Abstract
Apraxic patients are known for deficits in producing and comprehending skilled movements. Two experiments tested their implicit and explicit knowledge about manipulable objects in order to examine whether such deficits accompany impairment in the conceptual representation of manipulation features. An eyetracking method was used to test implicit knowledge (Experiment 1): Participants viewed a visual display on a computer screen and touched the corresponding object in response to an auditory input. Manipulation relationship among objects was not task-relevant, and thus the assessment of manipulation knowledge was implicit. Like the non-apraxic control patients, apraxic patients fixated on an object picture (e.g., “typewriter”) that was manipulation-related to a target word (e.g., ‘piano’) significantly more often than an unrelated object picture (e.g., “bucket”) as well as a visual control (e.g., “couch”). However, this effect emerged later than in the non-apraxic control group, suggesting impaired access to manipulation features in the apraxic group. In the semantic judgment task (Experiment 2), participants were asked to make an explicit judgment about the relationship of picture triplets of manipulable objects by choosing the pair with similar manipulation features. Apraxic patients performed significantly worse on this task than the non-apraxic control group. Both implicit and explicit measures of manipulation knowledge show that apraxia is not merely a perceptuomotor deficit of skilled movements, but results in a concomitant impairment in representing manipulation features and accessing them for cognitive processing.
Keywords: apraxia, manipulation knowledge, perceptuomotor experiences, implicit vs. explicit tasks, lexical-semantic representation
Introduction
Many everyday objects involve distinctive manipulations with certain motor movements appropriate to their intended usage. A doorknob, for instance, is typically associated with grabbing and turning motions. These motion properties are not only involved in manipulating an object physically, but also are intrinsic to the representation of the object itself as we think and talk about it. Supporting this line of thinking, Myung, Blumstein and Sedivy (2006) showed that the similarity of manipulation features among objects affects their lexical-semantic processing. In an auditory lexical decision task, participants made a significantly faster decision about the target word (e.g., ‘typewriter’) following a related prime that shared manipulation features with the target (e.g., ‘piano’) than an unrelated prime (e.g., ‘blanket’).1 In an eye tracking study, participants looked at the manipulation-related picture (e.g., “typewriter”) significantly more often than the unrelated ones (e.g., “bucket/chisel”) or the visual control (e.g., “couch”) when asked to touch the corresponding object on a display in response to an auditory input (e.g., ‘piano’). These results suggest that perceptuomotor-based experiences play a critical role in object representation and support the claims made by the perceptual symbol systems theory or the notion of cognitive embodiment (e.g., Barsalou, 1999; Barsalou, Simmons, Barbey, & Wilson, 2003; Garbarini & Adenzato, 2004).
The idea underlying the perceptual symbol systems theory or cognitive embodiment is not a recent development, but has a long history (see Barsalou, 1999 for an overview). Lissauer (1890), for instance, postulated a multi-modal account of object recognition and semantic memory, in which semantic information includes the visual and functional properties of an object as well as the sound and the characteristic motor patterns associated with it. Allport (1985) also proposed a distributed semantic architecture in which objects are represented by visual, tactile and motor/proprioceptive nodes in proportion to the extent to which these various sensorimotor systems were involved as the concept was initially acquired and further elaborated.
Similarly, the perceptual symbol systems theory postulates that perceptual and conceptual processes share cognitive and neural resources and/or mechanisms, and that conception is grounded in perception. In this view, conceptual tasks recruit the same brain areas (or at least the areas approximate to them) as those used in related perceptual processes). Thus, according to the perceptual symbol systems theory, a deficit in perceptuomotor processes should be accompanied by a deficit in accessing conceptual representations that make use of these processes. If this were the case then, a deficit in physically manipulating objects should result in a concomitant impairment in the semantic representations of objects in which manipulation features are an intrinsic property. Apraxia provides a unique means of investigating this issue.
Apraxia is a neurological disorder of skilled movements without musculature weaknesses or motor impairments. A patient with ideomotor apraxia exhibits an inability to pantomime the use of objects and has impaired gesture recognition. For example, patients may show an inability to correctly pantomime ‘how to salute,’ or ‘how to use a hammer.’ Ideomotor apraxic patients make spatiotemporal errors in praxis with regard to amplitude, trajectory, and timing, even when imitating (Buxbaum, 2001; Koski, Iacoboni, & Mazziotta, 2002). To examine the relationship between manipulation action (e.g., gesture) and manipulation knowledge, Buxbaum and Saffran (2002) tested a group of left hemisphere brain-damaged apraxic and non-apraxic subjects on tool and animal knowledge, body part knowledge, and manipulation and function knowledge. They hypothesized that given apraxic patients’ deficit in skilled movements, they should perform worse on tool knowledge, body part knowledge, and manipulation knowledge than non-apraxic patients. For each test, patients were given either a picture triplet or a word triplet or both and were asked to point to the two most similar items (e.g., “ruler,” “plug,” “tape measure” for tool knowledge; “raccoon,” “leopard,” “lion” for animal knowledge; ‘elbow,’ ‘knee,’ ‘neck’ for body part knowledge; egg beater, pencil sharpener, hedge clipper for manipulation knowledge; tape, stapler, pen for function knowledge). As predicted, apraxic patients performed worse on tool than animal knowledge (non-apraxics displayed the opposite pattern). Apraxics were also more impaired in body part knowledge than non-apraxics. Most importantly, apraxics were relatively impaired in manipulation knowledge, while non-apraxics tended to be relatively impaired in function knowledge. These patterns indicate an association between gestural praxis impairment and a deficit in the conceptual representation of skilled movements, consistent with the theoretical framework of the perceptual symbol systems. Furthermore, the lesions of the apraxic patients in the left frontoparietal areas either overlapped or were in close proximity to those involved in conceptually processing manipulable objects (e.g., Boronat et al., 2005; Chao & Martin, 2000; Kellenbach, Brett, & Pattern, 2003).
Buxbaum and Saffran’s study used explicit semantic judgment tasks, however, and it is often the case that brain-injured patients show differences in their performance on explicit versus implicit tasks. For instance, Wernicke’s patients, who are severely impaired in auditory comprehension and show poor performance on explicit semantic judgment tasks, exhibit semantic priming in a lexical decision task where the semantic relationship between the prime and target word pairs is implicit. Such findings suggest that these patients are able to access lexical semantic relations, although they may fail to explicitly act on them (e.g., Milberg & Blumstein, 1981; Blumstein, Milberg, & Shrier, 1982). It is possible that apraxic patients who fail to show sensitivity to manipulation features in an explicit task may show intact manipulation knowledge using an implicit task; this would imply that implicit manipulation knowledge is preserved. Alternatively, a failure of these patients to show sensitivity to manipulation knowledge in an implicit task would provide strong support for the association between gestural praxis and manipulation knowledge, and more generally the association between perceptuomotor processes and their conceptual representations.
