Abstract
Association of chronic inflammation with an increased risk of cancer is well established but the contributions of innate versus adaptive immunity are not fully delineated. There has furthermore been little consideration of the role played by chronic inflammation-associated antigens, including cancer antigens, and the possibility to use them as vaccines to lower the cancer risk. We studied the human tumor antigen MUC1 that is abnormally expressed in colon cancers and also in inflammatory bowel disease (IBD) that gives rise to colitis associated colon cancer (CACC). Using our new mouse model of MUC1+ IBD that progresses to CACC, IL-10−/− mice crossed with MUC1 transgenic mice, we show that vaccination against MUC1 delays IBD and prevents progression to CAAC. One mechanism is the induction of MUC1-specific adaptive immunity (anti-MUC1 IgG, anti-MUC1 CTL) that appears to eliminate abnormal MUC1+ cells in IBD colons. The other mechanism is the change in the local and the systemic microenvironments. Compared to IBD in vaccinated mice, IBD in control mice is dominated by larger numbers of neutrophils in the colon and myeloid-derived suppressor cells (MDSC) in the spleen, which can compromise adaptive immunity and facilitate tumor growth. This suggests that the tumor-promoting microenvironment of chronic inflammation can be converted to a tumor-inhibiting environment by increasing adaptive immunity against a disease-associated antigen.
Keywords: MUC1, vaccine, tumor antigen, colon cancer, chronic inflammation
Introduction
Patients with chronic inflammatory disorders have an increased incidence of cancer development at the affected site (1). There is evidence that chronic inflammation may serve as the carcinogenic event (1–3). On the other hand, oncogenic events, such as RET/PTC gene rearrangement found in thyroid cancer (4) or activation of Myc and Ras found in many cancers, can initiate inflammatory transcriptional programs in transformed cells or the surrounding stromal cells, generating chronic inflammation at the tumor site (5, 6). Thus, regardless of the order of events, inflammation preceding cancer or cancer preceding inflammation, the result can be a tumor-promoting chronic inflammatory microenvironment. Chronically inflamed sites contain a diverse population of leukocytes that express and secrete an assortment of mediators that foster cell proliferation and genomic instability (2, 7). We wanted to know whether this microenvironment could be altered by the presence of strong adaptive immunity specific for an antigen expressed at the disease site. Our hypothesis was that early in inflammation, effective priming of the adaptive immunity to many potential neoantigens at the affected site, or a prompt recall response to some of those antigens encountered previously, may clear the site and prevent chronic inflammation altogether, or prevent development of cancers that would be expected to express some of the same neoantigens. We have tested this hypothesis in a mouse model of inflammatory bowel disease and colitis associated colon cancer (CACC) that we previously described (8). As a target antigen for the adaptive immune response, we utilized the mucin glycoprotein MUC1 (9, 10).
MUC1 is expressed at low levels on the apical surface of ductal epithelial cells in several organs including on the colonic epithelium (11). The extracellular domain is dominated by a variable number of tandem repeats (VNTR) region composed of an average of 80–200 repeats that are 20 amino acids (a.a.) in length. The 20 a.a. peptide sequence HGVTSAPDTRPAPGSTAPPA has five potential O-glycosylation sites that on healthy epithelia are heavily glycosylated. In the majority of adenocarcinomas and their precursor lesions, and in various inflammatory diseases including IBD, MUC1 is overexpressed and its VNTR region is profoundly hypoglycosylated (8, 9, 12–15). The reduced glycosylation exposes the peptide backbone resulting in peptide epitopes as well as truncated novel glycopeptide epitopes that are processed and presented to the immune system (16–18). Furthermore, we and others discovered that this hypoglycosylated MUC1 has chemotactic properties for the cells of the innate immune system that have multiple receptors capable of binding this abnormal form (16, 19–21). Abnormal expression of MUC1 in IBD thus provides a source of predictable disease associated neoepitopes, that could exert important influence on the innate immune system as well as be targets of adaptive immunity.
We previously characterized a mouse model of MUC1 positive human IBD (MUC1+ IBD) (8) derived by crossing human MUC1 transgenic mice (22) that faithfully replicate tissue specific MUC1 expression in humans, with interleukin-10 knockout (IL-10−/−) mice that develop spontaneous IBD. De novo expression of abnormally glycosylated MUC1 on affected colonocytes resulted in worse inflammation and in increased cancer development (8). In the current study we used the same mouse model to show that vaccine-elicited adaptive immunity to MUC1 early in the disease process leads to delayed and tempered development of IBD, changes in the inflammatory microenvironment and complete protection from colon cancer.
Materials and Methods
Mice
Interleukin-10 knockout mice (IL-10−/−) on a C57Bl/6 background were purchased from The Jackson Laboratory and MUC1-transgenic (Tg) mice on a C57Bl/6 background were originally purchased from Dr. S.J. Gendler (The Mayo Clinic, Scottsdale, AZ) and are bred at the University of Pittsburgh. IL-10−/− mice (homozygous for the IL-10 deletion) develop spontaneous colitis with pathological changes similar to human inflammatory bowel disease. Human MUC1 Tg mice express full length human MUC1 in the same spatial and tissue distribution as the endogenous protein in humans (22). These mice are maintained in a heterozygous state for human MUC1. The MUC1+/IL-10−/− progeny (25% of total) generated by crossing IL-10−/− mice with MUC1-Tg mice, develop MUC1 positive inflammatory bowel disease (8). Littermates that do not carry this genotype are used as controls thus keeping the living conditions of all mouse groups equal. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (Pittsburgh, PA).
