Abstract
The relationship of circumcision with the acquisition and clearance of HPV infection was examined in a cohort of 357 adult males followed at 2-month intervals for an average of 431 days. There were no differences in HPV acquisition by circumcision status. Clearance of HPV infection, including oncogenic types, was slower in the glans/coronal sulcus of the penis of uncircumcised compared to circumcised men. The median duration of HPV infection in the glans/coronal sulcus was significantly longer among uncircumcised men (154 days) compared to circumcised men (91 days) (p=0.04). Circumcision may protect against HPV-associated disease by enhancing the resolution of infection.
Keywords: human papillomavirus, circumcision, men, clearance, duration
Introduction
There is growing evidence that circumcision may protect against human papillomavirus (HPV) infection and penile cancer [1-5]. Partners of uncircumcised men have an elevated risk of cervical cancer [3], which suggests that circumcision may also reduce the transmission of HPV. We recently demonstrated that compared to circumcised men, those who are uncircumcised have a higher prevalence of HPV infection, specifically of the glans/coronal sulcus of the penis [6]. We pursued these findings further through an examination of the relationship of circumcision status to the acquisition and clearance of HPV infection of the external genitalia in a cohort of adult males.
Methods
Enrollment and follow-up
The study was approved by the Committee on Human Studies of the University of Hawaii. Written, informed consent was obtained from all study subjects. Study participants were primarily recruited from a university population in Hawaii, U.S.A. Eligible men were at least 18 years old, English speaking, and had no history of blood-clotting disorders (due to collection of blood for serology). Between July 2004 and December 2006, 445 adult males were initially enrolled and 357 were subsequently followed at 2-month intervals. The 88 men who did not complete more than 1 visit were excluded from the present analysis. Study visits were conducted at the University of Hawaii Health Services and the Cancer Research Center of Hawaii.
Interview
A structured questionnaire was administered by a trained interviewer at each study visit. The questionnaires covered demographic information and medical, sexual, and reproductive characteristics.
Specimen collection
Trained clinicians collected exfoliated cell samples from the external genitals. Circumcision status was determined by the clinician obtaining the specimen. Separate specimens were collected from the glans penis and corona sulcus, penile shaft, and scrotum. For uncircumcised men, an additional specimen was obtained from the inner foreskin. Visible warts and lesions were avoided in sampling the genitals. Disposable gloves worn by clinicians were changed between sampling of each site to minimize the risk of contamination between sites. The sampling procedure utilized textured paper and saline-moistened swabs and has been previously described [6-8].
HPV DNA testing and genotyping
DNA was extracted from each specimen by use of commercial reagents (Qiagen). The polymerase chain reaction used PGMY09/PGMY11 primers to amplify a 450-bp region of the L1 HPV genome. HPV-positive specimens were subsequently genotyped using a reverse line blot detection method for 37 different HPV types. HPV-positive specimens that were subsequently found to be negative in the genotyping assay were considered to be unclassified HPV positive specimens. All specimens were also tested using GH20 and PC04 primers to amplify a 268-bp region of the human beta-globin gene as an internal control for sample sufficiency. Specimens testing negative for beta-globin were considered to be insufficient and were excluded from analyses.
Statistical analysis
Twenty-eight men self-reporting to be HIV-positive were excluded from all analyses because of the strong confounding effect of HIV on HPV infection [9]. HPV DNA results were evaluated by anatomic site (glans/corona, shaft, scrotum). HPV results from individual sites were also combined for analysis of any genital HPV infection. HPV results from the inner foreskin were excluded from analysis of any genital infection for lack of a comparable site in circumcised men. HPV genotypes were grouped as follows: (1) any HPV, including unclassified types; (2) oncogenic HPV; and (3) other HPV. Oncogenic types included HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 [10]. Other HPV included non-oncogenic HPV and HPV of undetermined risk status: HPV 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 (subtype HPV 82), and CP6109 (HPV 89).
Analyses of HPV acquisition and clearance were limited to incident HPV infections, defined as the presence of an HPV genotype not identified on a previous visit.
HPV clearance was defined as the acquisition of infection followed by the lack of detection of that HPV genotype at two or more subsequent visits. The timing of viral clearance was defined as the time of the first negative visit. We assessed the overall differences in clearance of HPV infection between circumcised and uncircumcised individuals by the Kaplan-Meier (product-limit) method and Wilcoxon test for the equality of strata.