To this end, Experiment 1 examined whether apraxic patients would show a deficit in manipulation knowledge when performing an implicit task. In an earlier study, Myung and colleagues (2006) showed that the eye tracking paradigm provides a sensitive measure of manipulation knowledge representation without requiring an explicit response about the manipulation properties of the stimuli. Moreover, this paradigm has been used successfully with brain-injured patients to examine on-line lexical processing (Yee, Blumstein, & Sedivy, 2008). Experiment 2 tested the same patients as in Experiment 1 using an explicit semantic judgment task comparable to the explicit task used by Buxbaum and Saffran (2002). Thus, Experiments 1 and 2 aimed to explore implicit and explicit processing of manipulation knowledge in apraxic patients and to elucidate whether a specific deficit in producing and/or comprehending skilled movements is associated with a deficit in the lexical-semantic representation of manipulable objects—even in an implicit task. Experiment 1 was conducted prior to Experiment 2 to make certain that any effect found in the implicit task of Experiment 1 was not contaminated with conscious activation of manipulation features called for in the explicit semantic judgment task of Experiment 2.
Experiment 1
Experiment 1 sought to determine whether apraxic patients would show an effect of manipulation similarity in an implicit task using the eye tracking paradigm. In order to investigate this question, it is necessary to ensure that a failure to show sensitivity to manipulation knowledge is not secondary to a more generalized deficit in access to lexical-semantic information. Therefore, in addition to investigating sensitivity to manipulation knowledge, Experiment 1 also assessed access to lexical-semantic information more generally in apraxic patients by examining whether they would show sensitivity to a stimulus that was semantically related to an auditory target word (Semantic post-test). Yee and colleagues (Yee et al., 2008), using an eye tracking paradigm, showed that when aphasic patients heard an auditory target (e.g., ‘scissors’), both Broca’s and Wernicke’s aphasics fixated on semantically-related pictures (e.g., “knife”) significantly more often than on unrelated pictures (e.g., “coat”). Since Wernicke’s aphasics, who are known to have auditory comprehension deficits, showed sensitivity to semantically related stimuli, there is no a priori reason to expect that apraxic patients would fail to show a semantic relatedness effect. Nevertheless, the inclusion of the Semantic post-test provides a means of assessing apraxic patients’ general semantic processing ability. It was not necessary to test the non-apraxic aphasic patients on the Semantic post-test because Yee and colleagues (2008) had already found that aphasic patients showed a semantic relatedness effect. If apraxic patients show sensitivity to semantically related stimuli in the Semantic post-test, but fail to show an effect of manipulation similarity, the basis of the impairment cannot be attributed to a more global lexical-semantic processing impairment.
Methods
Participants
Patients were recruited either at Brown University or at the Moss Rehabilitation Research Institute. All of the patients recruited at Brown University were tested in their homes and gave written informed consent, as approved by the Human Subjects Committees of both Brown University and one of the medical institutions where they were originally recruited for the study (the Harold Goodglass Aphasia Research Center, the Boston VA Medical Center, Rhode Island Hospital, Roger Williams Medical Center, Memorial Hospital of Rhode Island or Jordan Hospital at Plymouth, MA). All of the patients recruited at the Moss Rehabilitation Research Institute were tested at the Moss Rehabilitation Research Institute and consented to the study in accordance with the Institutional Review Board guidelines of the Albert Einstein Healthcare Network.
For the apraxic group, a total of five patients were recruited, four at the Moss Rehabilitation Research Institute, and one at Brown University. The data from one patient were not included in the analyses because his eye movements could not be tracked reliably. All apraxic patients except for one (A1) had unilateral lesions, and none had an associated dementia. All of the apraxic patients had lesions involving the inferior parietal lobule. The precentral gyrus and the premotor cortex were affected in two patients (A3 and A4). All were native speakers of American English and had normal or corrected-to-normal vision and no reported hearing deficits. The apraxic patients were 9 years post-stroke on average. The average age of the apraxic patients was 61 (range: 41 ~ 77, SD: 15).
For the non-apraxic brain-damaged control group, a total of eight patients were recruited, seven at Brown University, and one at the Moss Rehabilitation Research Institute. These patients were selected because, similar to the apraxic patients, they had lesion sites that included the left frontoparietal areas. The experimental data from three patients were not included in the analyses for the following reasons. One patient had such a severe comprehension deficit that he was not able to accurately point to the target picture in Experiment 1. Another patient failed to reach calibration criteria in Experiment 1, indicating that his eye movements could not be tracked reliably. The last patient suffered from palinopsia (a visual disturbance that causes images to persist even after their corresponding stimulus has disappeared) and thus showed delayed eye movements. With the exception of this patient, all of the non-apraxic aphasic patients had normal or corrected-to-normal vision. All were native speakers of American English and had no reported hearing deficits. None of the patients had an associated dementia. They were 11 years post-stroke on average. The average age of the non-apraxic aphasic patients was 60 (range: 57 ~ 65, SD: 2.97). All participants were paid for their participation.
For both groups of patients, presence of aphasia was confirmed with the administration of the Boston Diagnostic Aphasia Examination (BDAE, Goodglass & Kaplan, 1972). The word discrimination subtest of the BDAE was used as a baseline comparison measure for the apraxic and aphasic groups. It was important that the two groups did not differ in their ability to recognize pictures of auditorily presented words. In the word discrimination subtest of the BDAE, the patients were presented with a set of two cards containing drawings of objects grouped by semantic category such as objects and actions, and asked to point to the items named by the examiner. This subtest is similar to the eye tracking task in which the patients were asked to point to a picture from an array of four that matched the auditory input. Results indicated no significant difference in performance on the BDAE subtest between the apraxic group (average z-score = 0.32) and the non-apraxic control group (average z-score = 0.77).
The Florida Apraxia Battery (FAB, Rothi et al., 1992) screening test was used to assess the presence or absence of apraxia in all participants. The average score of the non-apraxic control group was 27 out of 30 (SD = 1.9), while the average score of the apraxic group was 15 (SD = 4.3). All of the apraxic patients performed with an error rate more than two standard deviations greater than the mean for the non-apraxic control group. Table 1 provides a summary of demographic, clinical, test, and lesion information of the aphasic and apraxic participants whose data are included in the analyses.
