lymph nodes and lamina propiria, and modulates CD3+ T cells that express tumor necrosis factor-alpha and interferon gamma. Markers of inflammation and inflammatory stress (p53 and p53-Phospho-Serine 15), are also down regulated by resveratrol. Since chronic colitis drives colon cancer risk, we carried out experiments to determine the chemopreventive properties of resveratrol. Tumor incidence is reduced from 80% in mice treated with Azoxymethane (AOM) + DSS to 20% in AOM + DSS + Resveratrol (300 p.p.m.) treated mice. Tumor multiplicity also decreased with resveratrol treatment. AOM + DSS treated mice had 2.4 ± 0.7 tumors per animal compared with AOM + DSS + 300 p.p.m. resveratrol, which had 0.2 ± 0.13 tumors per animal. The current study indicates that resveratrol is a useful, non-toxic complementary and alternative strategy to abate colitis and potentially colon cancer associated with colitis.
Keywords: Inflammation, Resveratrol, Colitis, Colon Cancer
Introduction
Resveratrol (3,4,5-Trihydroxy-trans-stilbene) is a naturally occurring compound, often derived from the Japanese (bushy) knotweed, but is also found in the skin of red grapes and is a constituent of red wine. Resveratrol has been shown to extend the life span of yeast and mice (1). In murine experiments, anti-aging, anti-inflammatory, anti-cancer, anti-neurodegenerative, blood-sugar-lowering, chelating and other beneficial cardiovascular effects of resveratrol have been reported (2–7). Although resveratrol has been studied little in humans, it appears to have cardioprotective effects ex vivo (8, 9). At daily doses equivalent to the amount of resveratrol in over 1000 bottles of red wine, resveratrol appears to be safe (10), but resveratrol undergoes extensive metabolism in humans, which limits the availability of the parent molecule at organs remote from the site of absorption (10). It is therefore particularly appealing when studying gastrointestinal tract diseases.
Resveratrol has been shown to suppress several autoimmune diseases, including experimental encephalomyelitis (11, 12), arthritis (11), myocarditis (13), and diabetes (14). The capability of resveratrol to suppress chronic inflammatory diseases associated with a high cancer risk, such as inflammatory bowel disease (IBD), has only been explored in rats by one other group (15, 16).
IBD consists of two forms Ulcerative colitis (UC) and Crohn’s Disease (CD), which are dynamic, idiopathic, chronic inflammatory conditions associated with a high colon cancer risk (17). Conventional treatment of colitis can reduce periods of active disease and help to maintain remission, but these treatments often bring marginal results, patients become refractory and there are side effects. For this reason, many colitis sufferers turn to unconventional treatments in hopes of abating symptoms of active disease and it is estimated that 40% of IBD patients use some form of megavitamin therapy of herbal/dietary supplement (18, 19). We have recently shown that Ginkgo biloba (EGb 761) and American ginseng extracts can suppress colitis in mice (20, 21). Because of the strong anti-inflammatory properties of resveratrol, we hypothesized that this supplement will also work against colitis. Here, we provide data supporting such studies, indicating that resveratrol administered in the basal diet suppresses DSS-induced colitis and colon cancer associated with colitis in mice.
Methods
Resveratrol Characteristics
We used resveratrol obtained from Sigma Chemical Co. This is 3,4,5-Trihydroxy-trans-stilbene (Trans-Resveratrol Aglycone), which is a purified compound with the molecular formula C14H12O3. The Certificate of Origin indicates it was originally purified and extracted from the Bushy Knotweed plant, and is found to be greater than 99% pure by both gas chromatography and thin-layer chromatography. The absorbance spectrum is consistent with its structure, and is EmM (218 nm) = 21.5 (ethanol), EmM (306 nm) = 30.0 (ethanol), EmM (320) = 29.1 (ethanol). The conditions used for gas chromatography were as follows: Capillary column: 30 m × 0.32 mm ID with 0.25 μm particles; Temperature (EC): injector 280 detector 280; Flow rate: 25 cm/s He; Column temp program: 260 to 280EC @ 4 deg/min; Solvent: Sigma Sil A 10 mg/mL; Volume Injected 1.0 μL; Retention time: approximately 5 min. The conditions used for thin layer chromatography were as follows: System: Silica gel plates; Solvent: 30 parts n-butanol/20 parts pyridine/20 parts water/6 parts glacial acetic acid; Detection: iodine vapor or methanolic sulfuric acid spray; Sample dissolved at 50 mg/mL in acetone, spotted from 0.5 to 250 μg; Rf approximately 0.7. It is a white powder with yellow cast, and has a molecular weight of 228.2 g/mol. This product is stable at −20°C for at least two years. When added to the basal diet, we stored the diet at 4°C, and changed the basal diet every 2 days during experiments. The diet comes from Research Diets, Inc. Resveratrol from Sigma is sent to Research Diets, Inc., and mixed with a standard AIN-93M diet. Briefly, the diet preparation operator at Research Diets, Inc. carefully weighs out each ingredient. With micro-ingredients, such as the resveratrol, vitamin mix, and mineral mix, a premix is created to optimize the microingredient particle distribution in the diet. This micro premix is then mixed with the main ingredients of the diet, and after diet is homogenously mixed, it is sent for pelleting. The final amount of resveratrol was 75–300 p.p.m. As indicated in Figure 1, although doses of 75 p.p.m. were inadequate at suppressing colitis, doses of 150 p.p.m. and 300 p.p.m. were successful. We therefore used these doses in subsequent mechanistic experiments. 150 p.p.m. were used for mechanistic experiments described in Figures 2 and 3; 300 p.p.m were used for mechanistic experiments described in Figures 4 and 5. Importantly, after the resveratrol is mixed with the basal diet, it maintains its biological activity. The basal diet has a 4 month expiration date from the time it is produced. We have tested the biological activity in 5 separate batches, and it maintained its biological activity in that it causes a 2–3 fold reduction in mouse colitis at 300 p.p.m.
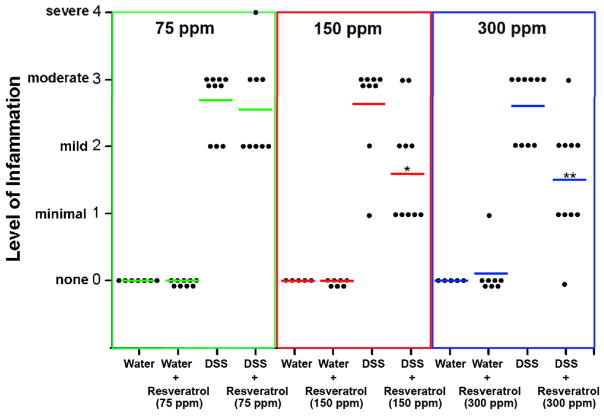
Figure 1. Resveratrol inhibits colitis in mice exposed to DSS.
