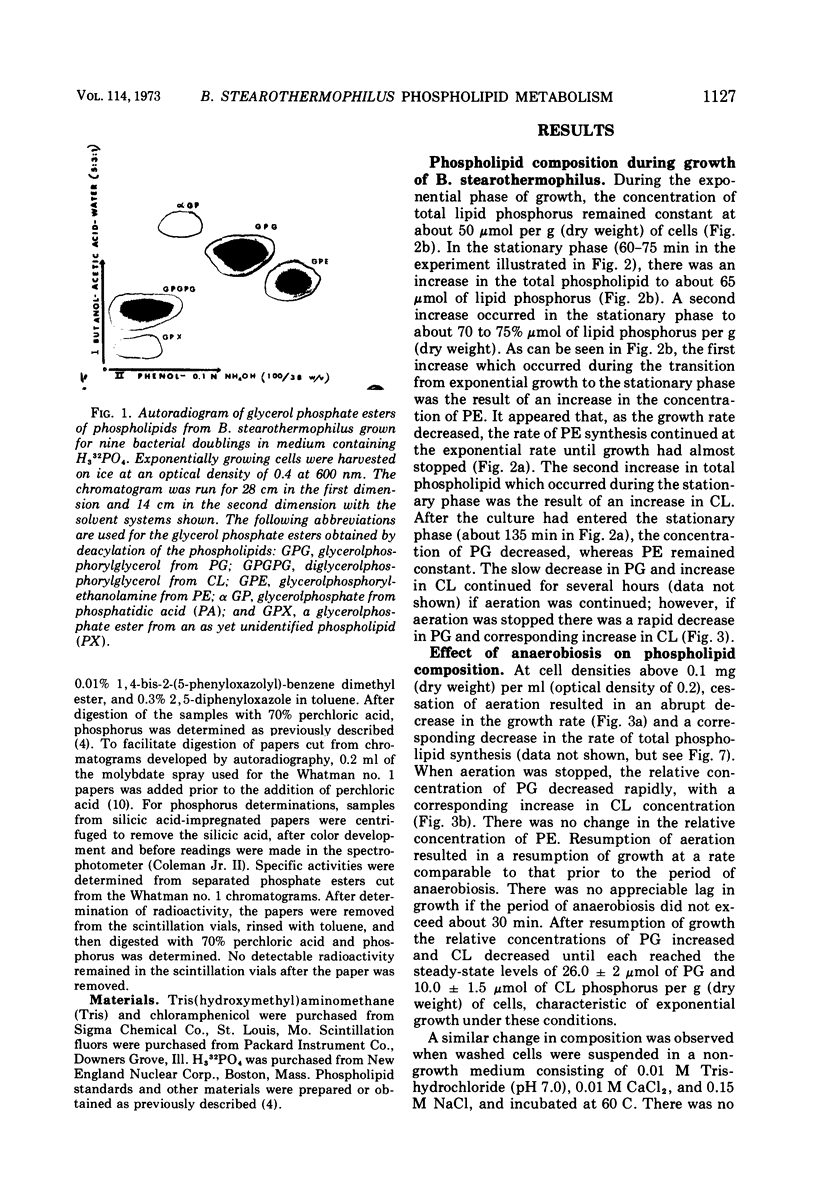
Abstract
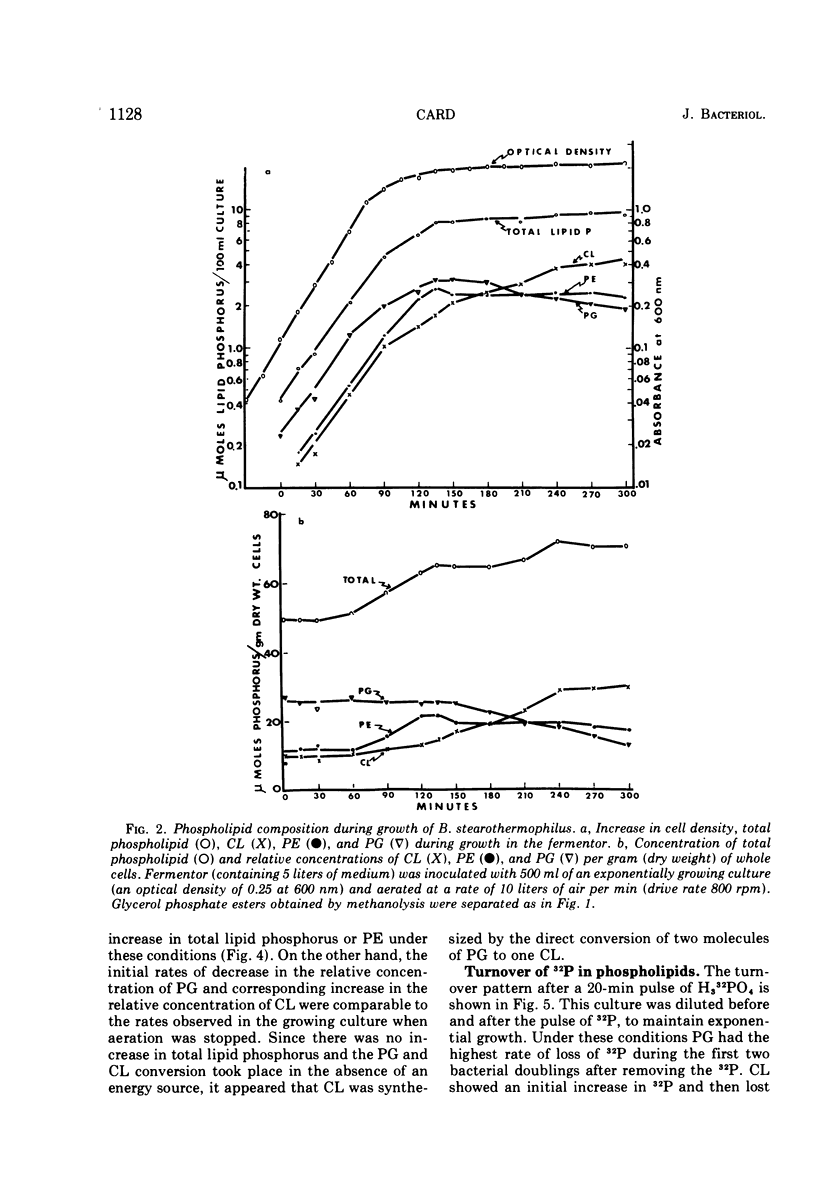
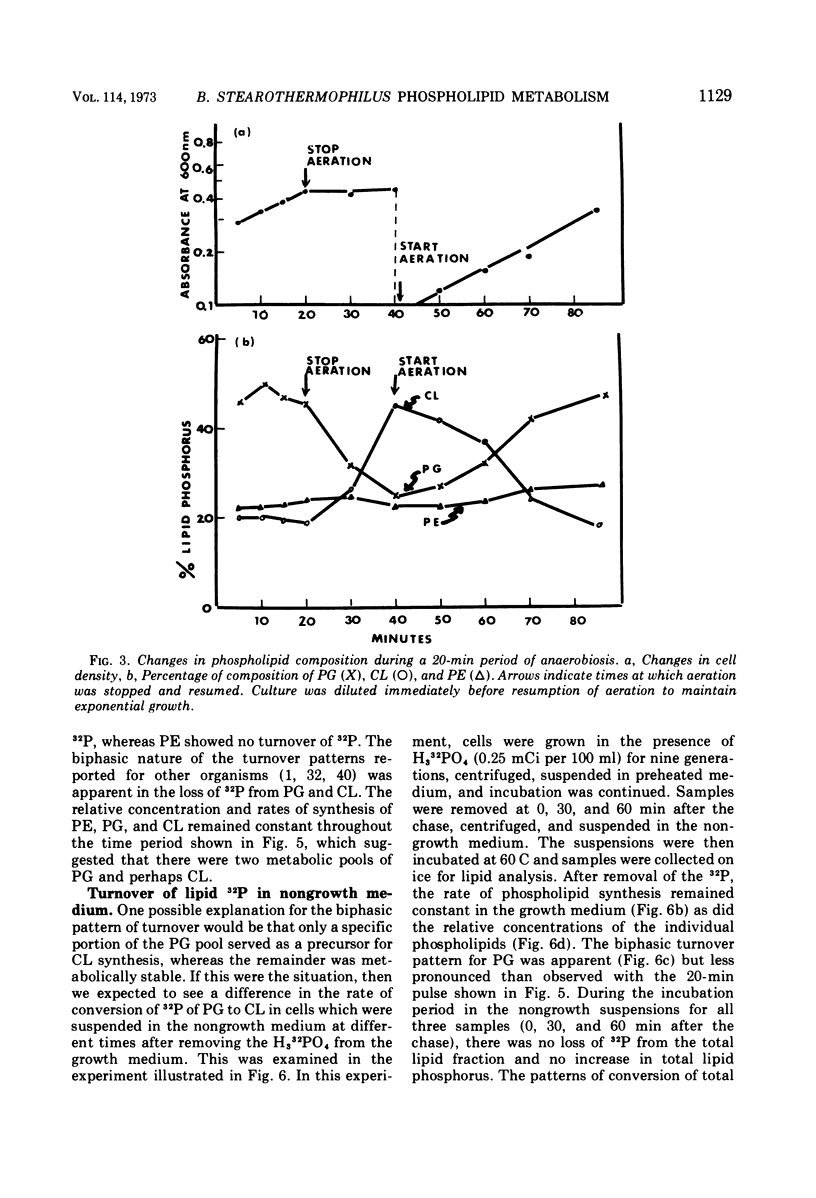
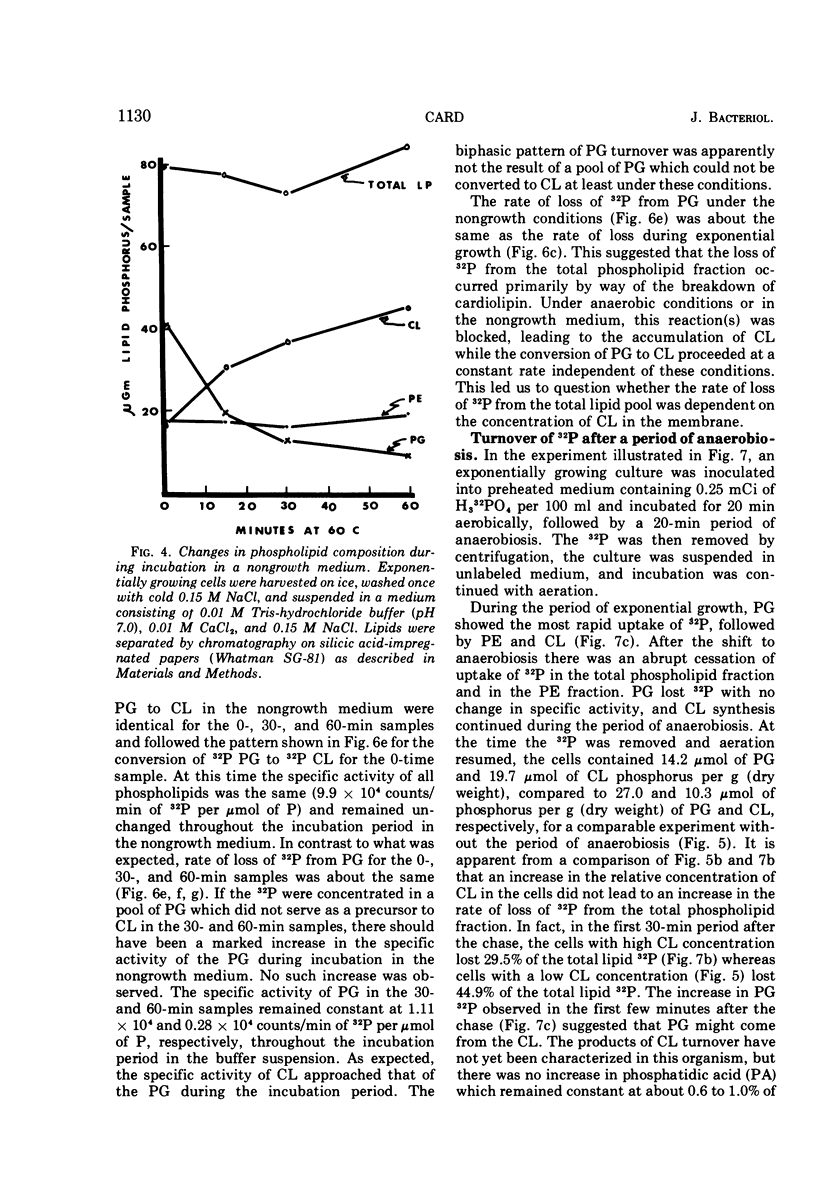
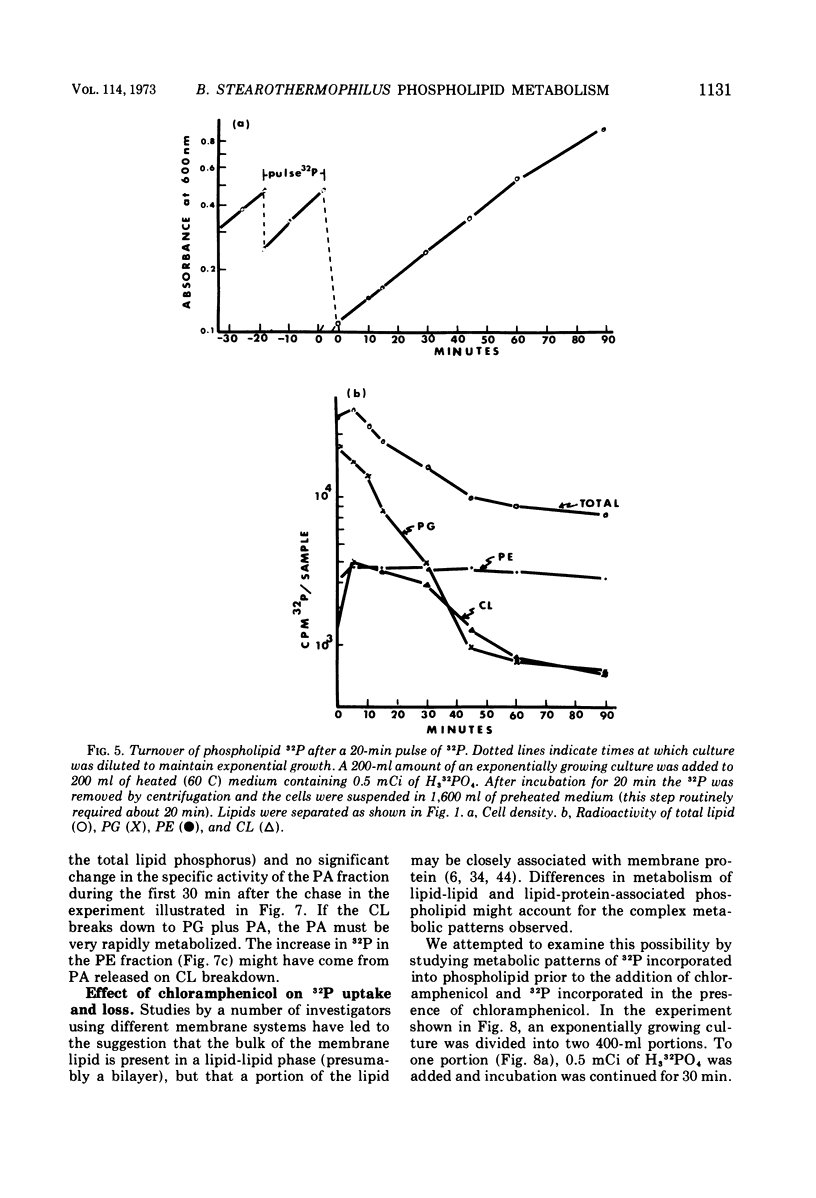
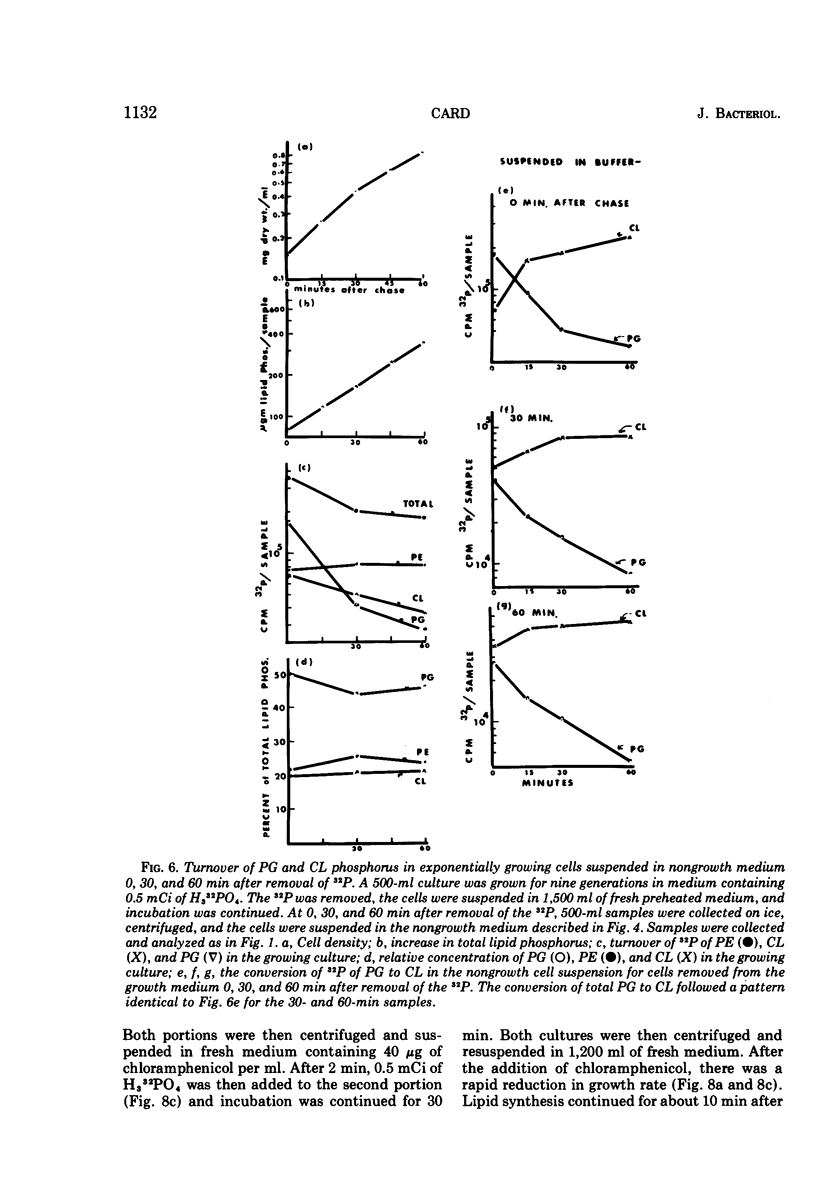
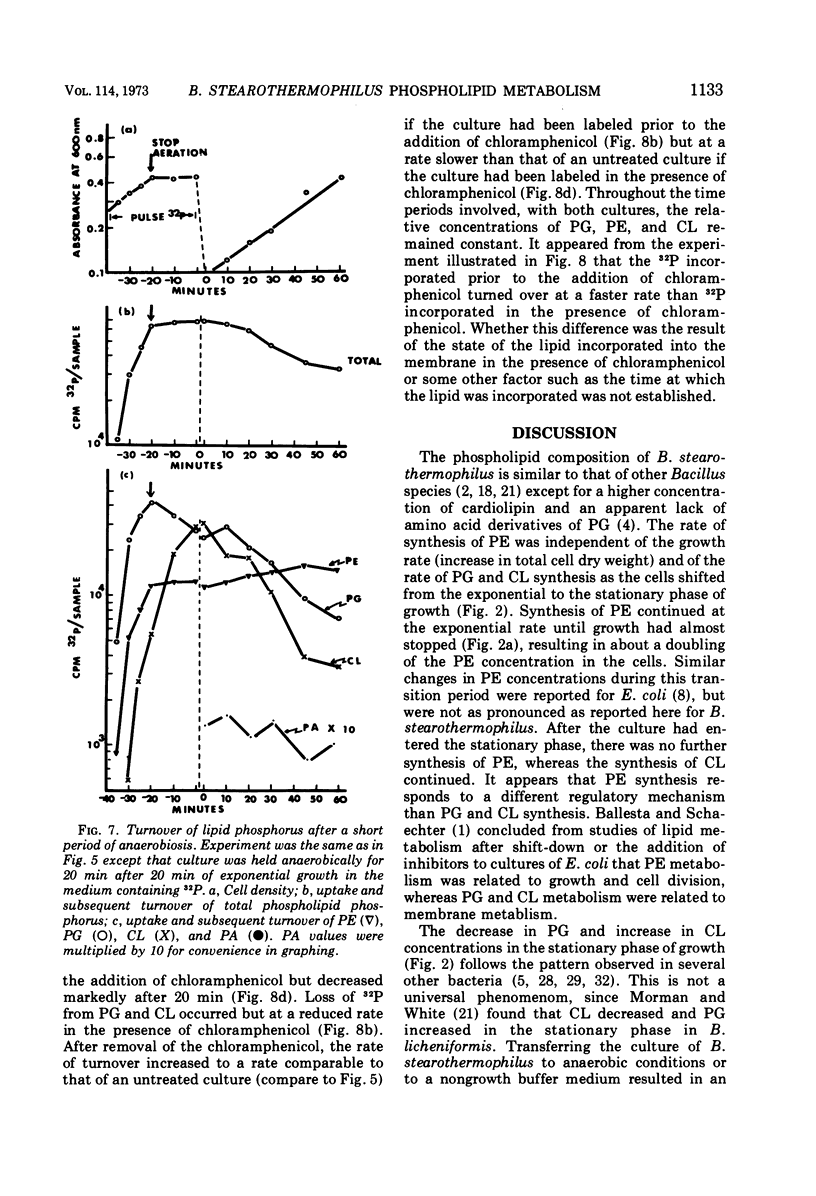
The total phospholipid content of Bacillus stearothermophilus was constant during exponential