Abstract
Object
Muscimol is a potent γ-aminobutyric acid-A receptor agonist (GABAA) that temporarily and selectively suppresses neurons. Targeted muscimol-suppression of neuronal structures could provide insight into the pathophysiology and treatment of a variety of neurologic disorders. To determine if muscimol delivered to the brain by convection-enhanced delivery (CED) could be monitored using a co-infused surrogate magnetic resonance (MR)-imaging tracer, we perfused the striata of primates with tritiated muscimol and gadolinium-DTPA.
Methods
Three primates underwent convective co-infusion of 3H-muscimol (0.8 μM) and gadolinium-DTPA (−5 mM) into the bilateral striata. Primates underwent serial MR-imaging during infusion and animals were sacrificed immediately after infusion. Post-mortem quantitative autoradiography and histological analysis was performed.
Results
MR-imaging revealed that infusate (tritiated muscimol and gadolinium-DTPA) distribution was clearly discernible from the non-infused parenchyma. Real-time MR-imaging of the infusion revealed the precise region of anatomic perfusion in each animal. Imaging analysis during infusion revealed that the distribution volume of infusate linearly increased (R=0.92) with volume of infusion. Overall, the mean (±S.D.) volume of distribution to volume of infusion ratio was 8.2±1.3. Autoradiographic analysis revealed that MR-imaging of gadolinium-DTPA closely correlated with the distribution of 3H-muscimol and precisely estimated its volume of distribution (mean difference in volume of distribution, 7.4%). Quantitative autoradiograms revealed that muscimol was homogeneously distributed over the perfused region in a square-shaped concentration profile.
Conclusions
Muscimol can be effectively delivered to clinically relevant volumes of the primate brain. Moreover, the distribution of muscimol can be tracked by co-infusion of gadolinium-DTPA using MR-imaging. The ability to accurately monitor and control the anatomic extent of muscimol distribution during its convection-enhanced delivery will enhance safety, permit correlations of muscimol distribution with clinical effect, and should lead to an improved understanding of the pathophysiologic processes underlying a variety of neurologic disorders.
Keywords: Brain, convection-enhanced delivery, imaging, muscimol, treatment
Introduction
Selective manipulation of neuronal populations within diseased circuits using neuroactive drugs represents a powerful tool for gaining insight into the pathophysiology and improving treatment of a variety of central nervous system (CNS) disorders. Specifically, the targeted use of a selective neural suppressive agent may provide an opportunity to medically manipulate pathophysiologic activity in a manner that is not available with currently existing technology. Previous studies have shown that muscimol is a short-acting potent γ-aminobutyric acid-A receptor agonist (GABAA) that selectively suppresses neuronal activity. This finding indicates that targeted delivery of muscimol could help elucidate the role of specific neuronal structures and pathologic circuits involved in CNS disorders.
Previously, it was not possible to target focal diseased neuronal populations with putative neuroactive compounds, because CNS drug distribution techniques relied on either systemic delivery or drug administration into cerebrospinal fluid spaces (intrathecal or intraventricular administration).6,13,15 Drug distribution with each of these delivery methods is limited by their intrinsic properties. Neither technique allows for targeted delivery. Systemic delivery of many compounds is restricted by systemic toxicity, ineffective penetration of the blood–brain barrier and inability to target specific anatomic treatment sites within the CNS. Because intrathecal or intraventricular delivery is driven by diffusion, drug distribution is severely constrained (2 to 4 millimeters from the CNS surface), non-targeted (covering the entire CNS surface) and heterogeneous.
Over the last several years, studies have revealed that convection-enhanced delivery (CED) can overcome many of the barriers limiting drug delivery to the CNS.2 Because CED uses a stereotactically placed cannula and depends on a small hydrostatic pressure gradient to transport compounds by bulk flow, it bypasses the blood-nervous system barrier and directly delivers infusate homogeneously in a targeted way to the extracellular space of the CNS.2,13 Recent studies have shown that co-infusion of surrogate imaging tracers may permit real-time magnetic resonance (MR)-imaging of convective drug delivery in the CNS. 11,12,14
To determine if CED of muscimol can be effectively monitored using MR-imaging, we imaged the brains of primates during co-infusion of 3H-muscimol and gadolinium-DTPA into the striatum.