| Patient classification |
Code | Gender | Age | Time post onset |
Aphasia Test |
Apraxia Test |
Lesion Loci (Left Hemisphere) |
|---|---|---|---|---|---|---|---|
| Aphasic | C1 | F | 59 | 7 yr | 0.78 | 29 | temporal/inferior parietal |
| Aphasic | C2 | M | 61 | 20 yr 3 mo | 0.96 | 25 | medial temporal, insula, caudate, globus pallidus, anterior internal capsule, periventricular white matter |
| Aphasic | C3 | M | 57 | 9 yr 4 mo | 0.18 | 28 | parietal |
| Aphasic | C4 | F | 60 | 7 yr 2 mo | 0.96 | 28 | inferior frontal (motor and premotor) |
| Aphasic | C5 | M | 65 | 9 y 9 mo | 0.96 | 25 | posterior inferior frontal, inferior/middle/superior temporal, temporal pole, inferior parietal, occipital |
|
| |||||||
| Aphasic & apraxic |
A1 | F | 77 | 4 yr 11 mo | 0.66 | 9.5 | subcortical between medial geniculate of thalamus and middle temporal, posterior temporal, inferior parietal |
| Aphasic & apraxic |
A2 | M | 65 | 7y 3 mo | 0.81 | 20 | inferior/middle/superior temporal, anterior/superior/inferior parietal, occipital |
| Aphasic & apraxic |
A3 | M | 61 | 7 y | 0.18 | 16 | middle/posterior frontal, middle/superior temporal, anterior/superior/inferior parietal, occipital |
| Aphasic & apraxic |
A4 | M | 41 | 17 y 2 mo | −0.36 | 15 | inferior/middle/posterior frontal, inferior/middle/superior temporal, anterior/superior/inferior parietal, occipital |
Apparatus
EyeLink I, a head-mounted eye tracking system by SR Research, was used to monitor eye movements of the patients recruited at Brown University. It has a high resolution (noise-limited at <0.01°) and a fast data acquisition rate (250 samples per second). For the patients recruited at the Moss Rehabilitation Research Institute, EyeLink II, a newer version of the head-mounted eye tracking system, was used. It has the same high resolution (noise-limited at <0.01°) as EyeLink I, but a faster data acquisition rate (500 samples per second). For both the apraxics and the non-apraxics, stimuli were presented on a 15″ Elo Entuitive 1525C touch screen monitor using PsyScript 5.1 for stimulus presentation.
Stimuli
As in Myung et al.’s study (2006), manipulation was defined operationally as general actions on an object that involve body movements and are associated with its intended usage. For instance, a piano and a typewriter require similar hand positions and movements despite the difference of their intended usages. It was the similarity of the general action patterns among objects rather than that of detailed finger positions that defined common manipulation movements.
The stimuli were taken from Myung and colleagues’ eyetracking study (see Experiment 2 in Myung et al., 2006 for details). There were twenty pairs of manipulation-related stimuli for the manipulation-related condition (e.g., “piano” – “typewriter”), and twenty pairs of unrelated stimuli for the control condition (e.g., “piano” – “couch”) (see Appendix A for the list of stimuli). Two stimulus lists were prepared and counterbalanced in terms of target frequency and duration. Each participant was shown only one list so that no item was seen or heard more than once.
For the displays, color pictures were selected from a commercial clip art collection and from a picture library (Rossion, & Pourtois, 2004). In the manipulation-related condition (10 of 60 trials), one of the pictures in the display was related to the target in terms of manipulation features. In the control condition (10 of 60 trials) and in filler trials (40 trials) no pictures in the display were related in terms of manipulation features. However, in the control condition one of the non-target items was matched to the manipulation-related item of the target in terms of visual similarity (e.g., “couch” as a visual match for the “piano”–“typewriter” pair). These trials were included to ensure that the manipulation-related pictures did not draw fixations merely because of their visual similarity to the targets. Since manipulation-related objects tend to have a similar appearance, it was critical to control for the potential effect of visual similarity. Picture positions were balanced so that the manipulation-related items were equally distributed in each corner of the display across the participants. Trial order was randomized for each participant. Patients had six practice trials before the experiment.
The stimuli for the Semantic post-test were taken from Yee et al.’s eye tracking study (2008) (see Appendix B for the list of the stimuli). Some of the unrelated pictures were replaced to avoid a repetition of the same stimuli in the Manipulation condition and the Semantic post-test. The experimental stimuli consisted of fourteen semantically-related trials in which one of the pictures in the display (e.g., “knife”) was semantically related to the auditory target (e.g., ‘scissors’), and twenty filler trials in which no pictures in the display were related to the target. Patients had four practice trials before the experiment.
Procedure
The auditory stimuli for the Manipulation condition were recorded by a male native speaker of American English in a sound-attenuated booth. They were recorded onto magnetic tape using a Sony Walkman Professional tape recorder and a Sony stereo microphone and then digitized at a sampling rate of 20 kHz with a 9.0-kHz lowpass filter and a 14 bit quantization. For the Semantic post-test, the auditory stimuli from Yee et al.’s study (2008) were used. For each trial in both the Manipulation experiment and Semantic post-test, participants were presented with four pictures on a 3×3 array, with one picture in each corner. They were seated a comfortable distance from the monitor (about 16 inches), while the monitor was placed at their eye height. Each cell in the stimulus array was approximately 2×2 inches, thus subtending approximately 7 degrees of visual angle. Participants were given a preview time for 1000 ms. To ensure that each picture in the display was of equal distance for participants’ fixations, participants were asked to touch a red square in the center of the grid that appeared after the preview. As soon as participants touched the red square, they heard the target word. They were instructed to touch the target item as quickly and accurately as possible upon hearing the target. Importantly, no information was given about the manipulation features or semantic relations of the objects during the instructions. Also, none of the participants reported that they had noticed any relationship among the objects presented in the experiment.
The Semantic post-test for the apraxics was always conducted following the Manipulation experiment. In an earlier study, Taylor (2005) showed that the presence of semantically related stimuli was able to override the effects of perceptually related stimuli when the former condition preceded the latter condition. Because of concerns that manipulation features are perceptually-based characteristics, the Manipulation condition preceded the Semantic post-test.
Results
The data were analyzed separately for each patient group. The proportions (across trials) of fixations on each picture type (target, manipulation-related, unrelated) were computed in each 32 ms frame for each condition for each participant. That is, at each time bin we computed the proportion of trials that contained a fixation to the target, the manipulation-related picture, and to the average of the two unrelated pictures. A fixation to a particular picture was operationally defined as the time that a saccade moved the eye into the specific region until a saccade moved the eye out of that region. Thus, saccades in which the eye did not move out of the region were included as part of the fixation time for that region. The statistical analysis was conducted on the averages of every five 32 ms frames (160 ms time bin). When the sphericity assumption was violated, a Greenhouse-Geisser correction was applied. For the time course analysis with paired t-tests on manipulation-relatedness, visual similarity, and semantic relatedness effects, one-tailed significance values are reported.
1) Results for the Non-apraxic Group
Two error trials (2%) were not included in the analysis. Another two trials (2%) did not provide any data because no eye movements were made after the onset of the target word; that is, in these trials patients were already fixating on the target picture at the onset of the target word. For the remaining data, fixation proportions that deviated from the mean by more than two SDs were substituted with the mean of the remaining fixation proportions for that frame of that condition (0% and 5.8% of fixation proportions for participants and items respectively).
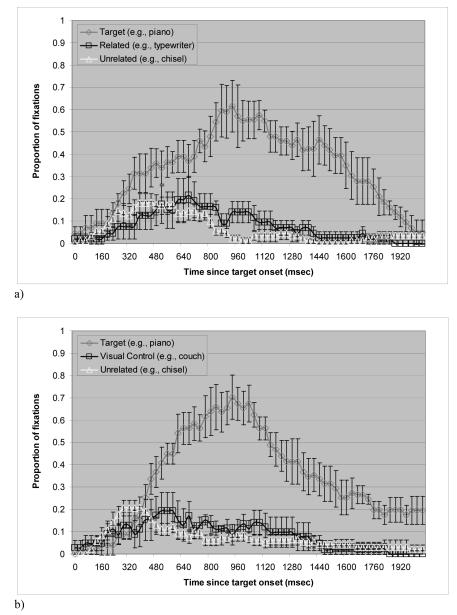
Figure 1a shows the mean proportion of trials in which the non-apraxic control patients fixated on each of the picture types over time (from target onset to 2080 ms after onset) in the manipulation-related condition. A trial was defined as starting 160 ms after the onset of the target word since it takes approximately 180 ms to launch an eye movement (Altmann & Kamide, 2004). A trial ended 1760 ms after target onset. Around 1760 ms, the probability of fixating on the target dropped below 30%, which was approximately half of the proportion of fixations on the target at the peak. The proportions do not add up to 1 because fixations outside of the critical regions are not plotted and because fixations on the two unrelated pictures are averaged. The average proportion was used for simplicity since there was no difference in fixations on the two unrelated pictures.