Mice were treated as described in the text. Results are presented as a dot-plot, with the lines representing the mean inflammation score among the group. Each dot represents an individual mouse, and the level of colon inflammation quantified and graded blindly and independently by two trained pathologists as described in the text. These data are presented in this fashion to reflect three separate studies that were done. Based on findings from other studies in our lab (20, 21), we initially used 75 p.p.m. resveratrol in the basal diet (green lines). This dose did not protect mice from DSS-induced colitis, so we initiated experiments with and without 150 p.p.m. (red lines), and with and without 300 p.p.m. (blue lines) in the basal diet. *, indicates 150 p.p.m. Resveratrol significantly reduced colitis compared with the DSS group alone (p < 0.05). **, indicates that 300 p.p.m. resveratrol significantly reduced colitis compared with the DSS only group (p < 0.01).
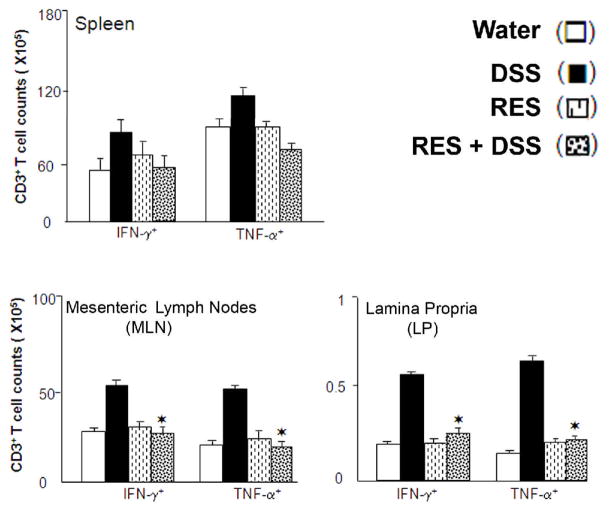
Figure 2. Resveratrol mediates T cells expressing IFN-γ, and TNF-α after DSS induction.
Splenic (A), Mesenteric Lymph Nodes (MLN) (B), and Lamina Propria (LP) (C) lymphocytes were isolated from C57BL/6 mice that received water (control open bar), 100 μl of resveratrol (RES) with water (open bar with dots) or water containing 1% DSS (solid bar), and 150 p.p.m. of RES with DSS (diagonal hash bars) in diets. Changes in number of T cells expressing IFN-γ, and TNF-α, were determined by flow cytometry and expressed as the total number of cells± SEM. Data represents the mean of three independent experiments involving 5 mice per group. For each experiment and group, the lymphocytes were pooled. *, indicates that 150 p.p.m. resveratrol significantly reduced colitis compared with the DSS only group (p < 0.05).
Figure 3. Resveratrol modulates DSS induced neutrophils during colitis.
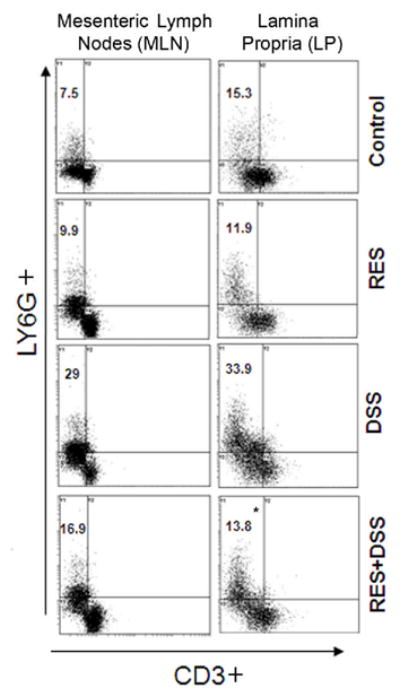
Mesenteric Lymph Nodes (MLN) and Lamina Propria (LP) lymphocytes were isolated from C57BL/6 mice that received water, 150 p.p.m. of resveratrol with water or water containing 1% DSS, 150 p.p.m. and of resveratrol with DSS and stained for CD3+ T cells and neutrophils. The MLN or LP-derived CD3− lymphocytes were characterized for neutrophils expression (LY6G) by flow cytometry. The numbers in the upper left quadrant indicate the total percentage of LY6G+ neutrophils. Data are a representative experiment of three independent experiments. Each experiment involved pooled lymphocytes from 5 mice per groups. *, indicates statistically significant differences (p < 0.01) between DSS + resveratrol (RES) treated groups compared with the DSS alone group.
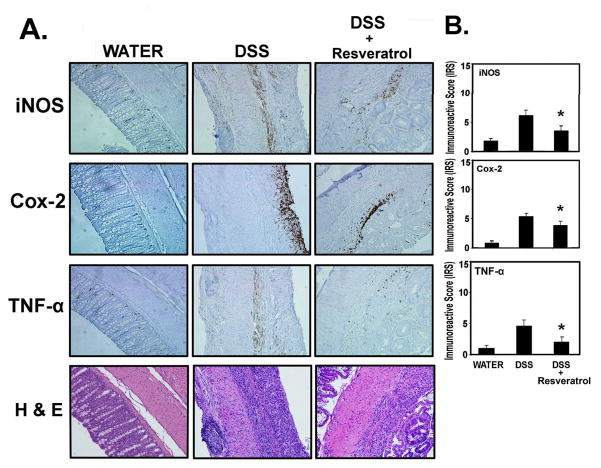
Figure 4. Resveratrol suppresses the players involved in inflammation (iNOS, COX-2 and TNF-α).
Tissues from experiments performed for Figure 1 (300 p.p.m. Resveratrol) were examined for iNOS, COX-2, and TNF-α by immunohistochemistry, using the Antibody Amplifier™ (ProHisto, LLC) rocked on a laboratory rocker to ensure even staining and reproducible results. (A) Representative staining of indicated end points in serial sections from water (n = 5), DSS (n = 10) and DSS + Resveratrol (n = 10) groups. Positive staining is brown colored. 200× magnification. (B) Quantification of indicated end points. All three markers were elevated in the DSS-treated group and suppressed when the DSS-treated group was fed Resveratrol (300 p.p.m.). *, indicates significant reduction in % positive cells in the Resveratrol + DSS group compared with the DSS group (p < 0.01).