Materials and Methods
Preparation of 3H-Muscimol / gadolinium-DTPA co-infusate
3H-muscimol (Perkin-Elmer; Boston, MA) and clinical grade gadolinium-DTPA (Berlex; Pointe-Claire, Quebec) were used in all infusions. 3H-muscimol and gadolinium-DTPA were diluted in phosphate buffered saline (PBS) (0.8 μM 3H-muscimol, 20 nCi/μl; 5 mM gadolinium-DTPA) before infusion.
Convective Co-Infusion of Muscimol and Gadolinium-DTPA
Three adult primates (Macaca fascicularis) underwent convective co-infusion of 13.5 to 35 microliters (Table 1) of 3H-muscimol and gadolinium-DTPA to the bilateral striata. The animals were placed under general endotracheal anesthesia. Vital signs were monitored during the operation and infusion. The head of the animal was secured in a stereotactic frame (Model 9-YSTI-35, Crist Instrument Co., Inc., Hagerstown, MD). A skin incision was made in the midline on the skull vertex to expose the skull. Two 8 mm burr holes were placed over the cannula entry points and the dura mater was opened. The outer guide cannulas (outer diameter 0.686 mm, inner diameter 0.508 mm) were bilaterally positioned along the target trajectory to a level 1.5 cm above the desired striatal target. The guide cannulas were secured with methylmethacrylate to the skull. The inner cannulas (outer diameter 0.356 mm, inner diameter 0.152 mm) were connected to the infusion tubing/syringe. They were then placed through the outer guide cannula to target.
Table 1.
Accuracy of gadolinium-DTPA as an imaging tracer for predicting 3H-muscimol distribution.
| Animal | Vi (microliters) | Striatum | *QAR Vd (mm3) | *MR-imaging Vd (mm3) | Percent difference | **Vd:Vi |
|---|---|---|---|---|---|---|
| 1 | 35 | Right | 228.7 | 228.1 | 0.2% | 6.5 |
| 35 | Left | 330.3 | 334.5 | 1.3% | 9.4 | |
| 2 | 27 | Right | 221.1 | 170.5 | −22.8% | 8.2 |
| 27 | Left | 261.1 | 297.8 | 14.0% | 9.7 | |
| 3 | 13.5 | Right | 111.5 | 106.0 | −5.0% | 8.3 |
| 18 | Left | 123.5 | 125.0 | 1.2% | 6.9 |
Volume of distribution (Vd) of radiolabeled 3H-muscimol as determined by quantitative autoradiography (QAR) compared with Vd of co-infused gadolinium-DTPA imaged using magnetic resonance (MR)-imaging. Volume of infusion (Vi).
QAR Vd was used in calculating this ratio.
Infusate was distributed to the striatum using convection using a previously described noncompliant, dead-volume free, gastight delivery system.12 A Harvard syringe pump (PHD 2000, Harvard Apparatus, Inc., South Natick, MA) was used to create a small hydrostatic pressure during the infusion. The pressure was transmitted to an infusate-filled syringe (250 microliter total volume) that was connected to polyethylene tubing (outer diameter 1.270 mm, inner diameter 0.584 mm) (Plastics One, Roanoke, VA). The inner infusion cannula was connected to the polyethylene tubing. Infusions were performed at 0.1 to 0.5 microliters/minute. Animals were euthanized upon completion of the infusions.
Imaging of Muscimol and Gadolinium-DTPA Distribution
MR images (T1-weighted in coronal plane) were obtained to determine the precise location of the inner infusion cannulas after they were placed to target. The infusions were started and T1-weighted MR-images were obtained in 3 planes (sagittal, axial, and coronal; slice thickness 1 mm, 0 mm spacing) using a 3 Tesla MR-scanner. Images were obtained at approximately 10 to 20 minute intervals until the infusions were complete.