PCR genotyping
PCR was used to identify the MUC1 transgene as well as the presence or absence of the IL-10 gene. The primer pairs for MUC1-Tg were 5′-CTTGCCAGCCATAGGACCAAG-3′ and 5′-CTCCACGTCGTGGACATTGATG-3′. The IL-10 primers were 5′-GTGGGTGCAGTTATTGTCTTCCCG and 5′-GCCTTCAGTATAAAAGGGGGACC and 5′-CCTGCGTGCAATCCATCTTG-3′. The PCR product of each reaction was analyzed on a 1% agarose gel. MUC1 amplification resulted in a 500-bp fragment and IL-10 amplification resulted in a 200-bp fragment if IL10+/+, 200 and 450-bp fragments if IL10+/−, and 450-bp fragment if IL10−/−.
Peptide synthesis and in vitro glycosylation
The TnMUC1-100mer peptide used for immunization corresponds to five tandem repeats of a 20-aa sequence HGVTSAPDTRPAPGSTAPPA from the extracellular variable number of tandem repeat (VNTR) region of MUC1. Enzymatic addition of GalNAc to the peptide was performed using recombinant UDP-GalNAc polypeptide N-acetyl-galactosaminyltransferases rGalNAc-T1 as previously described (41). The MUC1 peptide MUC1-9-5N9L and glycopeptide MUC1-10-5GalNAc correspond to the extracellular VNTR region of MUC1. The peptides were synthesized at the University of Pittsburgh Genomics and Proteomics Core Laboratories.
Vaccine protocol
Mice in the vaccine group received 20μl intranasally (10μl/nare) of 30μg of TnMUC1-100mer peptide mixed with 3μg of adjuvant E6020 in PBS. Adjuvant treated mice received 3μg of adjuvant E6020 in PBS. Mice received vaccine or adjuvant between 5–6 weeks of age and were boosted twice at 2-week intervals. The adjuvant E6020 is a synthetic, attenuated, TLR-4 agonist provided by Eisai Research Institute, Andover, MA (24).
Histology and inflammation scores
Colon was removed immediately after sacrifice, cut longitudinally, washed in cold 1X PBS, fixed in 5% buffered formalin, and embedded in paraffin. Five-μm-thick sections were stained with hematoxylin and eosin (H&E). Inflammation scores (0–4) were determined by a gastrointestinal pathologist who was blinded to the experimental protocol, using criteria previously reported (8). Briefly, 20–40 separate microscopic fields were evaluated for each mouse and graded 0–4. The total inflammation score for each sample was determined by taking the sum of the fields divided by the number of fields.
Immunohistochemistry
Five μm-thick tissue paraffin sections were deparaffinized by baking overnight at 59°C. Endogenous peroxidase activity was eliminated by treatment with 30% H2O2 for 15 minutes at room temperature. Antigen retrieval was performed by microwave heating in 0.1% citrate buffer. Nonspecific binding sites were blocked with Protein Blocking Agent (Thermo-Shandon). The anti-MUC1 antibody HMPV (BD Pharmingen) recognizes all forms of MUC1 by binding the epitope APDTR in the VNTR region in a glycosylation-independent manner. The anti-MUC1 antibody VU-4H5 (Santa Cruz Biotechnology) recognizes the epitope APDTRPAP in the VNTR region of hypoglycosylated MUC1. Staining was performed by the avidin-biotin-peroxidase method with a commercial kit (Vectastain ABC kit, Vector laboratories). Color development was performed using a 3,3′-diaminobenzidine kit (BD Pharmingen).
Microscopy and Image Acquisition
Histology sections were observed using an Olympus BX40 microscope. Images were acquired using a Leica DFC420 camera and Leica Application Suite version 2.7.1 R1.
Lactoferrin ELISA
Fecal samples were measured for the concentration of lactoferrin as previously described (31). Briefly, fecal pellets between 15–30mg were homogenized in 500μl of collection buffer. Collection buffer is 0.1% deoxycholate (Sigma D6750) in PBS, 10μM leupeptin (Sigma L2884), 1.6μM pepstatin A (Sigma P4265), and 5μg/ml aprotinin (Sigma A1153). 96-well Maxisorb plates (Fisher 44-24-04) were coated with 100μl/well of fecal homogenate or human lactoferrin standards (Sigma L0520) and incubated overnight at 4°C. Plates were washed with PBS/0.05% Tween 20 and blocked for 1 hour with BSA. Rabbit anti-human lactoferrin antibody (Sigma L3262) was diluted 1:500 in collection buffer and added at 100μl/well and incubated for 2 hours at room temperature. 100μl/well of HRP-labeled goat anti-rabbit antibody was added and incubated for 30 minutes at room temperature. For color development, 100μl/well of TNB substrate (BD OptEIA 555214) was added and incubated in the dark for 10–12 minutes. The reaction was stopped with 50μl/well of sulfuric acid. The plates were read at 450nm. Fecal lactoferrin concentration is based on the human lactoferrin standard curve and dividing the lactoferrin (ng) by the weight of the starting fecal sample. This gives nanogram of lactoferrin/gram of feces.
MUC1-specific ELISA
Blood was collected 5 weeks of age, prior to treatment and at the time of sacrifice. Serum was tested for the presence of MUC1-specific antibodies using a MUC1-specific ELISA. Briefly, 96-well plates were coated overnight at 4°C with 10μg/ml of MUC1-100mer peptide in PBS. Plates were washed with PBS/0.5% Tween 20 and incubated with mouse serum diluted at 1:50 for 1 hour at room temperature. After three washes with 0.5% Tween 20, plates were incubated with goat anti-mouse peroxidase conjugated secondary antibody (Sigma) for 1 hour at room temperature. Plates were washed three times with 0.5% Tween 20 and incubated with substrate reagent A&B (BD OptEIA, BD Biosciences) for 15 minutes. The reaction was stopped with 2.5M sulfuric acid and absorbance read at 450nm.