Figure 5. Resveratrol suppresses the players involved in inflammatory stress (p53 and p53-phospho-serine 15).
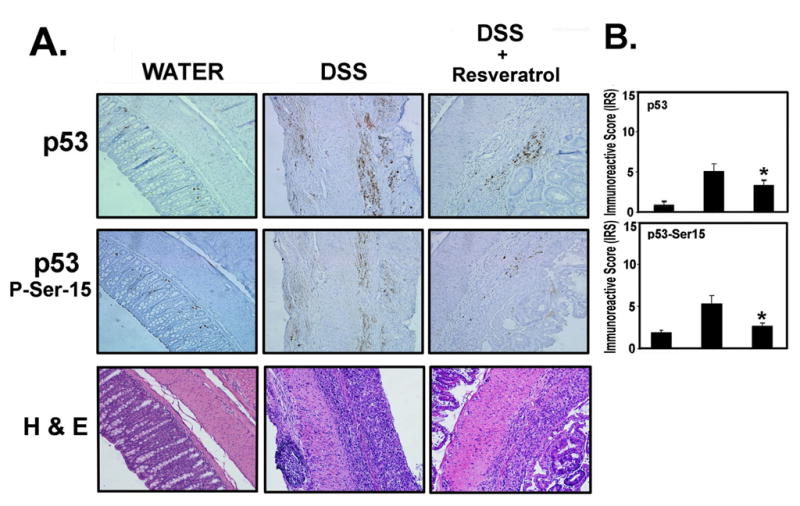
Tissues from experiments performed for Figure 1 (300 p.p.m. Resveratrol) were examined for p53 and p53-P-ser-15 by immunohistochemistry, using the Antibody Amplifier™ (ProHisto, LLC) rocked on a laboratory rocker to ensure even staining and reproducible results. (A) Representative staining of indicated end points in serial sections from water (n = 5), DSS (n = 10) and DSS + Resveratrol (n = 10) groups. Positive staining is brown colored. 200× magnification. (B) Quantification of indicated end points. Both markers were elevated in the DSS-treated group and suppressed when the DSS-treated group was fed Resveratrol (300 p.p.m.). *, indicates significant reduction in % positive cells in the Resveratrol + DSS group compared with the DSS group alone (p < 0.01).
The dose of resveratrol we used (75 – 300 p.p.m.) is the human equivalent dose of 58–232 mg daily, which is far below that considered safe in humans (up to 5000 mg) (10). Of note is that recommended dosages for oral dietary supplements range from 2.5 mg to 1 g for humans, and adverse effects of resveratrol have not been reported (10, 22). Also, of note, is that we used the commercially available aglycone formulation of resveratrol, whereas humans often consume resveratrol glucoside formulations. Resveratrol aglycone appears to represent the predominant form of resveratrol in tissues (23). Our calculation of the human equivalent amount of resveratrol consumed by mice uses the body surface area normalization method (24) with the following assumptions: a typical mouse eats 3.5 g diet daily and weighs 22 g; the average adult human weighs 60 kg. More specifically, here, diet contains 75 – 300 p.p.m. resveratrol. Seventy five p.p.m. equates to 75 mg/kg of diet. A mouse consumes 3.5 g diet daily. Therefore, 75 mg/1000 g daily diet × 3.5 g diet/day = 0.2625 mg resveratrol extract daily. If a mouse weighs on average 22 g, then 0.2625 mg/22 g × 1000 g/1 kg = 11.93 mg/kg daily. As discussed by Reagan-Shaw et al. (24), the human equivalent dose (mg/kg) = animal dose (mg/kg) × (animal Km/human Km). As such, human equivalent dose (mg/kg) for mouse = 11.93 mg/kg/(3/37) = 0.967 mg/kg. If an average human adult weighs 60 kg, this equates to 0.967 mg/kg × 60 kg = 58 mg daily for humans. The 58 mg (for 75 p.p.m.) × 4 = 232 mg daily for human for 300 p.p.m.. Mice consumed the same amount of diet daily (on average 3.5 g) regardless of it containing resveratrol (data not shown).
Animals
Male and female C57BL/6 mice, 8–12 weeks of age, weighing 20–25 g were obtained from Jackson labs, and are under a breeding protocol at the University of South Carolina. All mice are kept in dedicated animal quarters, and were provided food [AIN 93M, described previously (20, 21)] and water ad libitum. Routine changing of cages, food, and water was done twice weekly by trained personnel. Care and use of animals was overseen by the Animal Resource Facility (ARF) of the University of South Carolina, under the direction of our veterinarian. The ARF is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care-International (AAALAC), and is registered with the United States Department of Agriculture (56-R-003). The ARF also has an active letter of Assurance of Compliance on file at the National Institutes of Health (NIH).
DSS mouse model of colitis
We have previously described our DSS model of colitis (20, 21) (Supplementary Figure 1). Briefly, eight to twelve week-old C57BL/6 mice received either water ad libitum or water containing 1% DSS. Resveratrol was mixed into the AIN 93M diet of indicated groups at 75–300 p.p.m (Research Diets, Inc. New Brunswick, NJ), which is the human equivalent dose of approximately 58–232 mg daily for humans, as described above. Basal diet (± resveratrol) was initiated 1 week prior to the administration of DSS, and continued throughout the experiment. DSS was given over 2½ cycles (where each cycle in the DSS group consisted of 1% DSS in drinking water for 7 days, followed by a 7-day interval with normal drinking water). At the end of the experiment, blood was collected, and colon samples were washed with phosphate-buffered saline, cut longitudinally, swiss-roled, then formalin fixed and paraffin embedded.
Azoxymethane/DSS-induced colon cancer model
We followed a modified protocol outlined recently by the Neurath group (25). Supplementary Figure 2 outlines the time-line. Briefly, mice were weighed, and given a single intra-peritoneal injection of Azoxymethane (AOM; 10 mg/kg) or vehicle (Phosphate Buffered Saline) on experimental Day 1. One week later, animals received either 1% DSS or normal drinking water concurrently with AIN 93M basal diet containing 0 p.p.m. or 300 p.p.m. resveratrol. Chronic colitis and colon cancer was induced with a cyclical DSS treatment, which consisted of 7 days of 1% DSS followed by 14 days of normal water for a total of three cycles. The resveratrol was continued until the end of the experiment on Day 70.