The association between HPV acquisition or clearance and circumcision status was modeled through Cox regression using the days of HPV negative status (for acquisition) or days since infection acquisition (for clearance) as the time metric.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were used as measures of association comparing uncircumcised men to circumcised men. Separate infection paths were assigned to each HPV genotype detected. Estimation of risk associated with oncogenic types included specimens concurrently positive for other types. Likewise, risk estimation for “other” HPV included specimens concurrently positive for oncogenic types. Factors previously observed to be associated with circumcision and/or HPV infection [6] were included as covariates. These included age (continuous), race/ethnicity (white/non-white), birthplace (U.S./non-U.S.), education (<college, college degree), lifetime number of female partners, history of sex with men (yes/no), condom use during prior 4 months (yes/no), history of genital warts (yes/no). All p-values were two-sided, and p < 0.05 was defined as significant.
Results
The cohort included a total of 357 men followed for at least two visits including 290 circumcised men and 67 uncircumcised men. Men were followed for an average of 431 days (range 38-1262) over a mean of 7.2 visits (range 2-9). The mean age of the cohort was 29.2 years (range 18-79) years. The majority of men were white (58%), U.S.-born (81%), never married (82%), and college students (64%). Seventy-five percent were heterosexual with a median lifetime number of female partners of 6.5.
Overall, specimen sufficiency, measured by presence of human beta-globin, did not differ by circumcision status: for all genital specimens combined, 6,068/6,660 (91%) of those from circumcised men were beta-globin positive compared to 1,435/1,557 (92%) in uncircumcised men (p=0.18). For the glans/coronal sulcus specifically, beta-globin detection was lower in circumcised men (1,994/2,220, 90%) compared to uncircumcised men (481/519, 93%) (p=0.05). There were no differences in specimen sufficiency by circumcision status in shaft or scrotal specimens.
A total of 536 genotype-specific incident infections were observed across all genital sites during the period of follow-up. There were no differences in the risk of HPV acquisition by circumcision status at any genital site (data not shown).
The duration and risk of clearance of incident HPV infections were evaluated (Table 1). The duration of infection did not vary by circumcision status in the penile shaft, scrotum, or all genital sites combined. There were also no differences in the risk of HPV clearance by circumcision status at these sites. For the glans/coronal sulcus, the median duration of HPV infection was greater among uncircumcised (154 days) compared to circumcised men (91 days), although the confidence intervals overlapped. Product-limit estimates for the time to clearance of HPV infection in the glans/coronal sulcus were significantly longer among uncircumcised compared to circumcised men (p=0.04) (Figure 1). Uncircumcised men had a lower risk of HPV clearance in the glans/coronal sulcus compared to circumcised men. The lower risk of clearance was observed for any HPV (hazard ratio: 0.59, 95% CI: 0.36-0.98), oncogenic HPV (hazard ratio: 0.36, 95%: CI 0.14-0.91), and other HPV (hazard ratio: 0.50, 95% CI: 0.25-0.98).
Table 1. Duration and clearance of genital HPV infection in males by circumcision status, Hawaii, 2004-2008 (n=357).
| Site | HPV Duration | HPV Clearance | ||
|---|---|---|---|---|
| Any HPV1 | Any HPV1 | Oncogenic HPV2 | Other HPV3 | |
| Median days (95% CI) | Adjusted4 hazard ratio (95% CI) | Adjusted4 hazard ratio 95% CI | Adjusted4 hazard ratio 95% CI | |
| Any genital5 | ||||
| circumcised | 109 (89-126) | 1.00 (reference) | 1.00 (ref.) | 1.00 (ref.) |
| uncircumcised | 106 (64-146) | 1.04 (0.76-1.40) | 0.90 (0.48-1.68) | 1.15 (0.65-2.02) |
| Penis | ||||
| Foreskin | ||||
| circumcised | -- | |||
| uncircumcised | 112 (70-184) | -- | -- | -- |
| Glans /corona sulcus | ||||
| circumcised | 91 (71-111) | 1.00 (reference) | 1.00 (ref.) | 1.00 (ref.) |
| uncircumcised | 154 (68-248) | 0.59 (0.36-0.98) | 0.36 (0.14-0.91) | 0.50 (0.25-0.98) |
| Shaft | ||||
| circumcised | 119 (108-176) | 1.00 (reference) | 1.00 (ref.) | 1.00 (ref.) |
| uncircumcised | 105 (91-182) | 1.06 (0.71-1.58) | 0.60 (0.24-1.49) | 0.86 (0.41-1.80) |
| Scrotum | ||||
| circumcised | 91 (75-112) | 1.00 (reference) | 1.00 (ref.) | 1.00 (ref.) |
| uncircumcised | 70 (63-106) | 0.95 (0.67-1.35) | 1.24 (0.48-3.22) | 0.93 (0.54-1.60) |
Includes untyped HPV-positive specimens.