Chromium release assay
106 RMA and RMA-MUC1 target cells were labeled for 1 hour with radioactive sodium chromate (Na2CrO4), washed 3X with Dulbecco’s Modification of Eagle’s Medium (DMEM), resuspended at 106 cells/ml and plated in 96-well V-bottom microtiter plate at 2,000/well in 100μl. Effector cells were added in triplicate in DMEM at 105/well for 4 hours. Supernatant was harvested and analyzed on a Cobra II auto-gamma counter (Perkin-Elmer, MA). Specific Lysis was calculated as (lysis-spontaneous lysis)/(total lysis-spontaneous lysis). Average of triplicates was plotted.
Flow cytometry
Isolated cells from lymph nodes and spleens were washed and resuspended in FACs buffer (2% FBS in PBS) and plated at 0.5–1 × 106 cells/well. Surface Fc receptors were blocked with the addition of anti-CD16 (BD Biosciences) and incubated for 20 minutes. Cells were stained with anti-CD3, CD4, CD8, Gr1, and CD11b (BD Biosciences). DimerX, Soluble Dimeric Mouse H-2Kb:Ig Fusion Protein (BD Biosciences) was used according to manufacturer’s protocol to detect MUC1-specific CD8 T cells. Cells were analyzed on a LSR II Flow cytometry (BD Bioscience), running FACSDiva software.
Statistical analysis
We conducted statistical analyses with GraphPad software. We analyzed Kaplan-Meir curves with the log-rank test. We calculated mean scores and statistical significance with Student’s t test (unpaired two-tailed) and Fisher’s exact test (two-tailed).
RESULTS
MUC1 vaccine slows down IBD progression
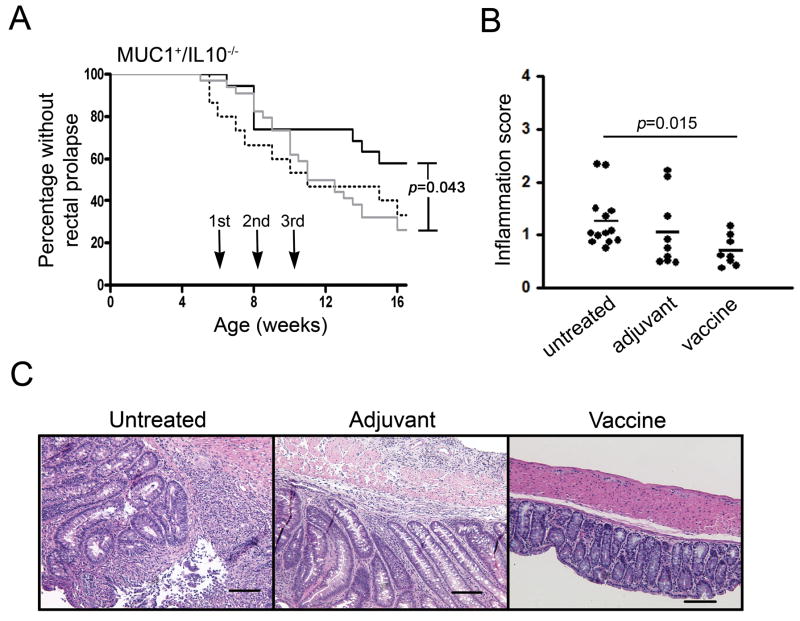
MUC1+/IL-10−/− mice develop spontaneous IBD around 6–8 weeks of age. Unlike IBD that develops in IL-10−/− mice, which has been used as one of the major models of human IBD, the disease that arises in MUC1+/IL-10−/− mice overexpresses the abnormal, hypoglycosylated MUC1 characteristic of and important for development of human IBD (8). MUC1+/IL-10−/− mice were started on a course of vaccination at 5–6 weeks of age. The vaccine consisted of a synthetic 100mer glycopeptide Tn-MUC1 corresponding to five 20 amino acid tandem repeats of MUC1, glycosylated in vitro to express the tumor associated glycan GalNAC (Tn), plus E6020 adjuvant, a synthetic, attenuated TLR-4 agonist that promotes both systemic and mucosal immunity (23–25). Mice received nasal administration of either the vaccine or the adjuvant alone as control and were boosted twice at two-week intervals. We chose nasal administration to promote mucosal immunity capable of homing to the colonic mucosa. As an additional control we included untreated mice. The three groups of mice were monitored until 16 weeks of age for the development of rectal prolapse as the first external symptom of IBD. Figure 1A shows that mice that received the vaccine developed rectal prolapse significantly later (p=0.043) compared to control mice. There was no significant difference between the two control groups. The difference between the vaccine group and the control groups emerged around 9 weeks of age at which time cases of rectal prolapse in the vaccine treated group reached a plateau while mice in the control groups continued to develop rectal prolapse. This plateau started after the second administration of the vaccine.
Figure 1.
Vaccination of MUC1+/IL-10−/− mice with a MUC1 vaccine slows down IBD development and decreases intestinal inflammation. (A) Kaplan-Meir curves representing development of rectal prolapse in vaccinated (solid line, n=19), adjuvant treated (dotted line, n=16) or untreated (gray line, n=34) mice. Arrows indicate administration of the vaccine or adjuvant alone at 6, 8, and 10 weeks of age. * p=0.043. P value was determined by the log-rank test. (B) Colonic inflammation scores formice that received vaccine (n=8), adjuvant alone (n=9), or remained untreated (n=13), sacrificed at 16 weeks of age. * p=0.015. P value was determined by unpaired two-tailed Student’s t test. (C) Representative H&E sections of colons from an untreated, an adjuvant treated, and a vaccinated MUC1+/IL-10−/− mouse. Scale bar, 50 μm.