On Day 70, the mice were weighed, euthanized, and their blood and tissues were harvested. Colons were cut longitudinally and fixed in 10% buffered formalin overnight. The colons were then stained with methylene blue and scored for the number of colonic neoplasms, to determine the incidence (number of animals with at least one tumor) and multiplicity (number of tumors per animal) of neoplasms. Tumor area, based on length and width, was also calculated. Following photography, colons were rinsed with ice-cold PBS and processed for histopathology and immunohistochemistry by paraffin embedding.
Quantifying inflammation
Slides were examined in a blind fashion by two individuals separately, as we have described previously (20, 21). Briefly, inflammation was graded by extent (focal, multifocal, diffuse or extensive areas) and depth/penetration of inflammation (lamina propria, into submucosa, into mucscularis propria, into subserosa), then given a numerical value of 0–4, where 0, is none observed, and 4 is severe inflammation and/or ulceration/erosion.
Immunohistochemical staining
For immunohistochemical staining, serial sections of mouse colon tissues (processed as described above) were incubated with antibodies against iNOS (Mouse monoclonal Clone 5D5-H7, Cat# MC-5245; diluted 1 in 10,000, Research & Diagnostic Antibodies), Cox-2 (Rabbit polyclonal, Cat#160126; diluted 1 in 20,000, Cayman Chemical), TNF- α (Mouse monoclonal, clone P/T2, cat# ab9579; diluted 1 in 50,000, Abcam), p53 (Mouse monoclonal, clone Pab 122, cat# X1494; diluted 1 in 1 million, Exalpha, Biologicals, Inc.), or p53-Phospho-Serine 15 (Mouse monoclonal, Anti-Phospho-Serine-15–53, clone 16G8, cat#9286S, diluted 1 in 20,000, Cell Signaling). To ensure even staining and reproducible results, sections were incubated by slow rocking overnight in primary antibodies (4°C) using the Antibody Amplifier™ (ProHisto, LLC, Columbia, SC, USA). Following incubation with primary antibody, sections were processed using EnVision+ System-HRP kits (DakoCytomation, Carpinteria, CA, USA) according to kit protocols; or using a mouse-on-mouse kit (Vector Labs, Burlingame, CA) if antibodies were mouse monoclonals. The chromogen was DAB and sections were counter-stained with 1% methyl green. The positive control tissue was colon cancer sections. These sections were highly positive for iNOS, Cox-2, TNF-α, p53 and p53-phospho-Serine 15.
Stained tissues were examined for intensity of staining using a method similar to that previously described (26). Intensity of staining in tumor sections were evaluated independently by blinded investigators. For each tissue section, the percentage of positive cells was scored on a scale of 0 to 4 for the percentage of tissue stained: 0 (0% positive cells), 1 (<10%), 2 (11% to 50%), 3 (51% to 80%), or 4 (> 80%). Staining intensity was scored on a scale of 0 to 3: 0-negative staining, 1-weak staining, 2-moderate staining, or 3-strong staining. The two scores were multiplied resulting in an immunoreactivity score (IRS) value ranging from 0 to 12.
Cell isolation
Spleens from individual mice were mechanically dissociated and RBC’s lysed with lysis buffer (Sigma St. Louis, MO). Single cell suspensions of spleen and colon mesenteric lymph nodes (MLN) were passed through a sterile wire screen (Sigma St. Louis, MO). Cell suspensions were washed twice in RPMI 1640 (Sigma St. Louis, MO) and stored in media containing 10% FBS on ice until used after one to two hours. The small intestine and colon was cut into 1-cm stripes and stirred in PBS containing 1mM EDTA at 37°C for 30 min. The cells from intestinal lamina propria (LP) were isolated as described previously (27). In brief, the LP was isolated by digesting intestinal tissue with collagenase type IV (Sigma St. Louis, MO) in RPMI 1640 (collagenase solution) for 45 min. at 37°C with moderate stirring. After a 45 min interval, the released cells were centrifuged, stored in complete media and mucosal pieces were replaced with fresh collagenase solution. This was repeated twice. LP cells were further purified using a discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient collecting at the 40–75% interface. Lymphocytes were maintained in complete medium, which consisted of RPMI 1640 supplemented with 10 ml/L of nonessential amino acids (Mediatech, Washington, DC), 1 mM sodium pyruvate (Sigma), 10 mM HEPES (Mediatech), 100 U/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamycin (Elkins-Sinn, Inc., Cherry Hill, NJ), 50 μM mercaptoethanol (Sigma) and 10 % FCS (Atlanta Biologicals).
Flow cytometry analysis
Cells from the spleen, MLN, and LP were freshly isolated as described above for each experimental group. Fluorescence-activated cell sorting (FACS) cell surface antigens staining cells were pre-blocked with Fc receptors for 15 min at 4°C. The cells were washed with FACS staining buffer (PBS with 1% BSA), and then stained with CY-conjugated anti-CD3 (145-2C11) and FITC conjugated LY6G (neutrophils) (BD-PharMingen, San Diego CA) for 30 min. with occasional shaking at 4°C. The cells were washed two times with FACS staining buffer and re-suspended in BD Cytofix/Cytoperm (BD-PharMingen, San Diego CA) solution for 20 min. Again cells were washed two times in BD perm/wash solution. For intracellular cytokines, re-suspended fixed permeabilized cell were stained with pre-determined APC flurochrome-conjugated anti-cytokine antibody (TNF-α, IFN-γ for 30 min at 4°C in the dark). Lymphocytes were then washed with FACS thoroughly staining buffer and analyzed by flow cytometry (FC 500by Beckman Coulter Fort Collins Co).
Statistical analysis
With inflammation as an endpoint, a chi-square contingency table analysis was done on the DSS and DSS plus resveratrol groups to determine if there was a statistically significant difference in their inflammation scores. For immunohistochemical quantification, mean differences between groups were compared by one-way ANOVA with Scheffe multiple comparison tests. For flow cytometry data, differences between groups were compared using a two-tailed paired student’s t-test or an unpaired Mann Whitney U-test. The results were analyzed using the Statview II statistical program (Abacus Concepts, Inc., Berkeley, CA) and Microsoft Excel (Microsoft, Seattle, WA) for Macintosh computers. Single-factor variance ANOVA analyses were used to evaluate groups respectively. Tumor incidence was examined using a Fisher’s exact test which is equivalent to a test for binomial proportions. Because the usual assumptions underlying the ANOVA test are not satisfied, in assessing the significance of the F-statistic value from the ANOVA table, instead of using the F-distribution, we used a permutation distribution to examine tumor multiplicity. Finally, a nonparametric Kruskal-Wallis test was used to compare mean tumor volume. The P-value chosen for significance in this study was 0.05.