Effectiveness of Gadolinium-DTPA as a Surrogate Imaging Tracer
Quantitative autoradiography (QAR)
Brains were sectioned in 20-micron-thick serial coronal sections. Autoradiograms were prepared from every tenth tissue section. Tissue sections and 3H-standards were then exposed on imaging plates (BAS-TR2025 plates; Fuji Medical Systems, Stamford, CT). Plates were developed using a BAS-5000 Bio-Imaging Analyzer (Fuji Medical Systems). Infusate area of distribution on each side (right and left) was determined (Image Gauge v 3.45 software program; Fuji Medical Systems) using a segmentation threshold of 10% of the maximum optical density in the region of interest. Volume of distribution (Vd) was determined by multiplying the sum of the areas of distribution on each side (right and left) by 0.2 mm.9,10,14
MR-Imaging Analysis
MR-images from the infusion were analyzed on an imaging work station (Sun workstation; Sun Microsystems, Inc., Palo Alto, CA). Vds from MR-images were calculated using image analysis software (MEDx 3.4; Sensor Systems, Inc., Sterling, VA). The MR-signal intensity segmentation threshold value was 2 standard deviations above the mean signal of the surrounding non-infused region.14 The Vd based on MR-imaging of gadolinium-DTPA and the Vd based on autoradiographic distribution of 3H-muscimol were compared.
Statistical Analysis
Statistical analysis was performed with commercial software (JMP 7.0, SAS Institute, Inc., Cary, NC) as described in the text.
Results
Convective Co-Infusion of Muscimol and Gadolinium-DTPA
Feasibility of imaging
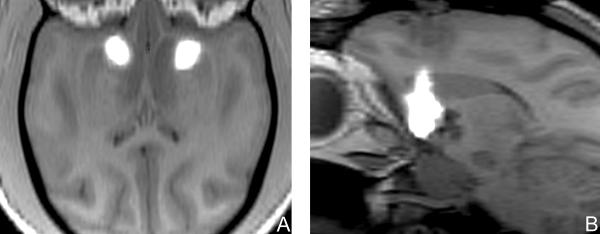
To establish the feasibility of convective co-infusion of 3H-muscimol and gadolinium-DTPA in the brain, we infused the bilateral striata of 3 primates with 13.5 to 35 microliters of 3H-muscimol (0.8 μM) and gadolinium-DTPA (1 mM) (Table 1). During convective co-infusion of gadolinium-DTPA and muscimol, MR-imaging revealed that the region of infusate perfusion was clearly demarcated from the immediately adjacent non-infused parenchyma (Figure 1). During infusion, the high-MR signal associated with the infusion steadily expanded around the tip of the cannula.
Figure 1.
(A) Axial and (B) left parasagittal T1-weighted magnetic resonance images of primate brain after perfusion of each striatum with 35 microliters of muscimol co-infused with gadolinium-DTPA. Gadolinium-DTPA tracer provided a distinct region of magnetic resonance T1-weighted hyperintensity (white area) compared to the surrounding tissue and could be seen filling the striata.
Vd:Vi ratio
To determine the Vd to Vi ratio during the infusion, volumetric analysis of real-time MR-imaging was performed. Based on the distinct imaging appearance of the co-infused gadolinium-DTPA, the volume of distribution in the perfused anatomic region was clearly depicted allowing for precise calculation of infusate volume of distribution. During the infusion, volumetric analysis of the perfused region revealed that the Vd of the gadolinium-DTPA and muscimol increased linearly with increasing Vi (R=0.92) (Figure 2). The final mean Vd to Vi ratio for the volumes infused was 8.2±1.3 (mean±S.D.) (Table 1).
Figure 2.
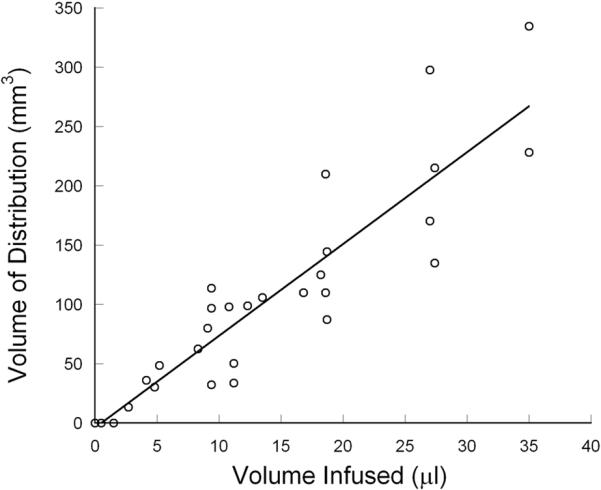
There was a linear relationship (R= 0.92) between volume of infusion (Vi) and volume of distribution (Vd) in primates infused with gadolinium-DTPA. The mean Vd:Vi ratio was 8.2± 1.3 (mean ± S.D.).