We performed the same experiment in IL-10−/− mice that do not express MUC1. As we previously published (8), in the absence of MUC1, the disease kinetics are different in these mice but we could nevertheless use them as controls for the antigen (MUC1)-specific effects of the vaccine. These mice also received nasal administration of the vaccine or adjuvant alone, or remained untreated, and were followed up to 16 weeks of age for the development of rectal prolapse. There was no statistically significant difference in disease progression between the vaccinated and the control groups (data not shown).
The differences between groups observed in MUC1+/IL-10−/− mice by monitoring the external signs of IBD were confirmed by pathology. Colons from each treatment group were collected at the time of sacrifice (16 weeks of age) and scored for inflammation grade by a pathologist blinded to the experimental protocol. Mice that received the vaccine had significantly lower inflammation scores (mean=0.699) compared to untreated mice (mean=1.256) (p=0.015) (Fig. 1B). Although mice that received adjuvant alone also had higher inflammation scores (mean=1.042) than the vaccinated mice, the difference did not reach statistical significance. This difference, however, was biologically significant. Representative tissue sections from a randomly selected mouse in each group (Figure 1C), viewed at same magnification, show increased cellular infiltrate and pronounced thickening of the colon from an untreated and an adjuvant treated mouse compared to a vaccinated mouse.
Induction of MUC1-specific immunity in response to IBD versus the vaccine
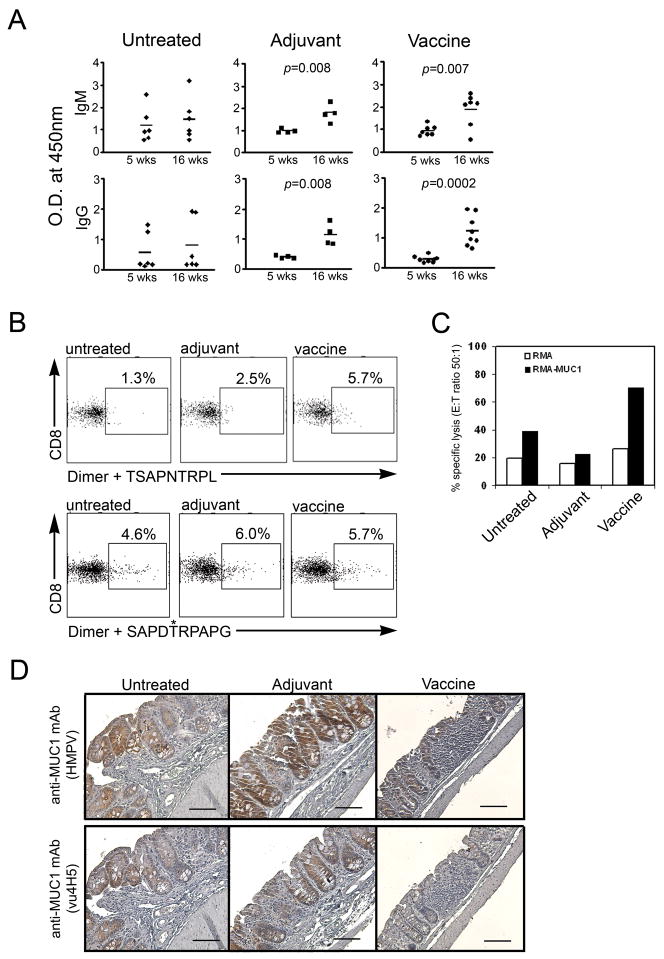
We evaluated anti-MUC1 immunity in all three groups of mice that was either generated spontaneously in response to its abnormal expression in IBD, further enhanced in the presence of adjuvant alone or elicited by the MUC1 vaccine. For measuring humoral responses, serum was collected at 5–6 weeks of age (prior to treatment) and again at 16 weeks of age, two weeks following the last injection in the treated groups. There was a significant increase in the ELISA O.D. values for anti-IgM in post-treatment samples compared to pre-treatment serum levels for both the vaccinated mice (p=0.007) and for the adjuvant treated mice (p=0.008 (Fig. 2A). We also detected a significant increase in the O.D. in post-treatment compared to pre-treatment serum levels of anti-MUC1 IgG in adjuvant treated mice (p=0.008) and a highly significant increase in the vaccinated mice (p=0.0002). Some of the untreated mice had high anti-MUC1 IgM and IgG responses at baseline but in contrast to treated mice there was no significant change between the two time points.
Figure 2.
MUC1-specific immune responses in vaccinated versus control MUC1+/IL-10−/− mice. (A) MUC1-specific antibodies in serum of untreated (n=6), adjuvant treated (n=4), and vaccinated (n=7) mice. IgM isotype, top panel; IgG isotype, bottom panel; Optical Density (O.D.) measured by ELISA at 450 nm. Serum samples were diluted 1:50. P value was determined by unpaired two-tailed Student’s t test. (B) Splenocytes from untreated, adjuvant treated, or vaccinated MUC1+/IL-10−/− mice were stained with MUC1 peptide loaded H2Kb-Ig dimer. Representative dot plots from two independent experiments. (C) Results of standard 51Cr release assay at an effector:tumor cell (E:T) ratio of 50:1. Effector cells are pooled lymph node (LN) from two mice per group. Targets are RMA (H2Kb) or RMA-MUC1 (H2Kb), transfected with human MUC1 cDNA. (D) Immunostaining (hematoxylin counterstained) of colon sections with anti-MUC1 antibodies. Scale bar, 50μm.