growth, increased during the transition from the exponential to stationary phase of growth, and then slowly increased during the stationary phase. The first increase was a result of an increase in phosphatidylethanolamine; the second was a result of an increase in cardiolipin. Cessation of aeration of an exponentially growing culture or suspension in a nongrowth medium resulted in an immediate reduction in the rate of total phospholipid and phosphatidylethanolamine synthesis and a quantitative conversion of phosphatidylglycerol to cardiolipin. Cardiolipin appeared to be synthesized by the direct conversion of two molecules of phosphatidylglycerol to cardiolipin. After a 20-min pulse of 32P, phosphatidylglycerol showed the most rapid loss of 32P followed by cardiolipin, whereas phosphatidylethanolamine did not lose 32P. The loss of 32P from the total lipid pool, phosphatidylglycerol, and cardiolipin was biphasic, with rapid loss during the first two bacterial doublings followed by a greatly reduced rate of loss. The major loss of 32P from the total phospholipid pool appeared to be by breakdown of cardiolipin. The loss of 32P from the lipid pool was energy dependent (i.e., did not occur under anaerobic conditions or in the absence of an energy source) and was dependent on some factor other than the concentration of cardiolipin in the cells. The apparent conversion of phosphatidylglycerol to cardiolipin was independent of energy metabolism. Chloramphenicol reduced the rate of turnover of both phosphatidylglycerol and cardiolipin. The rate of lipid synthesis (all phospholipid components) was constant for about 10 min after the addition of chloramphenicol but diminished markedly after 20 min. Turnover of 32P incorporated into phospholipid during a 30-min period prior to the addition of chloramphenicol was more rapid after the removal of chloramphenicol than that of 32P incorporated during a 30-min period in the presence of chloramphenicol.