Figure 1.
Proportion of fixations over time in the non-apraxic patients. Bars indicate standard errors. a) manipulation-related condition. b) control condition.
A two-way repeated measures analysis of variance (ANOVA) was conducted on the average fixations with manipulation-relatedness as one factor and time bin as the other to examine whether there was a manipulation-relatedness effect, and whether it interacted with time bin. There was a significant main effect of time bin in every analysis conducted for the study, but this effect was expected and will not be further discussed. The main effect of manipulation-relatedness failed to show significance both by participants and items. The interaction of manipulation-relatedness with time bin was not significant by participants (F1(10, 40) = 1.3, p = .31), but it was significant by items (F2 (10,180) = 2.5, p = .009). Considering the small number of participants (n = 5) and the considerable noise in the data, paired t-tests were nonetheless conducted on each 160 ms frame to determine whether significant effects emerged in specific time windows. Fixations on the manipulation-related pictures were not different from those on the unrelated pictures before 800 ms after target onset. In the time bins of 800 - 960 and 960 - 1120 ms, however, the manipulation-related pictures were looked at more often than the unrelated pictures in the display. The pattern of more fixations on manipulation-related pictures than on unrelated ones was significant in analysis by participants (p = .03 for 800 - 960 ms bin, p = .05 for 960 - 1120 ms bin). The analysis by items was also significant (p = .012 for 800 - 960 ms bin, p = .02 for 960 -1120 ms bin). Thus, a subtle effect of manipulation-relatedness emerged at around 800 ms and lasted for about 300 ms.
Figure 1b plots the mean proportion of trials in the control condition. Unlike the young normal group in Myung et al.’s study (2006), the pattern of fixations on the visual control stimuli appeared to differ from the pattern of fixations on the unrelated pictures for the non-apraxic group. A two-way repeated measures ANOVA was conducted on the average fixations with visual similarity as one factor and time bin as the other. The main effect of visual similarity was not significant either by participants (F1(1, 4) = .4, p = .56), or by items (F2(1, 18) = 2.1, p = .17). The interaction of visual similarity with time bin was not significant by participants (F1(10, 40) = .6, p = .78), but it was significant by items (F2(10, 180) = 2.3, p = .01). Paired t-tests that were conducted on each time bin of 160 ms showed that in the region of 640 - 800 ms, the effect of visual similarity approached significance in the analysis by participants (p = .09). The analysis by items, however, was significant or close to significant in the time bins from 480 - 640 and 640 - 800 ms (p = .04 for 480 ~ 640 ms bin, p = .06 for 640 - 800 ms bin). Thus, there was a subtle effect of visual similarity that emerged early around 480 ms and ended by 800 ms.
Of interest, the visual similarity effect arose around 480 ms, which is far earlier than the manipulation-relatedness effect for these patients that emerged around 800 ms. This difference in the time course suggests that the manipulation-relatedness effect and the visual similarity effect are from different sources, and that the manipulation-relatedness effect is not caused by visual resemblance between the manipulation-related stimuli.
2) Results for the Apraxic Group
Error trials (2.5%) were discarded from the analysis. Six trials (8.8%) did not provide any data because no eye movements were made after the onset of the target word. For the remaining data, fixation proportions that deviated from the mean by more than two SDs were substituted with the mean of the remaining fixation proportions of that frame for that condition (0% and 5.6% of fixation proportions for participants and items respectively).
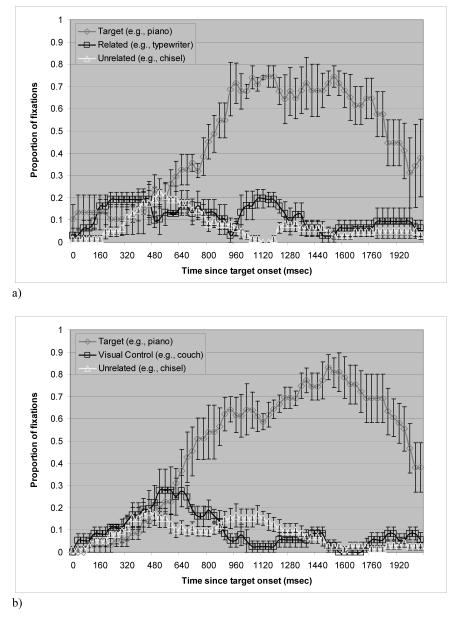
Figure 2a shows the mean proportion of trials in which the apraxic patients fixated on each of the picture types over time (from target onset to 2080 ms after onset) in the manipulation-related condition. As in the non-apraxic group, a trial was defined as starting 160 ms after the onset of the target word and ending at 1760 ms after target onset.
Figure 2.
Proportion of fixations over time in the apraxic patients. Bars indicate standard errors. a) manipulation-related condition. b) control condition.
A two-way repeated measures ANOVA was conducted on the average fixations with manipulation-relatedness as one factor and time bin as the other. The main effect of manipulation-relatedness was not significant by participants (F1(1, 3) = 3.2, p = .17), but approached significance by items (F2(1, 16) = 3.2, p = .09). The interaction of manipulation-relatedness with time bin was close to significant by participants (F1(10, 30) = 1.8, p = .1), and it was significant by items (F2(4.103, 65.654) = 3.6, p =.01). Follow-up paired t-tests were conducted on each time bin of 160 ms to determine the time frames in which the manipulation-relatedness effect emerged.
Fixations on the manipulation-related pictures were not different from those on the unrelated pictures before 960 ms after target onset. In the region of 960 - 1280 ms however, the manipulation-related pictures were looked at more often than the unrelated pictures in the display.2 This difference was significant or close to significant in the analysis by participants (p = .07 for 960 - 1120 ms bin, p = .003 for 1120 - 1280 ms bin). The analysis by items yielded similar results (p = .02 for 960 - 1120 ms bin, p = .02 for 1120 - 1280 ms bin).
It is interesting that the apraxic patients indeed showed a manipulation-relatedness effect in an implicit task. Of more interest is the time course of the manipulation-relatedness effect. Testing the young normal participants, Myung et al. (2006) showed that manipulation features of the objects related to the uttered word were available around 500 ms after target onset. Compared to the young normal participants and also the non-apraxic control patients (around 800 ms), the manipulation-relatedness effect emerged later for the apraxic patients (around 960 ms).