Using mouse MHC class I H2Kb dimers (BD DimerX®), MHC-immunoglobulin fusion proteins that detect antigen specific T cells (26), we looked for the induction of MUC1-specific CD8 T cells. Dimers were loaded with either the 9 amino acid peptide MUC1-9-5N9L or the 10 amino acid glycopeptide MUC1-10-5GalNAc, both derived from the MUC1 VNTR region sequence HGVTSAPDTRPAPGSTAPPA. In the peptide, the MHC class I anchor positions P5 and P9 in the native 9 amino acid peptide were changed from aspartic acid to asparagine and from alanine to leucine respectively, converting TSAPDTRPA to TSAPNTRPL, to increase binding affinity for class I (27, 28). The MUC1 10mer glycopeptide SAPDTRPAPG has the GalNAC (Tn) glycan attached to the threonine at position 5. Dimers were loaded overnight with either peptide, mixed with pooled splenocytes from each group of mice and analyzed by flow cytometry. Vaccinated mice had 5.7% of CD8 T cells that recognized the MUC1-9-5N9L peptide compared to 2.5% in the adjuvant group and 1.3% in the untreated group (Fig. 2B). Interestingly, all groups appeared to have CD8 T cells that recognized the MUC1-10-5GalNAc glycopeptide. We interpret this as an indication that MUC1 glycopeptide-specific CD8 T cells are generated in response to the disease and that their induction is further promoted in the presence of adjuvant. The administration of the MUC1 vaccine, on the other hand, resulted in an increased frequency of CD8 T cells specific for both the peptide and the glycopeptide.
To test if these CD8 T cells were functional CTL, we again pooled splenocytes from vaccinated, adjuvant treated or untreated mice and used them as effector CTL against chromium labeled targets, the mouse lymphoma cell line RMA and the same cell line transfected with human MUC1 cDNA (RMA-MUC1). The highest lytic activity was found in spleens from mice that received the vaccine. (Fig. 2C). This begged the question whether the presence of these CTL in vaccinated animals may lead to elimination of cells expressing MUC1 from the colons of mice with IBD. MUC1 expression in colons from the vaccinated group and the controls was evaluated by immunohistochemistry (Fig. 2D). Colon sections were stained with the anti-MUC1-specific mAb HMPV that recognizes all forms of MUC1 (top), and with the anti-MUC1-specific mAb VU4H5 that recognizes the abnormal hypoglycosylated form of MUC1 (bottom). Representative sections from each treatment group show that higher levels of MUC1 are observed in an untreated and adjuvant treated mouse colons compared to the vaccinated mouse (top panels). The MUC1 molecules in the untreated and adjuvant treated mice are the hypoglycosylated forms. In contrast, very little if any hypoglycosylated MUC1 was detected in vaccinated mice (bottom panels).
Vaccine elicited MUC1 immunity prevents colitis-associated colon cancer (CACC)
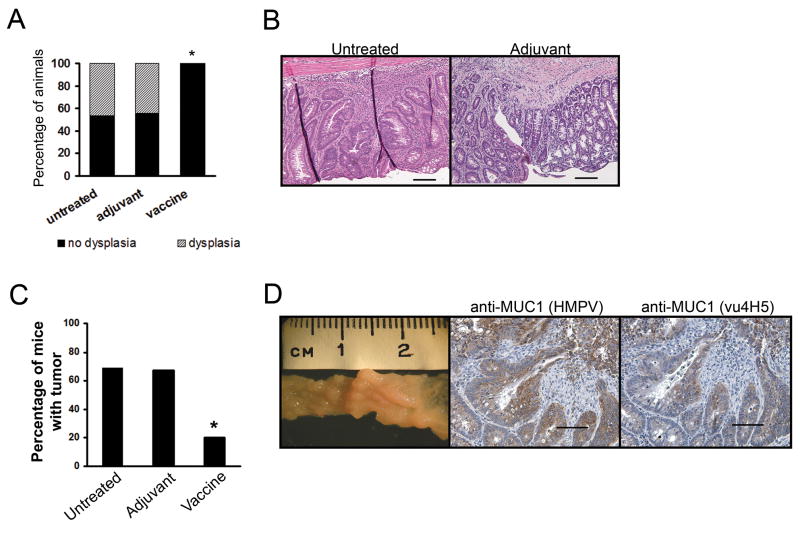
Given that our previous work showed that the majority of untreated MUC1+/IL-10−/− mice that developed IBD progressed to cancer (8), we evaluated the ability of the MUC1 vaccine to prevent early dysplastic lesions and CACC. The animals were matched by age and their colons examined early in the course of disease for the development of colonic dysplasia. Alternatively, animals were grouped by the extent of their disease and the number of tumor foci evaluated in vaccinated mice relative to untreated mice or to animals that received adjuvant only.
At 16 weeks of age, there was no evidence of colonic dysplasia or neoplasia in 8/8 animals in the vaccinated group (Fig. 3A). In contrast, six of thirteen untreated mice (46%) and four of nine adjuvant treated mice (44%) had colonic dysplasia. One mouse in the adjuvant group also had invasive colon cancer and one untreated mouse (not included in the graph) developed cancer at 12 weeks of age and had to be removed early from the protocol. Fig. 3B shows examples of areas of dysplasia in colons of an untreated and adjuvant treated mouse. We repeated the same analysis by focusing on colon tissue samples that had inflammation scores >1.0. Of 8 untreated mice in that category, six had dysplasia. The three adjuvant treated mice all had dysplasia with one displaying neoplasia as well. Two mice in the vaccinated group had inflammation scores >1.0 but neither of these colons had areas of dysplasia.
Figure 3.