Full text
PDF

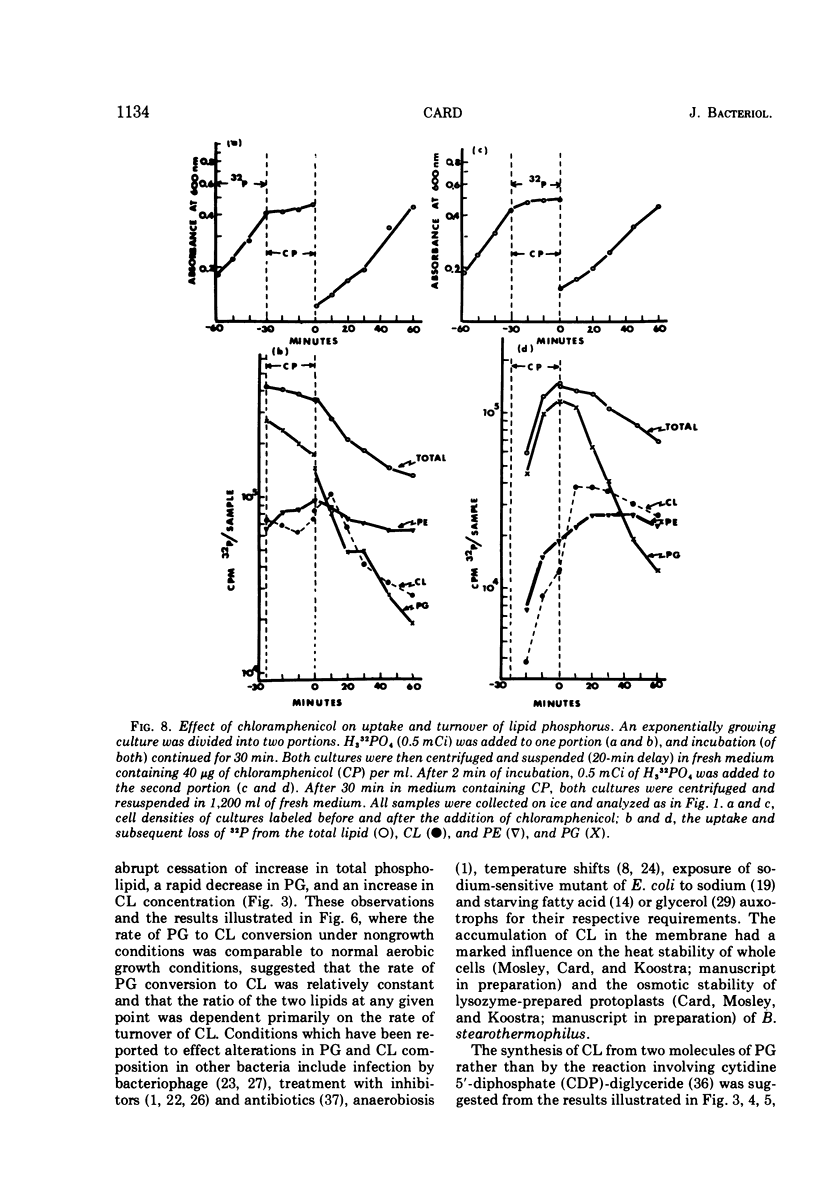



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., Schaechter M. Effect of shift-down and growth inhibition on phospholipid metabolism of Escherichia coli. J Bacteriol. 1971 Jul;107(1):251–258. doi: 10.1128/jb.107.1.251-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 1967 Nov;2(4):448–453. doi: 10.1111/j.1432-1033.1967.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Card G. L., Georgi C. E., Militzer W. E. Phospholipids from Bacillus stearothermophilus. J Bacteriol. 1969 Jan;97(1):186–192. doi: 10.1128/jb.97.1.186-192.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr Phospholipid alterations during growth of Escherichia coli. J Bacteriol. 1968 Jun;95(6):2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Daniels M. J. Some features of the bacterial membrane studied with the aid of a new fractionation technique. Biochem J. 1971 Apr;122(2):197–207. doi: 10.1042/bj1220197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J., Salton M. R. Biosynthesis of cardiolipin in the membranes of Micrococcus lysodeikticus. Biochim Biophys Acta. 1971 Jul 13;239(2):280–292. doi: 10.1016/0005-2760(71)90174-3. [DOI] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerman F. E., White D. C. Membrane lipid changes during formation of a functional electron transport system in Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1868–1874. doi: 10.1128/jb.94.6.1868-1874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz G. S. Lipids in membrane development. Adv Lipid Res. 1970;8:175–223. [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., van den Bosch H., van Deenen L. L. The mechanism of cardiolipin biosynthesis in liver mitochondria. Biochim Biophys Acta. 1972 Mar 23;260(3):507–513. doi: 10.1016/0005-2760(72)90065-3. [DOI] [PubMed] [Google Scholar]
- Hsu C. C., Fox C. F. Induction of the lactose transport system in a lipid-synthesis-defective mutant of Escherichia coli. J Bacteriol. 1970 Aug;103(2):410–416. doi: 10.1128/jb.103.2.410-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. H., White D. C. Effect of benzo(a) pyrene and piperonyl butoxide on formation of respiratory system, phospholipids, and carotenoids of Staphylococcus aureus. J Bacteriol. 1971 May;106(2):403–411. doi: 10.1128/jb.106.2.403-411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillich T. T., White D. C. Phospholipid metabolism in the absence of net phospholipid synthesis in a glycerol-requiring mutant of Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):790–797. doi: 10.1128/jb.107.3.790-797.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk J. E., Kennedy E. P. Altered phospholipid metabolism in a sodium-sensitive mutant of Escherichia coli. J Bacteriol. 1972 Mar;109(3):1034–1046. doi: 10.1128/jb.109.3.1034-1046.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Morman M. R., White D. C. Phospholipid metabolism during penicillinase production in Bacillus licheniformis. J Bacteriol. 1970 Oct;104(1):247–253. doi: 10.1128/jb.104.1.247-253.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Tropp B. E. Effects of phenethyl alcohol on phospholipid metabolism in Escherichia coli. J Bacteriol. 1972 Jan;109(1):162–168. doi: 10.1128/jb.109.1.162-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H. Phospholipid metabolism in Escherichia coli after a shift in temperature. Biochim Biophys Acta. 1969 Jan 21;176(1):125–134. [PubMed] [Google Scholar]