Figure 2b plots the mean proportion of trials in the control condition. Like the non-apraxic patients, the pattern of fixations on the visual control stimuli differed from those on the unrelated pictures. A two-way repeated measures ANOVA was conducted on the average fixations with visual similarity as one factor and time bin as the other. The main effect of visual similarity was not significant. However, there was a significant interaction of visual similarity with time bin by participants (F1(10, 30) = 2.9, p = .01). The interaction was not significant by items. Follow-up paired t-tests on each 160 ms frame yielded a significant result in the regions of 640 - 800 ms by participants (p = .008). The analysis by items showed a significant effect from 480 to 800 ms (p = .03 for 480 - 640 ms bin, p = .03 for 640 - 800 ms bin). Unexpectedly, follow-up tests also revealed a preference for the unrelated object at later time points (960 - 1280 ms). Although the reason for this preference is unclear, it appears to be largely driven by only a few (4) items.3 In fact, taking out those items from the analysis resulted in a loss of the later preference to the unrelated stimuli, but did not affect the visual similarity effect at the earlier time points. This indicates that the later preference to the unrelated stimuli is less reliable than the earlier visual similarity effect.
The pattern of the data shows that the apraxic patients are sensitive to visual similarity. This sensitivity contrasts with the absence of a visual similarity effect in the young normal participants in Myung et al.’s (2006) study, but parallels the results of the non-apraxic patients. Just like the non-apraxic patients, the visual similarity effect arose early – around 480 ms, which is earlier than the manipulation-relatedness effect that emerged around 960 ms.
Overall, the results of the Manipulation condition indicated that not only the non-apraxic patients, but also the apraxic patients were sensitive to manipulation similarity among objects. Nonetheless, this manipulation-relatedness effect arose 160 ms later in the apraxic patients compared to the non-apraxic aphasic patients and 460 ms later than normals (cf. Myung et al., 2006). One possible interpretation of the results is that the apraxic patients have a deficit in conceptual knowledge about manipulation, consistent with the previous studies on apraxia (e.g., Buxbaum & Saffran, 2002). However, an alternative possibility is that the delay reflects a more generalized deficit in accessing semantic features. To exclude this possibility, the apraxic patients were given the Semantic post-test described earlier. Only the apraxic patients who were tested at the Moss Rehabilitation Research Institute (n = 3) were given this post-test. In the analysis, error trials (9.5%) were not included. For the remaining data, fixation proportions that deviated from the mean by more than two SDs were substituted with the mean of the remaining fixation proportions for the frame of that condition (0% and 6.1% of fixation proportions for participants and items respectively).
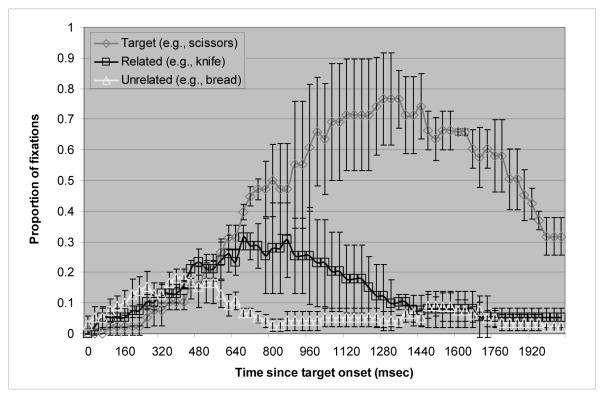
Figure 3 plots the mean proportion of trials in which the apraxic patients fixated on each of the picture types over time (from target onset to 2080 ms after onset). It is apparent that the apraxic patients showed sensitivity to the semantic relations of the pictures on the display. A two-way repeated measures ANOVA was conducted on the average fixations with semantic relatedness as one factor and time bin as the other. The main effect of semantic relatedness was close to significant by participants (F1(1, 2) = 10.5, p = .08) and significant by items (F2 (1, 13) = 12.1, p = .004 by items) as was the interaction of semantic relatedness with time bin (F1 (10, 20) = 2.02, p = .09 by participants, F2 (2.482, 32.263) = 4.8, p = .01 by items). Paired t-tests showed a significant difference in the proportion of looks from 640 - 960 ms after target onset (p = .04 for 640 - 800 ms bin; p = .09 for 800 - 960 ms bin) by participants. The analysis by items showed that from 640 to 1440 ms after the target onset the difference in the proportions of looks between the semantically related and the unrelated pictures was significant (p < .05).
Figure 3.
Proportion of fixations over time in the Semantic post-test in the apraxic patients. Bars indicate standard errors.
The results of the Semantic post-test are consistent with those of Yee and colleagues (Yee et al., 2008). Yee and colleagues reported no statistically significant difference among the groups (age-matched controls, Broca’s aphasics, and Wernicke’s aphasics) in terms of the magnitude of the semantic relatedness effect and did not make any specific claim about its time course. Nonetheless, the onset of the semantic relatedness effect in the apraxic patients (around 640 ms) was comparable to the semantic relatedness effect shown by Broca’s aphasics and the age-matched controls in Yee et al.’s study who appeared to show an effect shortly after 600 ms. Thus, this pattern indicates that the delay of the manipulation-relatedness effect in the apraxic patients is not caused by a general slowing of semantic processing or in accessing the semantic representations of words. Rather, it appears that access to manipulation features in particular is affected in apraxia.
3) Comparison of the Apraxic and Non-Apraxic Aphasic Groups
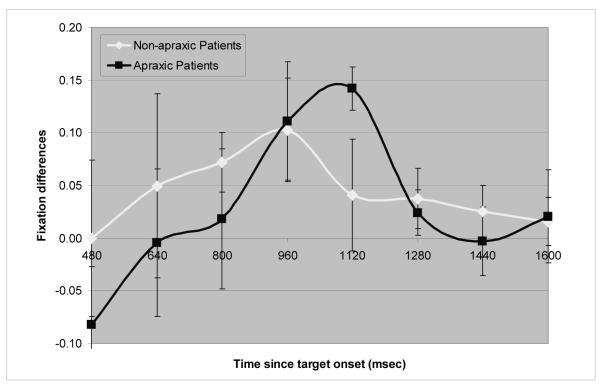
A crucial finding in Experiment 1 is that the apraxic patients and the non-apraxic control patients showed a manipulation-relatedness effect at different time points. The effect emerged later for the apraxic patients than their non-apraxic controls. To statistically assess this finding, the differences between the average fixations to the manipulation-related pictures and the unrelated pictures were calculated for each patient group for the time bins of 800 – 1280 ms, namely the time frame during which at least one of the two patient groups showed a significant effect. A two-way ANOVA was conducted on the fixation differences with time bin as one factor and patient group as the other. Significant differences in the time course between the two groups would be revealed by an interaction between group and time bin. Figure 4 shows the results for both patient groups. Of importance, there is a noticeable shift between the graphs of the two patient groups, indicating that the time course of the manipulation-relatedness effect differs between the two groups. The shift is most evident at the points where the graphs cross the x-axis and at their peaks.
Figure 4.
Differences between the average fixations to the manipulation-related pictures and the unrelated pictures in each patient group. Bars indicate standard errors.