Vaccination against MUC1 prevents the development of dysplasia and CACC. (A) Percentage of untreated (n=13) and adjuvant treated (n=9) mice with dysplasia at 16 weeks of age. Vaccinated mice (n=8) had no areas of dysplasia. *, p=0.046. P value was determined by two-tailed Fisher’s exact test. (B) Representative H&E stained colon sections showing dysplastic lesions. Scale bar, 50μm. (C) Percentage of untreated (n=16), adjuvant treated (n=6), or vaccinated (n=10) mice with tumors. *, p= 0.041. P value was determined by two-tailed Fisher’s exact test. (D) Representative colon section showing multiple tumors in the colon of an untreated mouse. Immunostaining (hematoxylin counterstained) for MUC1 of a representative colon tumor from an untreated mouse. Scale bar, 100μm.
The second analysis was performed on mice that had rectal prolapse for at least two weeks prior to sacrifice. Colons from such mice from all three groups were evaluated for the presence of neoplasia by a pathologist blinded to the experimental protocol. We found that vaccinated mice had statistically significantly fewer tumors compared to untreated mice (p=0.041) (Fig. 3C). Eight of 10 mice that received the vaccine were tumor free (80%), while only five of 16 mice in the untreated group (31%) and two of six mice (33%) in the adjuvant group remained free of tumors. In Figure 3d we show a macroscopic view of a representative colon bearing multiple tumors from an untreated mouse. Colons in tumor bearing mice were shortened in length and multiple tumors were macroscopically visible. These tumors expressed high levels of MUC1 and the majority was the abnormal form (representative sections showed in Fig. 3D).
MUC1 vaccination changes the inflammatory microenvironment
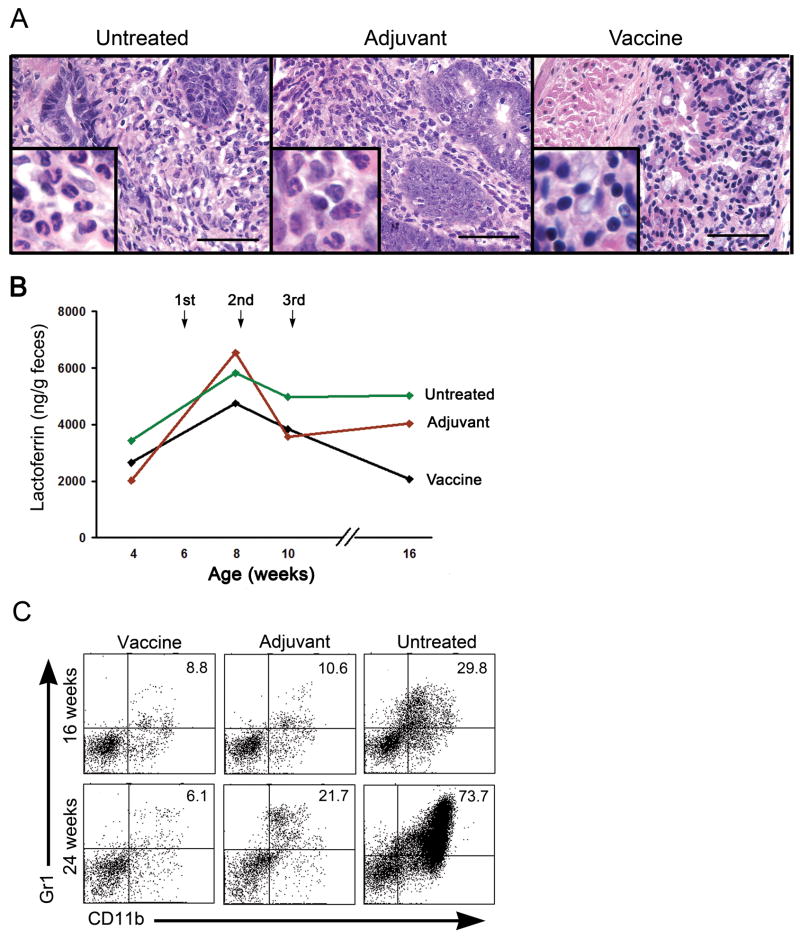
Neutrophils are a prominent cellular component in chronic inflammation and have been shown to play a significant role in establishing a tumor-promoting microenvironment through the production of cytokines and reactive oxygen metabolites (ROM) (29, 30). Representative tissue sections in Figure 4A show a predominant granulocytic infiltrate, including neutrophils in the dysplastic lesions of untreated and adjuvant treated mice at 16 weeks of age. In contrast, smaller mononuclear cells are the predominant population in the vaccinated mice. We postulated that the anti-tumor effects we observed in vaccinated mice reflected differences in the IBD cellular infiltrate and that measuring neutrophil infiltration over time might provide a marker of that difference. We thus followed the kinetics and the extent of neutrophil infiltration using iron-binding protein lactoferin-specific ELISA (31). Lactoferrin is stored in the secondary granules in neutrophils and secreted upon their activation. Fecal samples were collected from individual mice at 4–5 weeks of age, early in disease and prior to any treatment, and then at 8, 10, and 16 weeks of age, later in the disease process for untreated mice and post treatment for the adjuvant treated and vaccinated mice. Prior to treatment, mice had an average of 2600 ng of lactoferrin per gram of feces. In all three groups there was an increase in lactoferrin levels between 4 and 8 weeks (Fig. 4B), followed by a decrease between 8 and 10 weeks. However, between the 10 and the 16-week time points lactoferrin levels remained high or increased in mice that were untreated or received adjuvant alone but decreased in the vaccinated group. This decrease followed the third vaccine treatment and corresponds to the plateau in the development of rectal prolapse in the vaccinated group shown earlier in Figure 1A.
Figure 4.