Homogeneity of infusion
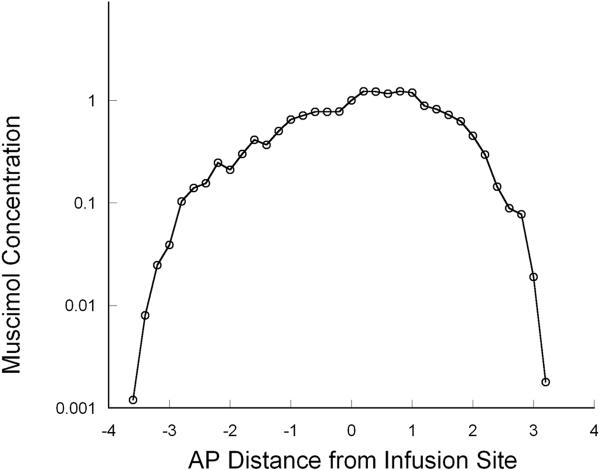
To determine the homogeneity of muscimol distribution throughout the perfused region, we analyzed the concentration gradient of muscimol over the infused area in the striata by quantification of the specific activity of 3H-muscimol in tissue slices by QAR. The concentration of the delivered infusate as determined by autoradiographic analysis of the perfused tissues revealed that muscimol distribution was relatively homogeneous and the concentration profile was square-shaped with a steep drop in concentration gradient at the infused region limits (Figure 3).
Figure 3.
Autoradiographic measurement of tissue concentrations across the perfused region demonstrated a relatively homogeneous “square-shaped” distribution profile with a steep concentration drop-off at the margins of the perfused tissue at the end of the infusion. Positive numbers refer to anterior spread and negative numbers to posterior spread of muscimol from the site of infusion.
Effectiveness of Gadolinium-DTPA as a Surrogate Imaging Tracer
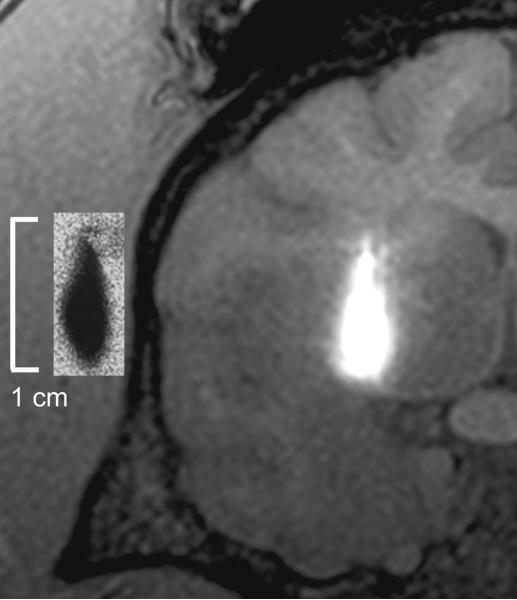
To establish the effectiveness of gadolinium-DTPA (MW 983 Da) as a surrogate imaging tracer for muscimol (MW 114 Da), we compared the volumetric distribution of co-infused 3H-muscimol and gadolinium-DTPA as measured by QAR and MR-imaging analyses. QAR and MR-images demonstrated near identical patterns of anatomic and volumetric infusate delivery (Figure 4). The mean difference between the Vds computed from MR-imaging of gadolinium-DTPA and autoradiography of 3H-muscimol infusion was 7.4% (p=0.39, paired t test; correlation 0.85) (Table 1). This difference in Vds between modalities resulted in a radial difference between imaging and autoradiographic distribution of less than 0.5 mm (Figure 4).