The main effects of patient group and time bin were not significant either by participants or items. However, the interaction of patient group by time bin approached significance by participants (F1(2, 14) = 2.5, p = .12) and was significant by items (F2 (2, 68) = 5.9, p = .004). Follow-up paired t-tests conducted on each of the three time bins showed that from 800 to 960 ms fixation differences were greater for the non-apraxics than the apraxics (p = .22 by participants, p = .07 by items), while from 1120 to 1280 ms the apraxics showed greater fixation differences than the non-apraxics (p = .08 by participants, p = .01 by items). These findings suggest that the manipulation-relatedness effect emerges later in the apraxic patients than the non-apraxic control patients. Nonetheless, it may appear surprising that, as shown in Figure 4, the magnitude of the fixation difference is larger for the apraxic than the non-apraxic group in one of the time bins (1120 ms). Why this difference at 1120 ms emerged is not clear. However, it is the case that overall, the apraxic patients showed a higher proportion of looks to the target and less variability than did the non-apraxic aphasic patients (see Figures 1a and 2a). In addition, the non-apraxic group showed the manipulation-relatedness effect earlier than did the apraxic group, with the magnitude of the effect diminishing by 1120 ms for the non-apraxic group, the time when it was largest for the apraxic patients. Thus, the difference in the magnitude of looks between the two groups was in some sense exaggerated at that time point.
4) The severity of apraxia on the manipulation-relatedness effect
It is possible that severity of apraxia might have an influence on the manipulation-relatedness effect in the apraxic group. We tested for such a relationship in two ways. First, we examined whether apraxia severity (as reflected in FAB scores) would predict the size of the manipulation-relatedness effect. Including apraxic patients and non-apraxic control patients, we found a weak negative relationship (r = −.14) between the patients’ FAB scores and the fixation difference in the manipulation-related condition (i.e., the difference between the average fixations to the manipulation-related items and the unrelated items during the entire trial (160 ms ~ 1760 ms) for each patient). Because the timing, rather than the size of the manipulation-relatedness effect seemed to be impacted by apraxia, we also tested whether more severe the apraxia (as reflected in lower FAB scores) would result in a more delayed onset of the manipulation-relatedness effect (operationally defined as the first peak point at which looks to the manipulation-related item clearly diverged from those of the unrelated items). We found a negative relationship (r = −.29) between the patients’ FAB scores and the onset of the manipulation-relatedness effect, but this correlation was not significant. Interestingly, an examination of the scatter plot revealed that one patient (A1) was an outlier with her effect onset time two SDs away from the mean of the group. With the patient excluded, we found a strong negative relationship (r = −.66), indicating that the more severe the apraxia the greater the delay in the onset of the manipulation effect. This correlation approached statistical significance (p = .11).
Discussion of Experiment 1
Both the apraxic patients and the non-apraxic aphasic control patients looked at manipulation-related pictures more often than unrelated pictures, indicating that they are sensitive to manipulation features even in an implicit task. Nonetheless, the apraxic patients performed differently from the non-apraxic patients, as they showed a delay in the onset of this effect. While the non-apraxic control patients exhibited a subtle effect of manipulation-relatedness around 800 ms after target onset, the same effect did not emerge for the apraxic patients until around 960 ms after target onset. These results suggest that the apraxic patients are sensitive to manipulation features, but that they have difficulty in accessing them. That said, these findings need to be interpreted cautiously given that overall the sample size was small and the statistical significance of the results was not robust.
It is important to note that the delayed time course of the manipulation-relatedness effect in the apraxic patients is not caused by an overall slowing of cognitive processing or accessing semantic features in general. Indeed, the pattern of results was similar for the apraxic patients and the aphasic controls in other conditions, such as the visual control condition and the Semantic post-test. In particular, the data from the visual control condition was similar in the two groups showing the emergence of a visual similarity effect at an earlier time point (around 480 ms) than the manipulation-relatedness effect (around 800 - 960 ms). More importantly, the apraxic patients showed a semantic relatedness effect that was similar to the non-apraxic aphasic patients. The semantic relatedness effect emerged around 640 ms, in contrast to the manipulation-relatedness effect which emerged around 960 ms. The time course of the semantic relatedness effect for the apraxic patients was similar to the semantic relatedness effect for aphasic patients shown by Yee and colleagues (Yee et al., 2008). Thus, the findings from Experiment 1 suggest that the delay in processing manipulation features in the apraxic patients is not secondary to a general slowing of semantic processing, but rather support the view that apraxic patients have difficulty in activating manipulation features. The correlation between apraxia severity and the delay of the manipulation-relatedness effect is also consistent with this view.
Experiment 2: Semantic judgment task
In a recent study, Buxbaum and colleagues (e.g., Buxbaum, Veramonti, & Schwartz, 2000; Buxbaum & Saffran, 2002) investigated apraxic patients’ sensitivity to manipulation features using an explicit task in which subjects were asked to choose the two items out of three that were most similar to each other in terms of their manipulation. Since such a task overtly requires patients to retrieve the manipulation features of the objects, the nature and demands of the task are very different from those of an implicit task that was used in Experiment 1. In Experiment 1 the presence of a manipulation relationship among the objects was implicit, and more importantly, manipulation features were not task relevant. Yet, the apraxic patients showed impaired sensitivity in accessing manipulation features. Experiment 2 used the items from Experiment 1 with the procedure employed by Buxbaum and colleagues (e.g., Buxbaum et al., 2000; Buxbaum & Saffran, 2002) not only to replicate previous findings, but more importantly to investigate whether differences would emerge for apraxic patients between implicit and explicit processing of manipulation features of identical items.
Methods
Participants
The same patients as in Experiment 1 took part in Experiment 2. The patients who were not included in the data analysis of Experiment 1 were excluded from the data analysis of Experiment 2 as well since the goal of Experiment 2 was to compare the same patients’ performance on implicit and explicit tasks.
Apparatus
Stimuli were presented on a 15″ Elo Entuitive 1525C touch screen monitor with the PsyScript 5.1.
Stimuli
The same stimuli as in Experiment 1 were used except that in Experiment 2 only three pictures (e.g., “piano” and “typewriter” as two manipulation-related pictures and “couch” as a distractor) appeared on a visual display. Distractor items were taken from the visual control items in the control condition of Experiment 1.
Procedure
The visual display was presented on a computer screen and remained on the screen until the participant’s responses were registered by the computer. Participants were asked to look at the pictures and point to the two pictures that were similar to each other in terms of manipulation features. To ensure that participants understood what was meant by manipulation features, the tester (J.M.) demonstrated the characteristic manipulation features of sample objects that were not included in either practice trials or experimental trials. Participants were encouraged to think of how they typically used the shown objects. It was emphasized that participants were not required to respond as quickly as they could, but rather to take their time and respond when they were certain of their response. There were three practice trials preceding the experimental trials.