Vaccination induces changes in the local and systemic microenvironments of MUC1+/IL-10−/− mice with IBD. (A) Representative H&E sections of colons from an untreated, an adjuvant treated, and a vaccinated MUC1+/IL-10−/− mouse. Scale bar, 100 μm. Inserts are oil immersion magnification ×1000 and show infiltration of neutrophils (lobular shaped nuclei and copious cytoplasm) in the colons of untreated and adjuvant treated mice and infiltration of lymphocytes (small round nuclei and little cytoplasm) in colons of vaccinated mice. (B) Neutrophil-produced lactoferrin levels in the feces collected at 4, 8, 10, and 16 weeks of age in vaccinated (n=7), adjuvant treated (n=6), and untreated (n=5) MUC1+/IL-10−/− mice, as measured by ELISA. (C) MDSC (Gr1+CD11b+) in spleens of indicated experimental groups. Representative dot plots of several independent experiments.
In addition to differences in the local microenvironment, we were interested in potential systemic differences that may account for different disease outcomes between the groups. Myeloid derived suppressor cells (MDSC) represent another cell population that is associated with chronic inflammation and a tumor-promoting microenvironment. MDSC are a heterogeneous population (32) characterized by the coexpression of Gr-1 and CD11b on the cell surface. MDSC have been shown to facilitate tumor development by exerting suppressor functions on an adaptive immune response (33, 34). These cells accumulate and or expand in the secondary lymphoid organs of mice developing IBD (35). Single-cell suspensions were prepared from the spleens of vaccinated, adjuvant only, and untreated mice at 16 and 24 weeks of age. At 16 weeks of age, the untreated mice had over 3-fold higher percentages of MDSC in the spleen compared to vaccinated mice and adjuvant treated mice (Fig. 4C). At 24 weeks, however, there was a 3.5-fold increase in spleen MDSC in adjuvant treated mice compared to vaccinated mice and a 12-fold increase in untreated controls.
DISCUSSION
Using IBD and CACC as a paradigm for chronic inflammation-associated carcinogenesis, and MUC1+/IL-10−/− mice, a new mouse model that spontaneously develops these diseases, we show that inflammation can be controlled and cancer prevented by strengthening adaptive immunity against an antigen that is known to be present at the disease site (the IBD affected colon) and on the future malignancy (CACC). We and others have previously shown that abnormal MUC1 expression is not limited to fully transformed cells, but that it can also be seen in chronic inflammation (8, 36–38). Thus in addition to being a tumor associated antigen, abnormal MUC1 is also an IBD associated antigen. We also previously reported that in the well-established IL10−/− mouse model of IBD, addition of MUC1 expression had an enhancing effect on IBD development, degree of inflammation and progression to CACC (8). This demonstrated that abnormal MUC1 expression was not only a marker, but also an active participant in the disease that could perpetuate chronic inflammation and drive cancer development at the disease site. In the current study, we used MUC1 as the IBD- associated antigen and a MUC1 vaccine to elicit and deliver adaptive immune response (anti-MUC1) to the site of chronic inflammation (e.g. IBD), otherwise characterized primarily by the presence of innate immune effectors. By changing the balance of innate and adaptive immunity at the disease site, we expected to change the nature of ongoing inflammation and alter the course of IBD as well as its cancer promoting potential.
We clearly show that vaccinated mice have a very different outcome than the controls in the course of their IBD, showing lesser inflammation and longer time to clinical disease. Furthermore, the controls progress to dysplasia and cancer and the vaccinated mice do not. Our data suggest that this may be the result of two different mechanisms, one a direct effector mechanism mediated by MUC1-specific adaptive immunity (anti-MUC1 IgG, MUC1-specific CTL) that appears to eliminate abnormal cells marked by abnormal expression of MUC1, and the other an indirect mechanism resulting from the changes in the local and systemic microenvironments. Locally, the pro-tumor environment of the inflamed colon, characterized by an ever-increasing neutrophil infiltration, appears to be replaced in vaccinated mice by an environment where neutrophils decrease and mononuclear cells dominate. Similarly, the systemic pro-tumor environment in the mouse with IBD, characterized by an ever-increasing number of MDSC in the spleen, is replaced in vaccinated mice by an environment lacking these cells.
Abnormal expression of normal cellular molecules can come about through many different events that affect normal cell functions, e.g. viral and bacterial infections, acute and chronic inflammations and malignant transformation (39). We have previously published that some events affecting epithelial cells, such as mastitis affecting the MUC1+ breast duct (36) or mumps infection of the MUC1+ salivary gland (unpublished) could lead to abnormal expression of MUC1 and an immune response against it. This immunity furthermore correlated to a reduced lifetime risk of MUC1+ ovarian cancer (40). The results we report here suggest that this naturally acquired anti-MUC1 immunity might also be protective of MUC1+ IBD or its progression.
In view of the accumulating evidence that cancer can result from a chronic inflammatory disorder, we can consider early stages of chronic inflammation as a premalignant condition. Many molecules that are abnormally expressed in chronically inflamed tissues and the malignancies that arise from those tissues may be playing a significant role in both diseases and thus can be effectively used as targets for immunotherapy of chronic inflammation and prevention of cancer. In particular we show that during the early stages of chronic inflammation, it might be feasible to change the maladaptive tumor-promoting microenvironment to one that is tumor-inhibiting by vaccinating to induce adaptive immune responses specific for one or more disease-associated molecules.
Acknowledgments
Financial Support: This study was funded in part by Cancer Prevention Foundation (P.L.B.), and RO1 56103 (O.J.F.).