Results
Resveratrol attenuates DSS-induced colitis in a dose-dependent manner
There is increasing evidence that resveratrol targets many key players in inflammation (28, 29). UC is a strong risk factor for colon cancer (17) with chronic inflammatory disease associated with overactive inflammatory cells infiltrating in the colon. Based on this information, we tested the hypothesis that resveratrol can suppress colitis in mice. To test this hypothesis, we used the DSS induced model of colitis, with and without resveratrol (75 p.p.m, 150 p.p.m. or 300 p.p.m.) treatment. We did not observe any phenotypic characteristics of toxicity (e.g. moribund and weight loss) at any doses of resveratrol used in our studies, and the ‘no observed adverse effect level’ (NOAEL) for murine species is 300 mg/kg/day (22). 300 p.p.m. (the highest dose we used) is equivalent to 42 mg/kg/day in mice, so this lack of toxicity was predicted.
Figure 1 shows that mice fed resveratrol were protected from DSS-induced colitis, at doses of 150 p.p.m. (p < 0.05 vs. DSS treated), and 300 p.p.m. (p < 0.01 vs. DSS treated) resveratrol. This data is supported by other endpoints in our experiment. Colon lengths were measured, because a short colon is indicative of heavy inflammation (20, 21). Compared with the control (water-treated) group (6.8 ± 0.1 cm), colon length decreased in the DSS-treated group (5.4 ± 0.1 cm) (p < 0.01), but not in the DSS + resveratrol-treated group (6.3 ± 0.2 cm). Interestingly, colon length increased significantly (p < 0.01) in the water + resveratrol group (8.1 ± 0.2 cm) compared with the water only group (6.8 ± 0.1 cm). These results clearly indicate that the resveratrol treatment prevents colitis progression and abrogate the disease as evidenced by the colitis score and colon length.
Characteristics of Th cells during DSS induced colitis after resveratrol treatment
Next, we examined whether resveratrol has any effect on systemic cytokine expression after DSS induction. Flow cytometry analysis revealed that CD3+ T cells (mainly CD4+ T cells) from the MLNs and LP express TNF-α and INF-γ during DSS-induced acute colitis. DSS induced mice with acute colitis shows increased numbers of CD3+ T cells that express TNF-α and INF-γ in the MLNs and the number of similar cells in the LP (Figure 2). However, resveratrol treatment significantly reduced the number of CD3+T cell infiltrates that express TNF-α and INF-γ in the MLNs, and LP with acute colitis after DSS induction as compared with the controls mice and/or mice that received DSS alone (Figure 2). We did not observe a decline in the number of splenic CD3+T cells expressing IFN-γ and/or TNF-α in mice treated with resveratrol compared with DSS-induced mice. Taken together, these results indicate that resveratrol treatment down regulates systemic TNF-α and INF-γ expressing T cells in the MLN and LP compared with similar cells obtained from DSS-induced mice with any treatment.
Resveratrol inhibits neutrophil infiltration in DSS induced mice
Neutrophils are among the first cell type to arrive at a site of inflammation. In UC, neutrophil activation, migration, and degranulation are important effector mechanisms of intestinal damage (30). In addition, circulating and activated neutrophils, a major source of inflammatory cytokines, are elevated in the UC patients. Further, it has been hypothesized in recent reports that neutrophils are involved in DSS-induced colonic mucosal injury. Several lines of evidence support this hypothesis: 1) colonic mucosal ICAM-1 expression is enhanced at an early stage of the inflammatory cascade in DSS-induced colitis (31); and 2) numerous neutrophils accumulate in DSS-treated colonic mucosa, and selective depletion of neutrophils by a monoclonal antibody reduces DSS-induced colitis (32). Based on this knowledge, we next examined the percentage changes in mucosal neutrophil expression after resveratrol treatment in DSS induced colitis. Interestingly, we noticed a dramatic increase in percentage of neutrophils in the DSS-challenged group of mice compared to the other groups of mice in the MLN and LP (Figure 3). At the same time resveratrol significantly reduced the percentage of neutrophils in MLN and LP compared with control or DSS alone group. Taken together these results indicate that the number of neutrophils increased both at MLN and LP sites after DSS induction and resveratrol significantly reduced these numbers.
Resveratrol suppresses markers of inflammation
To further quantify the impact of resveratrol on inflammatory markers in vivo, we examined iNOS, COX-2 and TNF-α expression. Tissues from the experiment using 300 p.p.m. resveratrol were used for these purposes. Immunohistochemical staining was accomplished by rocking slides using the Antibody Amplifier™ (ProHisto, LLC) to ensure even, consistent, sensitive and reproducible staining. Figure 4A shows representative sections of endpoint as indicated. Figure 4B shows quantification of each staining. Overall, iNOS, COX-2, and TNF-α levels were reduced in DSS-treated mice that consuming 300 p.p.m., resveratrol as compared with DSS-treated mice consuming regular basal diet.
Resveratrol suppresses markers of inflammatory stress
The p53 is a key biosensor of inflammatory stress. Because p53 is activated by phosphorylation at serine 15 during inflammatory stress (33), we also probed tissue sections for these markers. Figure 5A shows representative sections of endpoints as indicated. These were serial sections of the same mice shown in Figure 4A. Figure 5B shows quantification of staining. Overall, p53 and p53-phosph-serine-15 levels were reduced in DSS-treated mice consuming 300 p.p.m., resveratrol compared with DSS-treated mice consuming regular basal diet.
Resveratrol suppresses colon cancer associated with colitis
We have shown that resveratrol suppresses colitis. Because both mice and humans with chronic colitis are at a high risk for colon cancer, here, we tested the hypothesis that resveratrol prevents the onset of colon cancer in a mouse model of colitis-driven colon cancer. Table 1 show that the 80% of mice (8/10) treated with AOM + DSS had colon tumors. The mice treated with AOM + DSS + Resveratrol (300 p.p.m.) had a tumor incidence of 20% (2/10). This difference was statistically significant using a Fisher’s exact test (p < 0.05).
Table 1.
Tumor incidence and multiplicity in mice treated with AOM/DSS ± Resveratrol (Res).
| Group | N | % Animals with Colon Tumors (Incidence) | Number of Tumors per Animal (Multiplicity) | Avg. Size of Tumors (mm2) |
|---|---|---|---|---|
| Mean ± S.E. | Mean ± S.E. | |||
| AOM+DSS | 10 | 80% | 2.4 ± 0.7 | 1.4 ± 0.3 |
| AOM + Res | 10 | 0% | 0 | - |
| AOM + DSS + Res | 10 | 20%* | 0.2 ± 0.13* | 1.1 ± 0.4 |
, indicates significant difference from AOM + DSS treated group (see methods for statistics).