Includes oncogenic HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.
Includes non-oncogenic HPV and HPV of undetermined risk status:. HPV 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 (subtype HPV 82), and CP6109 (HPV 89)
Adjusted for age (continuous), race/ethnicity (white/non-white), birthplace (U.S./non-U.S.), education (<college, college degree), lifetime number of female partners, history of sex with men (yes/no), condom use during prior 4 months (yes/no), history of genital warts (yes/no).
Excludes foreskin specimens.
Figure 1. Clearance1 of HPV infection of the penile glans/coronal sulcus by circumcision status.
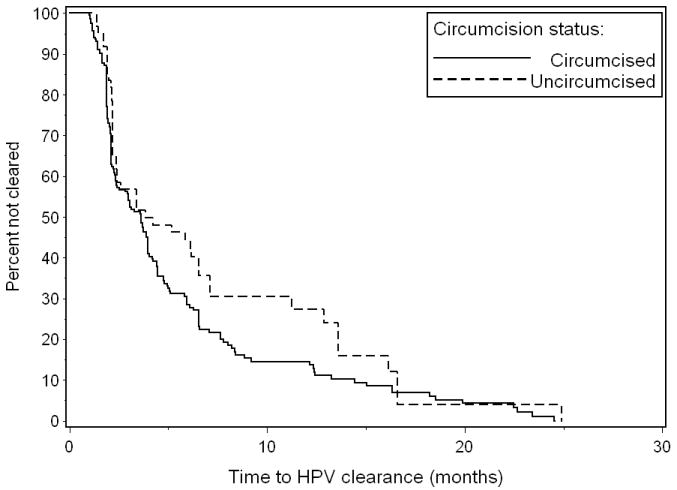
1Wilcoxon test for equality of time-to-clearance rates between circumcised and uncircumcised men: p=0.04
Discussion
Our results demonstrate that uncircumcised men have poorer ability to resolve HPV infections of the penis glans/coronal sulcus. The reduced viral clearance among uncircumcised men was observed for both oncogenic and non-oncogenic HPV types. We previously observed a higher prevalence of any HPV and oncogenic HPV infection in the glans/coronal sulcus of uncircumcised men [6]. The present analysis suggests that the higher prevalence of HPV may be attributed to longer duration of infection in the glans/coronal sulcus of uncircumcised men rather than to a greater rate of acquisition of infection.
Our results are consistent with two other longitudinal investigations of HPV in men which have demonstrated that reduced persistence and greater clearance of genital HPV among circumcised men [11, 12]. Lu et al similarly observed greater clearance of oncogenic HPV as well as any HPV [12]. In this study, however, site-specific estimations were not included as individual genital specimens (glans/coronal sulcus, shaft, and scrotum) were combined for testing.
A major limitation of our study as well as other longitudinal investigations of HPV is the inability to distinguish clearance of infection from failure to detect an infection. Lack of viral detection may be due to fluctuations in viral levels, sampling variability, as well as limitations in assay sensitivity.
It is not likely that the greater specimen sufficiency in the glans/corona of uncircumcised compared to circumcised men explains the observed differences in viral clearance. Measurement of HPV acquisition and clearance was limited to specimens that were positive for beta-globin. Moreover, a lower percentage of sufficient glans/corona samples among circumcised men would make it less likely to detect a clearance event, and therefore may lead to a longer estimated duration of HPV infection among circumcised men. However, the opposite was observed in our data, that is, circumcised men had a shorter duration of infection of the glans/corona.