- Onishi Y. "Phospholipids of virus-induced membranes in cytoplasm of Escherichia coli. J Bacteriol. 1971 Sep;107(3):918–925. doi: 10.1128/jb.107.3.918-925.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D of Haemophilus parainfluenzae. II. Characteristics and possible significance. J Bacteriol. 1970 Nov;104(2):712–718. doi: 10.1128/jb.104.2.712-718.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., White D. C. Consequences of the inhibition of cardiolipin metabolism in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1065–1071. doi: 10.1128/jb.108.3.1065-1071.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. H., Buller C. S. Phospholipid metabolism in T4 bacteriophage infected Escherichia coli K-12 (lambda). J Virol. 1969 May;3(5):463–468. doi: 10.1128/jvi.3.5.463-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Ray P. H., White D. C. Effect of glycerol deprivation on the phospholipid metabolism of a glycerol auxotroph of Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):668–677. doi: 10.1128/jb.109.2.668-677.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]
- Short S. A., White D. C. Biosynthesis of cardiolipin from phosphatidylglycerol in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):820–826. doi: 10.1128/jb.109.2.820-826.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol. 1971 Oct;108(1):219–226. doi: 10.1128/jb.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C. Metabolism of the glycosyl diglycerides and phosphatidylglucose of Staphylococcus aureus. J Bacteriol. 1970 Oct;104(1):126–132. doi: 10.1128/jb.104.1.126-132.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Stanacev N. Z., Chang Y. Y., Kennedy E. P. Biosynthesis of cardiolipin in Escherichia coli. J Biol Chem. 1967 Jun 25;242(12):3018–3019. [PubMed] [Google Scholar]
- Stárka J., Moravová J. Phospholipids and cellular division of Escherichia coli. J Gen Microbiol. 1970 Feb;60(2):251–257. doi: 10.1099/00221287-60-2-251. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Detection of a rapidly metabolizing portion of the membrane cardiolipin in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1058–1064. doi: 10.1128/jb.108.3.1058-1064.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Heterogeneity of phospholipid composition in the bacterial membrane. J Bacteriol. 1970 May;102(2):508–513. doi: 10.1128/jb.102.2.508-513.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Release of membrane components from viable Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 May;102(2):498–507. doi: 10.1128/jb.102.2.498-507.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskovsky V. E., Kostetsky E. Y. Modified spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res. 1968 May;9(3):396–396. [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during changes in the proportions of membrane-bound respiratory pigments in Haemophilus parainfluenzae. J Bacteriol. 1969 Jan;97(1):199–209. doi: 10.1128/jb.97.1.199-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Two-dimensional chromatography on silica gel-loaded paper for the microanalysis of polar lipids. J Lipid Res. 1966 Jul;7(4):544–550. [PubMed] [Google Scholar]