Tumor multiplicity (number of tumors per animal) also decreased with resveratrol treatment (Table 1). The total number of macroscopic lesions in the AOM + DSS group was 23 and the total number of macroscopic lesions in the AOM + DSS + resveratrol group was 2. Permutation distribution analysis found similar results to that of tumor incidence. The difference between the AOM + DSS (2.4 ± 0.7 tumors per animal) and AOM + DSS + 300 p.p.m. resveratrol (0.2 ± 0.13 tumors per animal) was statistically significant (p < 0.05). Finally, although tumor volume (mm2) was decreased with resveratrol treatment (Table 1), this difference was not statistically significant.
Supplementary Figure 3A are colon sections representative of the indicated group. Supplementary Figure 3 shows H&E histological sections of each group as indicated. Upon histological evaluation of the tumors, we found that there were no invasive cancers, consistent with other similar studies (34, 35). In the AOM + DSS treated group, 25% of the lesions were adenomas with low grade dysplasia, and 47% were adenomas with high grade dysplasia. The remaining lesions (28%) were carcinoma in-situ (CIS). In contrast, in the AOM + DSS + 300 p.p.m. resveratrol treated group more than double the lesions (58%) were adenomas with low grade dysplasia, and less lesions were adenomas with high grade dysplasia (16%) as well as CIS (26%). Although initially surprising that no adenocarcinomas were observed, the tumor histology varies greatly depending on many factors, including mouse strain (36), housing conditions affecting intestinal microflora (37), as well as the AOM/DSS treatment regimen. We used 1% DSS here, which is relatively low compared with other studies, which have used up to 4% DSS (38). A recent study on C57BL/6 mice (the same strain of mouse used here) using 3% DSS also found no evidence of invasive colorectal adenocarinomas (35).
Discussion
Although there has been progress into the treatment of UC (39), current strategies often bring side effects with marginal results and have population-specific efficacy. Alternative treatment strategies are therefore needed. We show here that the consumption of resveratrol, a popular ingredient in red wine and the skin of red grapes is non-toxic, and capable of suppressing colitis in mice at 150 p.p.m. and 300 p.p.m. in the diet. This is in agreement with one other group who has shown that resveratrol suppresses colitis induced by trinitrobenzenesulphonic acid (TNBS) in rats (15, 16). Resveratrol in those studies was administered by oral gavage at 5–10 mg/kg/day. By mixing resveratrol in the basal diet, we were able to generate similar results in mice, in that 150 p.p.m. and 300 p.p.m. (equivalent to 21 and 42 mg/kg/day in mice; 116 and 232 mg/kg/day in humans) suppresses DSS-induced colitis.
Consistent with the known anti-oxidant and anti-inflammatory properties of resveratrol, immunohistochemical staining for iNOS, Cox-2, and TNF-α was also reduced in mice drinking DSS-spiked water and consuming resveratrol. These endpoints (iNOS, Cox-2, and TNF-α) are key mediators of colitis (40–45). The mechanism of resveratrol in the inhibition of these molecules is a subject of further detailed investigation. Recent studies indicate that resveratrol inhibits the nuclear translocation and activation of nuclear factor kappaB (NF-κB), which transcriptionally regulates iNOS and Cox-2 (46–50). Similarly, suppression of resveratrol has been shown to suppress Cox-2 expression by blocking the activation of mitogen activated protein kinases (MAPKs) and activator protein -1 (AP-1) (51). Because TNF-α transcriptionally regulates iNOS and Cox-2 (52, 53), the ability of resveratrol to inhibit gene expression of TNF-α (54) may also be a mechanistic link between such molecules and the suppression of colitis.
Elevated TNF-α is associated with both human IBD and murine colitis (32). Reports from other studies indicate that TNF-α production plays an important role in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced chronic colitis (55). IFN-γ also plays a critical role in the induction and progression of colitis (56). In the present study, we demonstrate that mucosal and/or systemic TNF-α, and IFN-γ expression was decreased by resveratrol treatment in mice after DSS induction. These results are in agreement with previous published in vivo and in vitro data, where resveratrol has been shown to reduce the level of inflammatory cytokines and inflammatory cell infiltrates in the colon (15, 16, 57, 58).
Neutrophils significantly contribute to the pathogenesis of IBD. Disease activity in UC is linked to an influx of neutrophils in the mucosa and subsequently in the intestinal lumen, resulting in the formation of crypt abscesses. It has been reported in the past that in rats, monoclonal antibody-mediated depletion of neutrophils decreases several parameters of DSS-induced colitis (32). In another study it has been demonstrated that blockade of neutrophil adhesion with CD11b/CD18 Ab reduced the cellular infiltrates in rectal administration of TNBS-induced colitis (59). Recently, it has been shown that neutrophil elastase enzymes activity is significantly elevated in both plasma and colonic mucosal tissues in UC patients and a ONO-5046 (neutrophils elastase specific inhibitors) exerts therapeutic effect in DSS-induced colitis by correcting weight loss and inflammation scores (60). In the present study, we have shown that the percentage of neutrophils in MLN and LP significantly increased in the DSS induced mice as compared to naive mice. The resveratrol treatment significantly diminished the neutrophil numbers as compared to DSS-induced mice. The present study corroborates the above findings, and suggests that resveratrol might be a non-toxic, alternative medicine strategy for the treatment of DSS-induced colitis.
Inflammatory stress is associated with the phosphorylation of p53 and serine 15, and subsequent p53 stabilization (33). Therefore, another key finding is that mice drinking DSS water and consuming resveratrol have suppressed expression of p53 and p53-Serine-15 phosphorylation. This has been observed in other diseases associated with inflammatory stress. For example, resveratrol suppresses induced expression of p53 in a rat diabetic nephropathy model (61). The consequences of this finding are currently being explored. For example, it is possible that p53 is a target of resveratrol-induced apoptosis or senescence of inflammatory cells during colitis. To this end, resveratrol has been shown to induce apoptosis in cancer cells through a p53-mediated mechanism (62). Similarly, resveratrol induces cell cycle arrest in HCT 116 colon cancer cells, but not in their p53−/− isogenic counterpart (63); possibly through the promotion of binding of p53 to the cell cycle inhibitor, p21 (62, 64). All studies are consistent with the hypothesis that resveratrol not only induces p53 to drive apoptosis (65), but uses the p53 molecule as a molecular node to induce apoptosis.