A major strength of this investigation is the short interval between visits (2 months), which enhanced our ability to examine the acquisition of new infections and the duration of these incident infections. Estimates of viral duration and clearance events were made more robust by limiting these observations to incident infections and excluding prevalent HPV infections detected at baseline. We were able to account for sexual history, including condom use and number of sexual partners, and for other potential confounders related to either circumcision status or HPV infection. Site-specific sampling and testing allowed us to separately evaluate genital subsites.
Our study demonstrates that the apparent protective influence of circumcision against genital HPV infection may not involve a reduction in new infections but rather the enhanced ability to resolve existing HPV infections. Similar to what has been observed in females, HPV infections are generally transient infections in men [11]. It is not understood how circumcision facilitates greater clearance of HPV. It is possible that HPV persists more efficiently within the mucosal surface to the inner foreskin of uncircumcised men compared to the keratinized penile surface of circumcised men. Thorough washing of the genitals may be more difficult for uncircumcised men as this requires retraction of the foreskin to expose the inner surface [13]. To what extent genital washing influences HPV clearance is not known. Nonetheless, we did not observe a longer duration of HPV infection in the foreskin compared to the glans/corona of uncircumcised men indicating that the infections did not persist longer in the mucosal surface.
Our observation of reduced clearance time of oncogenic HPV infection specifically in the glans/corona of uncircumcised men has clinical significance. Uncircumcised men have an increased risk of penile cancer [1, 5] and the glans penis is the primary subsite of penile cancers [14, 15]. Partners of uncircumcised men have an increased risk of cervical cancer [3], underscoring the possibility that transmission of HPV to sexual partners is more efficient among uncircumcised men due to greater duration of their infection.
Strategies to minimize the persistence of HPV infection combined with those to prevent acquisition of infection may be the most effective means of controlling HPV-associated disease. Whether circumcision is an effective means to facilitate HPV clearance has yet to be demonstrated.
Acknowledgments
We extend our gratitude to the staff of the Cancer Research Center of the University of Hawaii (UH) and the UH University Health Services.
Financial support: Centers of Biomedical Research Excellence Program (award P20 RR018727 from the National Center for Research Resources, National Institutes of Health).
Footnotes
Potential conflicts of interest: none
References
- 1.Tsen HF, Morgenstern H, Mack T, Peters RK. Risk factors for penile cancer: results of a population-based case-control study in Los Angeles County (United States) Cancer Causes Control. 2001;12:267–77. doi: 10.1023/a:1011266405062. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, Vaught LC, Giuliano AR. Condom use and other factors affecting penile human papillomavirus detection in men attending a sexually transmitted disease clinic. Sex Transm Dis. 2004;31:601–607. doi: 10.1097/01.olq.0000140012.02703.10. [DOI] [PubMed] [Google Scholar]
- 3.Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 4.Svare EI, Kjaer SK, Worm AM, Osterlind A, Meijer CJ, van den Brule AJ. Risk factors for genital HPV DNA in men resemble those found in women: a study of male attendees at a Danish STD clinic. Sex Transm Infect. 2002;78:215–218. doi: 10.1136/sti.78.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606–616. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez BY, Wilkens LR, Zhu X, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008;197:787–94. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez BY, McDuffie K, Goodman MT, et al. Comparison of physician- and self-collected genital specimens for detection of human papillomavirus in men. J Clin Microbiol. 2006;44:513–517. doi: 10.1128/JCM.44.2.513-517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–685. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 9.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 10.Castle PE. The evolving definition of carcinogenic human papillomavirus. Infect Agent Cancer. 2009;4:7. doi: 10.1186/1750-9378-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lajous M, Mueller N, Cruz-Valdez A, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–1716. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 12.Lu B, Wu Y, Nielson CM, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: a prospective study. J Infect Dis. 2009;199:362–71. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- 13.O'Farrell N, Quigley M, Fox P. Association between the intact foreskin and inferior standards of male genital hygiene behaviour: a cross-sectional study. Int J STD AIDS. 2005;16:556–9. doi: 10.1258/0956462054679151. [DOI] [PubMed] [Google Scholar]
- 14.Rippentrop JM, Joslyn SA, Konety BR. Squamous cell carcinoma of the penis: evaluation of data from the surveillance, epidemiology, and end results program. Cancer. 2004;101:1357–1363. doi: 10.1002/cncr.20519. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113:2883–91. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]