Figure 4.
Coronal T1-weighted magnetic resonance imaging after convection-enhanced delivery of 13.5 microliters of radiolabeled 3H-muscimol co-infused with gadolinium-DTPA (surrogate-imaging tracer) into the right striatum. (Inset) Corresponding autoradiogram of 3H-muscimol distribution demonstrates the anatomical and spatial accuracy obtained when utilizing gadolinium-DTPA as a surrogate tracer for muscimol.
Discussion
Muscimol
We determined in primates the feasibility and accuracy of convective co-infusion of a MR-imaging surrogate tracer (gadolinium-DTPA) and similarly sized muscimol. Based on these findings, we hope to develop region-specific treatment paradigms that permit selective pharmacologic manipulation and/or treatment of pathologic foci within the CNS.
Description
Muscimol (3-hydroxy-5-aminomethylisoxazole) is a GABAA receptor agonist that has been used to study the effects and function of GABA within the CNS. GABA is the primary inhibitory neurotransmitter in the brain and is present in up to 70% of all brain synapses. GABA is an inhibitory neurotransmitter that causes hyperpolarization of the post-synaptic neuron by increasing chloride ion conductance. Muscimol is broken down more slowly than GABA and subsequently, has a more sustained suppressive effect than GABA.
Safety
Previously, intraoperative microinjections of muscimol into deep brain nuclei of Parkinson's disease patients have been performed.,8,16} In these studies, muscimol was used to transiently inhibit neurons within the globus pallidus interna or subthalamic nucleus and elicit a temporary clinical effect before lesioning or placement of a deep brain stimulator (DBS). Infusion of muscimol was safe and resulted in a marked reversal of Parkinson's disease symptomatology beginning 5 to 10 minutes after infusion and lasting for 10 to 60 minutes. The clinical improvements with muscimol infusion correlated closely with successful final treatment effects (either lesioning or deep brain stimulation) in all cases. While these studies provided preliminary data about the potential effect and utility of intracerebral infusion of muscimol, they did not provide insight into the relationship of drug distribution and specific symptom amelioration because anatomic distribution of drug was not assessed.
Accuracy of imaging of muscimol distribution
When CED is used in preclinical and clinical studies it will be critical to determine the treatment volume and region of perfusion of infusate. Specifically, it will be useful to co-administer an imaging agent whose imaged distribution is accurately correlated to that of drug perfusion. These data show that the imaged distribution of gadolinium-DTPA provides an accurate estimation of the Vd and anatomic region of co-infused muscimol (Table 1; Figure 4).
Muscimol and gadolinium-DTPA are highly water-soluble and without extensive hydrophobic regions. Because of this, hydrophobic interaction between the 2 agents is highly unlikely. Interaction of these compounds on the basis of charge is also unlikely. In solution, muscimol is a zwitterion, with no net charge, and gadolinium-DTPA is a salt that ionizes into 2 ions of N-methylglucamine, a positively charged quaternary amine, and gadopentatate, a negatively charged chelate of gadolinium. Gadolinium-DTPA does not bind to protein, in contrast to other gadolinium chelates which bind weakly or strongly to protein.3 These factors suggest that muscimol and gadolinium-DTPA should not interact on a molecular level. Gadolinium-DPTA has not been reported to react chemically with muscimol or similar compounds. It is therefore unlikely that gadolinium-DTPA would influence the volume of distribution of muscimol.