As a natural extension to our data, here, we also carried out experiments to determine the chemopreventive properties of resveratrol against colitis driven colon cancer. Although resveratrol has been shown to be protective against colon cancer previously (2), to our knowledge, this is the first time resveratrol has been shown to reduce tumorigenesis associated with colitis. This is consistent with the hypothesis that the ability of resveratrol to suppress colitis is responsible for its chemopreventive properties. This is not surprising, given the close link between inflammation and cancer (66, 67). Overall, results presented here indicate that resveratrol is a viable, non-toxic therapeutic agent for the treatment of UC, and is a potential candidate for the chemoprevention of colon cancer in this population.
Supplementary Material
Supplementary Figure 1. Experimental protocol to test the hypothesis that Resveratrol suppresses colitis.
Supplementary Figure 2. Experimental protocol to test the hypothesis that Resveratrol suppresses colon cancer associated with colitis.
Supplementary Figure 3. Tumor formation in an AOM + DSS mouse model of colon cancer. Tumor formation in colons of mice treated with AOM + DSS is prevented when these mice consume resveratrol (300 p.p.m.) in their basal diet. A. Colons stained with methylene blue. Arrows indicate macroscopic tumors. B. Representative H&E sections. The AOM + DSS group shows a polypoid adenoma with high grade dysplasia, characteristic of tumors in this group.
Acknowledgments
This work was supported by NIH grants 1R03CA141758-01 (LJH), and 1P01AT003961-01A1 (PN, LJH, MN). Thanks also to the Pathology Core (Dr. William Hrushesky, Director), Administrative Core (Dr. Frank Berger, Director), Mouse Core (Dr. Marj Pena, Director) and Imaging/Histology Core supported by the Center for Colon Cancer Research (supported by NIH grant P20RR17698-01).
Abbreviations
- DSS
Dextran Sulfate Sodium
- iNOS
Inducible Nitric Oxide Synthase
- Cox-2
Cyclooxygenase-2
- TNF-α
Tumor Necrosis Factor-Alpha
- IFN-γ
interferon gamma
- IBD
Inflammatory Bowel Disease
- UC
Ulcerative Colits
- p.p.m
Parts Per Million
- WBC
White Blood Cell
- IHC
Immunohistochemistry
References
- 1.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 2.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila Pa) 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 3.Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445–454. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 4.Rocha-Gonzalez HI, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther. 2008;14:234–247. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol. 2008;7:2–7. doi: 10.1111/j.1473-2165.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Das DK. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Patents Cardiovasc Drug Discov. 2007;2:133–138. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 7.Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Gresele P, Pignatelli P, Guglielmini G, et al. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr. 2008;138:1602–1608. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 9.Rakici O, Kiziltepe U, Coskun B, et al. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int J Cardiol. 2005;105:209–215. doi: 10.1016/j.ijcard.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Boocock DJ, Faust GE, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 11.Singh NP, Hegde VL, Hofseth LJ, et al. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imler TJ, Jr, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17(+)IL-10(+) T cells, CD4(−) IFN-gamma(+) cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9:134–143. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida Y, Shioi T, Izumi T. Resveratrol ameliorates experimental autoimmune myocarditis. Circ J. 2007;71:397–404. doi: 10.1253/circj.71.397. [DOI] [PubMed] [Google Scholar]
- 14.Aribal-Kocaturk P, Kavas GO, Buyukkagnici DI. Pretreatment effect of resveratrol on streptozotocin-induced diabetes in rats. Biol Trace Elem Res. 2007;118:244–249. doi: 10.1007/s12011-007-0031-y. [DOI] [PubMed] [Google Scholar]
- 15.Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Martin AR, Villegas I, Sanchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 18.Hilsden RJ, Verhoef MJ, Best A, Pocobelli G. Complementary and alternative medicine use by Canadian patients with inflammatory bowel disease: results from a national survey. Am J Gastroenterol. 2003;98:1563–1568. doi: 10.1111/j.1572-0241.2003.07519.x. [DOI] [PubMed] [Google Scholar]
- 19.Head K, Jurenka JS. Inflammatory bowel disease. Part II: Crohn’s disease--pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2004;9:360–401. [PubMed] [Google Scholar]
- 20.Jin Y, Kotakadi VS, Ying L, et al. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis. 2008;29:2351–2359. doi: 10.1093/carcin/bgn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotakadi VS, Jin Y, Hofseth AB, et al. Ginkgo biloba extract EGb 761 has anti-inflammatory properties and ameliorates colitis in mice by driving effector T cell apoptosis. Carcinogenesis. 2008;29:1799–1806. doi: 10.1093/carcin/bgn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haneke Trans-resveratrol: Review of Toxicological Literature. NIEHS; 2002. pp. 1–64. [Google Scholar]
- 23.Abd El-Mohsen M, Bayele H, Kuhnle G, et al. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br J Nutr. 2006;96:62–70. doi: 10.1079/bjn20061810. [DOI] [PubMed] [Google Scholar]
- 24.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb J. 2007;17:17. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 25.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 26.Denkert C, Koch I, von Keyserlingk N, et al. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol. 2006;19:1261–1269. doi: 10.1038/modpathol.3800645. [DOI] [PubMed] [Google Scholar]
- 27.Singh UP, Singh S, Taub DD, Lillard JW., Jr Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol. 2003;171:1401–1406. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]
- 28.Birrell MA, McCluskie K, Wong S, et al. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. Faseb J. 2005;19:840–841. doi: 10.1096/fj.04-2691fje. [DOI] [PubMed] [Google Scholar]
- 29.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 30.Verspaget HW, Pena AS, Weterman IT, Lamers CB. Diminished neutrophil function in Crohn’s disease and ulcerative colitis identified by decreased oxidative metabolism and low superoxide dismutase content. Gut. 1988;29:223–228. doi: 10.1136/gut.29.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breider MA, Eppinger M, Gough A. Intercellular adhesion molecule-1 expression in dextran sodium sulfate-induced colitis in rats. Vet Pathol. 1997;34:598–604. doi: 10.1177/030098589703400608. [DOI] [PubMed] [Google Scholar]