Results
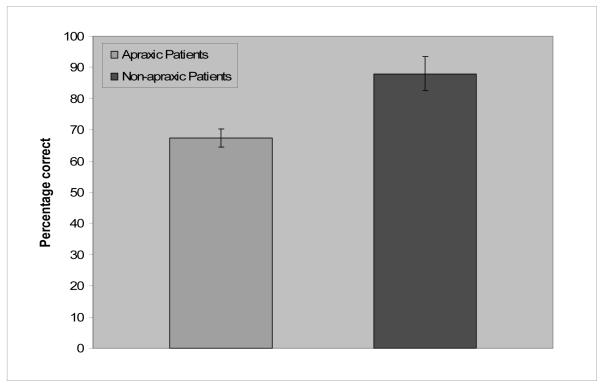
Semantic judgment responses were analyzed for percentage correct and the results are shown in Figure 5. A one-way ANOVA was conducted with participants ( F1) as a random variable. The results of the ANOVA showed a significant difference between the apraxic group (68%) and the non-apraxic group (88%) ( F1(1, 7) = 11.8, p = .01).
Figure 5.
Mean percentage correct of each patient group in the semantic judgment task. Bars indicate standard errors.
As anticipated, the apraxic patients performed more poorly in making a semantic judgment based on manipulation similarity than the non-apraxic control patients. These results are consistent with those of Buxbaum and colleagues (e.g., Buxbaum et al, 2000; Buxbaum & Saffran, 2002). Yet, it should be also noted that the apraxic patients’ performance (68% correct) is significantly better than chance (33% correct). Apraxia does not lead to a complete loss of manipulation features, even if it selectively affects them. This pattern was also apparent in Experiment 1, in which the apraxic patients showed sensitivity to manipulation features despite a delay in accessing them.
Discussion of Experiment 2
Similar to the findings of Buxbaum and colleagues (e.g., Buxbaum et al., 2000; Buxbaum & Saffran, 2002), the results of Experiment 2 showed that although their performance was better than chance, apraxic patients performed significantly worse at making a semantic judgment on manipulation similarity of concrete objects than did brain-damaged controls. These results are in line with the eyetracking results of Experiment 1 in which the apraxic patients showed a delayed effect of manipulation similarity. Thus, taken together with the results of Experiment 1, the findings in Experiment 2 suggest that the apraxic patients have a deficit in accessing manipulation features.
General Discussion
Experiments 1 and 2 investigated the processing of manipulation features in apraxic patients and non-apraxic brain-damaged controls. The results of Experiment 1 suggested that both the apraxic patients and the non-apraxic patients were sensitive to manipulation similarity among objects, but that this sensitivity was somewhat delayed in the apraxic patients. The results of Experiment 2 showed that the apraxic patients were significantly worse at making a semantic judgment on manipulation similarity of objects than were the non-apraxic control patients. Thus, apraxic patients appear to have difficulty in accessing information about manipulation features not only in an explicit task (Exp 2) but also in an implicit task in which manipulation features are neither task-relevant nor explicitly called for (Exp 1). Owing to the difficulties in finding patients who meet the various criteria for this study, it is the case that the results of the current study are based on a small group of subjects and the difference between the groups was statistically weak. Thus, a replication of these findings with a larger group of patients is in order, as would be using other methodologies, such as functional neuroimaging with normal individuals. Nonetheless, the results of this study suggest that apraxic patients do have deficit in implicit as well as explicit processing of manipulation features of objects.
What is the basis of this deficit? One possibility is that access to manipulation features is simply delayed in apraxic patients. If this were the case, given sufficient time, the apraxic patients should have been able to eventually fully access manipulation features, and thus they should have performed normally on the semantic judgment task in Experiment 2. However, this was not the case. Another possibility is that the general activation of manipulation features is reduced in apraxic patients. If true, it would not only take longer for the activation of the manipulation features to reach threshold, thereby delaying access to manipulation information, but it would also affect the extent to which such information is activated, affecting use of this information in explicit tasks. This explanation would account for why, even given sufficient time as in Experiment 2, apraxic patients showed deficits in making overt judgments on those features. Further research will be needed to illuminate the exact nature of the deficits shown by apraxic patients in Experiments 1 and 2.
In several studies, Buxbaum and colleagues showed that apraxic patients are impaired not only in generating and comprehending voluntary movements, but also in thinking and making judgments about them (e.g., Buxbaum et al., 2000; Buxbaum & Saffran, 2002). When apraxic patients had to make an explicit semantic judgment on objects in terms of manipulation, function, and manipulation and function, they were much worse in the manipulation condition than the other two conditions. The results from Experiments 1 and 2 are consistent with these findings by Buxbaum and colleagues, as they also indicate that the specific deficit of apraxia in skilled movements results in a concomitant impairment in the conceptual representation of manipulable objects. Thus, these results support the view that conceptual or cognitive processes are grounded in perceptual processes, and that a deficit in perceptuomotor processes leads to a deficit in conceptual processes about those perceptuomotor processes.
In fact, a growing body of research suggests that conceptual knowledge is not only grounded in perception and action, but that it also shares the neural substrates underlying perceptuomotor actions related to that knowledge (e.g., Kan, Barsalou, Solomon, Minor, & Thompson-Schill, 2003; Pecher, Zeelenberg, & Barsalou, 2004; Zwaan, Stanfield, & Yaxley, 2002). The action or motor system has also been hypothesized to underlie language processing. The “motor resonance” theory (for review, see Fischer & Zwaan, 2008) postulates that comprehending linguistic descriptions of an action requires an internal simulation of the described action, and thus a deficit in the action or motor system would accompany a deficit in conceptual processing of motor processes. Support for this hypothesis comes from a study by Boulenger et al. (2008) on Parkinson’s disease (PD) patients who have impaired motor skills. Boulenger and colleagues demonstrated that PD patients’ ability to capture information from a masked prime depended on word meaning: When the patients were on dopaminergic treatment that improved their motor function, they showed priming for both concrete nouns and action verbs. However, when they were off dopaminergic treatment and their motor function was impaired, patients showed priming for concrete nouns, but not for action verbs. That is, they showed a selective deficit for action word priming. Thus, the results strongly indicated a close link between lexical-semantic processing and the motor systems.
The results from Experiments 1 and 2 present further evidence for a link between lexical-semantic processing and the sensory and motor systems. Manipulation features of an object appear to be affected by damage to the sensory and motor systems that are active in the use of the object in daily life. Those sensory and motor systems are affected in apraxia, thus leading to a deficit not only in physically manipulating objects but also in conceptually processing manipulation features. The apraxic patients’ impairment in accessing manipulation features, even in an implicit task, demonstrates a close link between perception and conceptualization.
Acknowledgments
This research was funded by NIH Grant DC00314 to Sheila E. Blumstein, NIH Grant MH62566 to Julie Sedivy, NIH Grant MH070850 to Sharon L. Thompson-Schill, and NIH Grant NS36387 to Laurel Buxbaum. We would like to thank Kathleen M. Kurowski, Audrey Kittredge, and Kathleen Kyle for their generous help with testing patients.