Gadolinium-DTPA and muscimol interact dissimilarly with brain structures. Gadolinium-DTPA passes through the extracellular space of the brain until it is absorbed into the blood stream or CSF. In contrast, muscimol passes through the extracellular space until it binds to the GABA-A receptor on the surface of neurons, is enzymatically degraded, or is absorbed into the blood stream or CSF. Despite these differences, the distribution of tritiated muscimol measured by autoradiography was nearly identical to the distribution of gadolinium signal recorded by MR-imaging, presumably because binding of some muscimol molecules to GABA-A receptors did not substantially reduce the distribution of unbound muscimol. 1,18
Volume of muscimol infusion
The Vd of tissue perfusion increased linearly with increasing Vi of muscimol and gadolinium-DTPA. The overall Vd:Vi ratio was 8.2±1.3. Because the CED distributes infusate within the interstitial spaces of the CNS, the Vd:Vi ratio mirrors the extracellular fraction of tissue volume. The Vd:Vi ratio in this study is similar to a previous study that examined CED of gadolinium-DTPA within grey matter of the brain (Vd:Vi ratio, 7.0±0.4).11 Vi and subsequent Vd obtained in these animals and reported in other studies indicate that it is feasible to perfuse clinically relevant volumes of neuronal structures in the human brain. Specifically, deep brain nuclei including the subthalamic nucleus, globus pallidus internus and thalamus in humans could be completely perfused by infusion volumes similar to those used in this study. 17 Similarly, much larger Vds could be obtained using larger Vi as demonstrated by the linear relationship between Vi and Vd in this study, as well as in long-term infusion studies of muscimol in primates.5
Homogeneity of infusion
Concentration of the infusate (muscimol) in perfused regions was homogeneous. QAR analysis of muscimol concentration revealed uniformity and a steep fall-off at the margins of the perfused area. These findings are consistent with the known bulk flow properties of CED and previous studies that have described CED of molecules to the CNS.2,4,9,10 These results confirm that this technique may be used to distribute infusate in homogeneous concentrations over targeted areas in the CNS. 7 Because muscimol is a small molecule, it will diffuse rapidly from the perfused region at the completion of infusion and the slope of the concentration gradient at the margins will decrease with time. Subsequently, delayed interpretation of muscimol distribution after infusion completion or with prolonged infusions may differ from short term infusions.
Potential Applications
Imaging of convective muscimol distribution will allow direct anatomic-physiologic correlation to clinical effect. Data from this paradigm should lead to deeper understanding of CNS pathophysiology, lead to development of new diagnostic methods and permit the development of new pharmacologic-based treatments.
Pathophysiologic investigations
The ability to selectively suppress neurons and neuronal circuits provides an opportunity to study the impact that site-specific neuronal suppression has on pathophysiologic circuitry. Specifically, focal suppression of deep brain nuclei (e.g., globus pallidus interna, thalamus or subthalamic nucleus) and selected portions of other nuclei or brain regions, as determined by real-time imaging in 1) Parkinson's disease, 2) psychiatric disorders, 3) movement disorders other than Parkinson's disease, 4) pain syndromes and 5) epilepsy, should give direct insights into the role that specific neurons play in the circuitry of these disorders.
Understanding of the mechanisms of symptom alleviation by selective neuronal suppression will also provide critical insights into the specific therapeutic mechanisms of existing treatments, including DBS. Currently, the mechanism of clinical improvement associated with DBS remains unknown (e.g., does symptom improvement result from electrical suppression of neurons, white matter tracts or both?). Deeper understanding of the mechanisms of improvement and potential treatment complications should (at a minimum) lead to improved use of this treatment modality and avoidance of complications.
Diagnostic applications
Because the effect of muscimol is temporary, selective perfusion of neuronal targets could provide useful diagnostic and/or predictive (for therapeutic success or potential complications associated with other surgical treatments) information in various neurologic disorders. An ideal disorder for application of this diagnostic paradigm is medically intractable epilepsy. Short-term (days) co-infusion of muscimol and imaging tracer during continuous electrocorticographic monitoring could be used to evaluate suspected epileptogenic foci. Accurate localization of a site of perfusion that ameliorated seizures or epileptogenic activity would encourage a tailored resection of this region. Alternatively, perfusion of muscimol and tracer could potentially be used to predict complications associated with resection of the perfused region.
Treatment applications
To develop surgical interventions that are disease (or cell) specific, increasingly flexible and reversible, intracerebral infusions have enormous potential for selective therapeutic manipulations of diseased neurotransmitter systems. Muscimol delivered by intermittent or continuous infusion via implanted pumps could represent a new targeted, pharmacologic, and neuromodulatory treatment paradigm. Because its effects are transient, untoward effects of muscimol can be reversed by discontinuing its infusion. Success of any therapeutic compound that is infused into the brain ultimately depends on the accurate delivery and known distribution of the agent being infused.