- 32.Natsui M, Kawasaki K, Takizawa H, et al. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J Gastroenterol Hepatol. 1997;12:801–808. doi: 10.1111/j.1440-1746.1997.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 33.Hofseth LJ, Saito S, Hussain SP, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:143–148. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clapper ML, Gary MA, Coudry RA, et al. 5-aminosalicylic acid inhibits colitis-associated colorectal dysplasias in the mouse model of azoxymethane/dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2008;14:1341–1347. doi: 10.1002/ibd.20489. [DOI] [PubMed] [Google Scholar]
- 35.Onizawa M, Nagaishi T, Kanai T, et al. Signaling pathway via TNF-{alpha}/NF-{kappa}B in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G850–859. doi: 10.1152/ajpgi.00071.2008. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki R, Kohno H, Sugie S, et al. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 37.Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Chang WC, Coudry RA, Clapper ML, et al. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis. 2007;28:2375–2381. doi: 10.1093/carcin/bgm134. [DOI] [PubMed] [Google Scholar]
- 39.Targan SR. Current limitations of IBD treatment: where do we go from here? Ann N Y Acad Sci. 2006;1072:1–8. doi: 10.1196/annals.1326.032. [DOI] [PubMed] [Google Scholar]
- 40.Zingarelli B, Szabo C, Salzman AL. Reduced oxidative and nitrosative damage in murine experimental colitis in the absence of inducible nitric oxide synthase. Gut. 1999;45:199–209. doi: 10.1136/gut.45.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kankuri E, Vaali K, Knowles RG, et al. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(aminomethyl)benzyl]acetamidine. J Pharmacol Exp Ther. 2001;298:1128–1132. [PubMed] [Google Scholar]
- 42.Beck PL, Li Y, Wong J, et al. Inducible nitric oxide synthase from bone marrow-derived cells plays a critical role in regulating colonic inflammation. Gastroenterology. 2007;132:1778–1790. doi: 10.1053/j.gastro.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 43.El-Medany A, Mahgoub A, Mustafa A, et al. The effects of selective cyclooxygenase-2 inhibitors, celecoxib and rofecoxib, on experimental colitis induced by acetic acid in rats. Eur J Pharmacol. 2005;507:291–299. doi: 10.1016/j.ejphar.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 44.Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murthy S, Flanigan A, Coppola D, Buelow R. RDP58, a locally active TNF inhibitor, is effective in the dextran sulphate mouse model of chronic colitis. Inflamm Res. 2002;51:522–531. doi: 10.1007/pl00012423. [DOI] [PubMed] [Google Scholar]
- 46.Kim YA, Kim GY, Park KY, Choi YH. Resveratrol inhibits nitric oxide and prostaglandin E2 production by lipopolysaccharide-activated C6 microglia. J Med Food. 2007;10:218–224. doi: 10.1089/jmf.2006.143. [DOI] [PubMed] [Google Scholar]
- 47.Nunokawa Y, Oikawa S, Tanaka S. Human inducible nitric oxide synthase gene is transcriptionally regulated by nuclear factor-kappaB dependent mechanism. Biochem Biophys Res Commun. 1996;223:347–352. doi: 10.1006/bbrc.1996.0897. [DOI] [PubMed] [Google Scholar]
- 48.Zamamiri-Davis F, Lu Y, Thompson JT, et al. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic Biol Med. 2002;32:890–897. doi: 10.1016/s0891-5849(02)00775-x. [DOI] [PubMed] [Google Scholar]
- 49.Kim YA, Lim SY, Rhee SH, et al. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in beta-amyloid-treated C6 glioma cells. Int J Mol Med. 2006;17:1069–1075. [PubMed] [Google Scholar]
- 50.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kundu JK, Chun KS, Kim SO, Surh YJ. Resveratrol inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets. Biofactors. 2004;21:33–39. doi: 10.1002/biof.552210108. [DOI] [PubMed] [Google Scholar]
- 52.Kaviani A, Ohta M, Itani R, et al. Tumor necrosis factor-alpha regulates inducible nitric oxide synthase gene expression in the portal hypertensive gastric mucosa of the rat. J Gastrointest Surg. 1997;1:371–376. doi: 10.1016/s1091-255x(97)80059-5. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 54.Kowalski J, Samojedny A, Paul M, et al. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep. 2005;57:390–394. [PubMed] [Google Scholar]
- 55.Nakai M, Sudo K, Yamada Y, et al. The role of the tumor necrosis factor receptor in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice. Dig Dis Sci. 2005;50:1669–1676. doi: 10.1007/s10620-005-2913-1. [DOI] [PubMed] [Google Scholar]
- 56.Obermeier F, Kojouharoff G, Hans W, et al. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao X, Xu YX, Janakiraman N, et al. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol. 2001;62:1299–1308. doi: 10.1016/s0006-2952(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 58.Bi XL, Yang JY, Dong YX, et al. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Palmen MJ, Dijkstra CD, van der Ende MB, et al. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin Exp Immunol. 1995;101:351–356. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morohoshi Y, Matsuoka K, Chinen H, et al. Inhibition of neutrophil elastase prevents the development of murine dextran sulfate sodium-induced colitis. J Gastroenterol. 2006;41:318–324. doi: 10.1007/s00535-005-1768-8. [DOI] [PubMed] [Google Scholar]
- 61.Tikoo K, Singh K, Kabra D, et al. Change in histone H3 phosphorylation, MAP kinase p38, SIR 2 and p53 expression by resveratrol in preventing streptozotocin induced type I diabetic nephropathy. Free Radic Res. 2008;42:397–404. doi: 10.1080/10715760801998646. [DOI] [PubMed] [Google Scholar]
- 62.Lin HY, Sun M, Tang HY, et al. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J Cell Biochem. 2008;104:2131–2142. doi: 10.1002/jcb.21772. [DOI] [PubMed] [Google Scholar]
- 63.Heiss EH, Schilder YD, Dirsch VM. Chronic treatment with resveratrol induces redox stress- and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. J Biol Chem. 2007;282:26759–26766. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- 64.Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–998. [PubMed] [Google Scholar]
- 65.She QB, Bode AM, Ma WY, et al. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–1610. [PubMed] [Google Scholar]
- 66.Hofseth LJ, Ying L. Identifying and defusing weapons of mass inflammation in carcinogenesis. Biochim Biophys Acta. 2006;1765:74–84. doi: 10.1016/j.bbcan.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nature Reviews Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Experimental protocol to test the hypothesis that Resveratrol suppresses colitis.
Supplementary Figure 2. Experimental protocol to test the hypothesis that Resveratrol suppresses colon cancer associated with colitis.
Supplementary Figure 3. Tumor formation in an AOM + DSS mouse model of colon cancer. Tumor formation in colons of mice treated with AOM + DSS is prevented when these mice consume resveratrol (300 p.p.m.) in their basal diet. A. Colons stained with methylene blue. Arrows indicate macroscopic tumors. B. Representative H&E sections. The AOM + DSS group shows a polypoid adenoma with high grade dysplasia, characteristic of tumors in this group.