Appendix A
Appendix A
| Target | Related | Visual Control | Distractor | Distractor |
|---|---|---|---|---|
| baby carriage | lawn mower | spinning wheel | football | ladder |
| broom | hockey stick | crutches | flask | ashtray |
| camera | perfume | handcuffs | dustpan | anchor |
| clip | clothespin | headphones | dice | scarf |
| corkscrew | lightbulb | alarm clock | box | toilet paper |
| dart | paper plane | desk lamp | tissue | scale |
| doorknob | egg timer | cassette | abacus | bandaid |
| faucet | bottle cap | bell | yarn | magnifying glass |
| grenade | baseball | drum | clothes iron | thumbtack |
| handbrake | pliers | hourglass | wok | microphone |
| balloon | whistle | necklace | bouquet | hurdle |
| drill | spray bottle | safety pin | basket | picture frame |
| key | screwdriver | dumbbell | sled | frisbee |
| lighter | stopwatch | harmonica | palette | cotton swab |
| nutcracker | gas pump | extension cord | vacuum cleaner | blackboard |
| pencil sharpener | eggbeater | lawn chair | swimming goggles | strainer |
| piano | typewriter | couch | canoe | chisel |
| straw | pipe | mirror | easel | coat hanger |
| toaster | flush | spool | camcorder | sword |
| wheelchair | shopping cart | oven | birdhouse | microscope |
Appendix B
Appendix B
| Target | Related | Distractor | Distractor |
|---|---|---|---|
| muffin | donut | snail | calculator |
| cat | mouse | pump | printer |
| grapes | wine | cap | train |
| pie | ice cream | monkey | clover |
| pants | shirt | bricks | salt |
| sock | shoe | beer | bird |
| scissors | knife | coat | bread |
| wallet | purse | lawn | rope |
| telescope | binoculars | pumpkin | crow |
| window | door | gun | dog |
| robe | slippers | clam | wreath |
| teepee | igloo | paperclip | donkey |
| hammer | nail | elephant | bee |
| tape | glue | celery | violin |
Footnotes
Single quotes signify a stimulus word, double quotes signify a stimulus picture, and italics signify a conceptual representation. Thus, ‘piano’ signifies the word piano, “piano” signifies a picture of a piano, and piano signifies the corresponding concept.
Compared to the non-apraxic control group, the apraxic group appears to show a larger manipulation-relatedness effect. However, there are some points to be considered. First of all, the apraxic group showed higher proportions of fixations on the critical regions in general than the non-apraxic control group, which is most apparent in the proportions of target fixations. Furthermore, the apraxics showed the manipulation-relatedness effect later than the non-apraxics. Since the effect does not occur at the same time window across the groups and the apraxics showed higher proportions of fixations in general, it is hard to draw any conclusion based on the magnitude of the effect per se.
It seems that these four items were visually distinctive from other stimuli because of visual complexity (e.g., “anchor” with a rope around it compared to “dustpan” or “handcuffs”) or the size difference of actual objects represented by pictures (e.g., “chisel” compared to “couch” or “canoe”).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allport DA, Epstein R. Distributed memory, modular subsystems and dysphasia. In: Newman SK, editor. Current perspectives in dysphasia. Churchill Livingstone; Edinburgh: 1985. [Google Scholar]
- Altmann G, Kamide Y. Now you see it, now you don’t: mediating the mapping between language and the visual world. In: Henderson J, Ferreira F, editors. The interface of language, vision, and action. Psychology Press; 2004. [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behavioral and Brain Sciences. 1999;22:577–660. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences. 2003;7(2):84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Blumstein S, Milberg W, Shrier R. Semantic processing in aphasia: Evidence from an auditory lexical decision task. Brain and Language. 1982;17:301–315. doi: 10.1016/0093-934x(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Boronat C, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, Detre JA. Distinctions between function and manipulation knowledge of objects: Evidence from functional magnetic resonance imaging. Cognitive Brain Research. 2005;23:361–373. doi: 10.1016/j.cogbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, Nazir TA. Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008;46:743–756. doi: 10.1016/j.neuropsychologia.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor Apraxia: a Call to Action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Saffran EM. Knowledge of object manipulation and object function: dissociations in apraxic and non-apraxic subjects. Brain and Language. 2002;82:179–199. doi: 10.1016/s0093-934x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Veramonti T, Schwartz MF. Function and manipulation tool knowledge in apraxia: knowing “what for” but not “how”. Neurocase. 2000;6:83–97. [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Fischer MH, Zwaan RA. Embodied language: a review of the role of the motor system in language comprehension. Quarterly Journal of Experimental Psychology. 2008;61:825–850. doi: 10.1080/17470210701623605. [DOI] [PubMed] [Google Scholar]
- Garbarini F, Adenzato M. At the root of embodied cognition: Cognitive science meets neurophysiology. Brain and Cognition. 2004;56:100–106. doi: 10.1016/j.bandc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Lea and Febiger; Philadelphia: 1972. [Google Scholar]
- Kan IP, Barsalou LW, Solomon KO, Minor JK, Thompson-Schill SL. Role of mental imagery in a property verification task: fMRI evidence for perceptual representations of conceptual knowledge. Cognitive Neuropsychology. 2003;20:525–540. doi: 10.1080/02643290244000257. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Pattern K. Actions speak louder than functions. The importance of manipulability and action in tool representation. Journal of Cognitive Neuroscience. 2003;15:30–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Koski L, Iacoboni M, Mazziotta JC. Deconstructing apraxia: understanding disorders of intentional movement after stroke. Current Opinion in Neurology. 2002;15:71–77. doi: 10.1097/00019052-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Lissauer H. Ein Fall von Seelenblindheit nebst einem Beitrag zur Theorie derselben. Archiv für Psychiatrie und Nervenkrankheit. 1890;21:222–270. [Google Scholar]; Shallice T, Jackson M. Lissauer on agnosia. Cognitive Neuropsychology. 1988;5:157–192. Edited and reprinted in translation by. [Google Scholar]
- Milberg W, Blumstein SE. Lexical decision and aphasia: Evidence for semantic processing. Brain and Language. 1981;14:371–385. doi: 10.1016/0093-934x(81)90086-9. [DOI] [PubMed] [Google Scholar]
- Myung J, Blumstein SE, Sedivy JC. Playing on the Typewriter, Typing on the Piano: Manipulation Knowledge of Objects. Cognition. 2006;98:223–243. doi: 10.1016/j.cognition.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Pecher D, Zeelenberg R, Barsalou LW. Sensorimotor simulations underlie conceptual representations: Modality-specific effects of prior activation. Psychonomic Bulletin & Review. 2004;11:164–167. doi: 10.3758/bf03206477. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: The role of surface detail in basic level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Rothi LJG, Raymer AM, Ochipa C, Maher LM, Greenwald ML, Heilman KM. Florida Apraxia Battery, Experimental Edition. 1992.
- Taylor JR. Unpublished doctoral dissertation. Brown University; Providence: May, 2005. On the perceptual basis of semantic memory: Representation, process, and attentional control revealed by behavior and event-related potentials. 2005. [Google Scholar]
- Yee E, Blumstein SE, Sedivy JC. Lexical-semantic activation in Broca’s and Wernicke’s Aphasia: Evidence from eye movements. Journal of Cognitive Neuroscience. 2008;20:592–612. doi: 10.1162/jocn.2008.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan RA, Stanfield RA, Yaxley RH. Language comprehenders mentally represent the shapes of objects. Psychological Science. 2002;13:168–171. doi: 10.1111/1467-9280.00430. [DOI] [PubMed] [Google Scholar]