Conclusions
Muscimol can be effectively delivered to clinically relevant volumes of the primate brain. The distribution of muscimol can be monitored by co-infusion of gadolinium-DTPA and MR-imaging. The ability to accurately trace muscimol delivery through the use of surrogate markers will enhance safety, permit direct anatomic-physiologic correlation with clinical effect and should provide insight into a variety of neurologic disorders.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
References
- 1.Baraldi M, Grandison L, Guidotti A. Distribution and metabolism of muscimol in the brain and other tissues of the rat. Neuropharmacology. 1979;18:57–62. doi: 10.1016/0028-3908(79)90009-1. [DOI] [PubMed] [Google Scholar]
- 2.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavagna FM, Maggioni F, Castelli PM, Dapra M, Imperatori LG, Lorusso V, et al. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol. 1997;32:780–796. doi: 10.1097/00004424-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg. 1999;90:315–320. doi: 10.3171/jns.1999.90.2.0315. [DOI] [PubMed] [Google Scholar]
- 5.Heiss JD, Walbridge S, Morrison P, Hampton RR, Sato S, Vortmeyer A, et al. Local distribution and toxicity of prolonged hippocampal infusion of muscimol. Journal of Neurosurgery. 2005;103:1035–1045. doi: 10.3171/jns.2005.103.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer R. New methods of drug delivery. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 7.Laske DW, Morrison PF, Leiberman DM, Corthesy ME, Reynolds JC, Stewart-Henney PA, et al. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg. 1997;87:586–594. doi: 10.3171/jns.1997.87.4.0586. [DOI] [PubMed] [Google Scholar]
- 8.Levy R, Lang AE, Dostrovsky JO, Pahapill P, Romas J, Saint-Cyr J, et al. Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain. 2001;124:2105–2118. doi: 10.1093/brain/124.10.2105. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 10.Lonser RR, Gogate N, Morrison PF, Wood JD, Oldfield EH. Direct convective delivery of macromolecules to the spinal cord. Journal of Neurosurgery. 1998;89:616–622. doi: 10.3171/jns.1998.89.4.0616. [DOI] [PubMed] [Google Scholar]
- 11.Lonser RR, Schiffman R, Robison RA, Butman JA, Quezado Z, Walker ML, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 12.Lonser RR, Walbridge S, Garmestani K, Butman JA, Walters HA, Vortmeyer AO, et al. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. Journal of Neurosurgery. 2002;97:905–913. doi: 10.3171/jns.2002.97.4.0905. [DOI] [PubMed] [Google Scholar]
- 13.Morrison PF. Distributed models of drug kinetics. In: Atkinson A, Daniels CE, Dedrick RL, Grudzinskas CV, Markey SP, editors. Principles of Clinical Pharmacology. Academic Press; New York: 2001. [Google Scholar]
- 14.Nguyen TT, Pannu YS, Sung C, Dedrick RL, Walbridge S, Brechbiel MW, et al. Convective distribution of macromolecules in the primate brain demonstrated using computerized tomography and magnetic resonance imaging. Journal of Neurosurgery. 2003;98:584–590. doi: 10.3171/jns.2003.98.3.0584. [DOI] [PubMed] [Google Scholar]
- 15.Pardridge WM. Drug delivery to the brain. Journal of Cerebral Blood Flow & Metabolism. 1997;17:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Penn RD, Kroin JS, Reinkensmeyer A, Corcos DM. Injection of GABA-agonist into globus pallidus in patient with Parkinson's disease [letter] Lancet. 1998;351:340–341. doi: 10.1016/S0140-6736(05)78336-7. [DOI] [PubMed] [Google Scholar]
- 17.Yelnik J. Functional anatomy of the basal ganglia. Mov Disord. 2002;17(Suppl 3):S15–21. doi: 10.1002/mds.10138. [DOI] [PubMed] [Google Scholar]
- 18.Zhang HL, Ersoy H, Prince MR. Effects of gadopentetate dimeglumine and gadodiamide on serum calcium, magnesium, and creatinine measurements. J Magn Reson Imaging. 2006;23:383–387. doi: 10.1002/jmri.20517. [DOI] [PubMed] [Google Scholar]