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Sato Y, Takahashi S, Kinouchi Y, et al. IL-10 deficiency leads to somatic mutations in a model of IBD. Carcinogenesis. 2006;27:1068–73. doi: 10.1093/carcin/bgi327. [DOI] [PubMed] [Google Scholar]
- 4.Borrello MG, Alberti L, Fischer A, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102:14825–30. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–8. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 7.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: transgenic expression of human MUC1 in IL-10−/− mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179:735–9. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 9.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 10.Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41:189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 11.Croce MV, Isla-Larrain M, Rabassa ME, et al. Lewis x is highly expressed in normal tissues: a comparative immunohistochemical study and literature revision. Pathol Oncol Res. 2007;13:130–8. doi: 10.1007/BF02893488. [DOI] [PubMed] [Google Scholar]
- 12.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–95. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 13.Ajioka Y, Allison LJ, Jass JR. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J Clin Pathol. 1996;49:560–4. doi: 10.1136/jcp.49.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furr AE, Ranganathan S, Finn OJ. Aberrant Expression of MUC1 Mucin in Pediatric Inflammatory Bowel Disease. Pediatr Dev Pathol. 2008:1. doi: 10.2350/08-06-0479.1. [DOI] [PubMed] [Google Scholar]
- 15.Ho SB, Ewing SL, Montgomery CK, Kim YS. Altered mucin core peptide immunoreactivity in the colon polyp-carcinoma sequence. Oncol Res. 1996;8:53–61. [PubMed] [Google Scholar]
- 16.Ryan SO, Vlad AM, Islam K, Gariepy J, Finn OJ. Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice. Biol Chem. 2009 doi: 10.1515/BC.2009.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanisch FG, Ninkovic T. Immunology of O-glycosylated proteins: approaches to the design of a MUC1 glycopeptide-based tumor vaccine. Curr Protein Pept Sci. 2006;7:307–15. doi: 10.2174/138920306778018034. [DOI] [PubMed] [Google Scholar]
- 18.Vlad AM, Muller S, Cudic M, et al. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–46. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aarnoudse CA, Garcia Vallejo JJ, Saeland E, van Kooyk Y. Recognition of tumor glycans by antigen-presenting cells. Curr Opin Immunol. 2006;18:105–11. doi: 10.1016/j.coi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Carlos CA, Dong HF, Howard OM, Oppenheim JJ, Hanisch FG, Finn OJ. Human tumor antigen MUC1 is chemotactic for immature dendritic cells and elicits maturation but does not promote Th1 type immunity. J Immunol. 2005;175:1628–35. doi: 10.4049/jimmunol.175.3.1628. [DOI] [PubMed] [Google Scholar]
- 21.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 22.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–21. [PubMed] [Google Scholar]
- 23.Ishizaka ST, Hawkins LD. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev Vaccines. 2007;6:773–84. doi: 10.1586/14760584.6.5.773. [DOI] [PubMed] [Google Scholar]
- 24.Przetak M, Chow J, Cheng H, Rose J, Hawkins LD, Ishizaka ST. Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine. 2003;21:961–70. doi: 10.1016/s0264-410x(02)00737-5. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins LD, Ishizaka ST, McGuinness P, et al. A novel class of endotoxin receptor agonists with simplified structure, toll-like receptor 4-dependent immunostimulatory action, and adjuvant activity. J Pharmacol Exp Ther. 2002;300:655–61. doi: 10.1124/jpet.300.2.655. [DOI] [PubMed] [Google Scholar]
- 26.Schneck JP, Slansky JE, O’Herrin SM, Greten TF. Monitoring antigen-specific T cells using MHC-Ig dimers. Curr Protoc Immunol. 2001;Chapter 17(Unit 17):2. doi: 10.1002/0471142735.im1702s35. [DOI] [PubMed] [Google Scholar]
- 27.Apostolopoulos V, Yuriev E, Ramsland PA, et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc Natl Acad Sci U S A. 2003;100:15029–34. doi: 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisarev VM, Kinarsky L, Caffrey T, et al. T cells recognize PD(N/T)R motif common in a variable number of tandem repeat and degenerate repeat sequences of MUC1. Int Immunopharmacol. 2005;5:315–30. doi: 10.1016/j.intimp.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Gungor N, Godschalk RW, Pachen DM, Van Schooten FJ, Knaapen AM. Activated neutrophils inhibit nucleotide excision repair in human pulmonary epithelial cells: role of myeloperoxidase. FASEB J. 2007;21:2359–67. doi: 10.1096/fj.07-8163com. [DOI] [PubMed] [Google Scholar]
- 30.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 31.Logsdon LK, Mecsas J. A non-invasive quantitative assay to measure murine intestinal inflammation using the neutrophil marker lactoferrin. J Immunol Methods. 2006;313:183–90. doi: 10.1016/j.jim.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Bronte V, Chen SH, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 34.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haile LA, von Wasielewski R, Gamrekelashvili J, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–81. 81, e1–5. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 36.Jerome KR, Kirk AD, Pecher G, Ferguson WW, Finn OJ. A survivor of breast cancer with immunity to MUC-1 mucin, and lactational mastitis. Cancer Immunol Immunother. 1997;43:355–60. doi: 10.1007/s002620050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longman RJ, Poulsom R, Corfield AP, Warren BF, Wright NA, Thomas MG. Alterations in the composition of the supramucosal defense barrier in relation to disease severity of ulcerative colitis. J Histochem Cytochem. 2006;54:1335–48. doi: 10.1369/jhc.5A6904.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Finn OJ. Immunological weapons acquired early in life win battles with cancer late in life. J Immunol. 2008;181:1589–92. doi: 10.4049/jimmunol.181.3.1589. [DOI] [PubMed] [Google Scholar]
- 40.Cramer DW, Titus-Ernstoff L, McKolanis JR, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–31. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 41.Brokx RD, Revers L, Zhang Q, et al. Nuclear magnetic resonance-based dissection of a glycosyltransferase specificity for the mucin MUC1 tandem repeat. Biochemistry. 2003;42:13817–25. doi: 10.1021/bi0353070. [DOI] [PubMed] [Google Scholar]