Abstract
Background
Differences in atrial fibrillation (AF) cycle length (CL) between the left (LA) and right (RA) atrium and coronary sinus (CS) may help separate paroxysmal from persistent AF and identify patients most likely to respond to pulmonary vein isolation, though cannot be measured non-invasively.
Methods and Results
We developed methods to estimate regional intra-atrial AF CL from the surface electrocardiogram (ECG) in 30 patients with persistent and 10 patients with paroxysmal AF prior to ablation. Intra-atrial AF CL was measured near the LA appendage, mid-CS and lateral RA. In simultaneous filtered ECG AF CL was estimated using autocorrelation. The mean of ECG-derived AF CL in leads V5, I and aVL was used to estimate LA CL, leads aVF, II and III for CS CL, and V1, V2 and aVR for RA CL. ECG CL estimates for the LA, CS, and RA had R2>0.91 versus measured CL (all p<0.001). Though magnitudes of left-vs.-right AF CL gradients were small in this series, the ECG predicted the direction of gradients in 62% of measurements (p<0.05). When the gradient was >10 ms, the direction was accurately predicted in 8 of 11 patients. The accuracy of AF CL estimates was not adversely affected by AF type or LA dilatation (≤40 or > 40 mm). The ECG estimated AF-CL showed high 5 min temporal stability (p<0.001 each chamber).
Conclusions
Left and right atrial AF CL, and their gradients, can be accurately determined from the ECG using autocorrelation analysis. This approach may be a helpful guide prior to ablation procedures.
Keywords: atrial fibrillation, electrocardiogram, signal processing, spectral analysis, atrial fibrillation ablation
Introduction
Recent intracardiac mapping observations suggest that atrial fibrillation (AF) is supported by several mechanisms. In particular, a shorter AF cycle length (CL) in the left compared with the right atrium identifies patients with paroxysmal AF,1 and those with persistent AF most likely to respond to pulmonary vein isolation,2 while lengthening of the AF CL in the coronary sinus (CS) predicts favorable post-ablation outcomes.3 AF CL also lengthens preceding pharmacologic cardioversion to sinus rhythm.4 However, bedside electrocardiogram (ECG) indices that measure regional intracardiac AF CL have not been developed.
Previous studies have used ECG lead V1 to estimate the right atrial AF CL, following spectral dominant frequency analysis on QRS subtracted ECG.4 However, high-resolution atrial mapping suggests that this method, applied to different ECG leads, does not estimate LA AF CL.5 It is unclear if this results from technical limitations, a posterior displacement of an enlarged LA away from precordial leads, or other factors. Conversely, we have recently demonstrated that autocorrelation may increase the accuracy of spectral estimates of AF CL when applied to the ECG6 or intracardiac signals.7
We hypothesized that the application of autocorrelation to multiple vectorially-selected ECG leads to minimize the impact of anatomic variability, would estimate the regional AF CL non-invasively. We tested our hypothesis in patients with paroxysmal and persistent AF prior to ablation procedures.
Methods
Patient Recruitment
We studied 30 consecutive patients with paroxysmal (n=10) or persistent (n=20) AF referred for catheter ablation at the San Diego Veterans Affairs Medical Center. The study protocol was approved by the joint Institutional Review Board of the Veterans Affairs and University of California Medical Center, San Diego, and all patients provided written informed consent to participate.
Electrophysiologic Recordings
The patients underwent electrophysiologic studies in the post-absorptive state. Anti-arrhythmic medications were discontinued for at least 5 half-lives, and amiodarone was withheld for 30 days. All patients were anticoagulated or had a normal trans-esophageal echocardiogram.
Using femoral venous access, a duo-decapolar catheter was placed along the lateral right atrium and in the CS. Left atrial (LA) recordings were made near the ostium of the LA appendage, using a 64-pole basket (Constellation, Boston Scientific, Natick, MA) advanced via transseptal puncture through an SL1 sheath to the left atrium. The 12-lead surface ECG was recorded simultaneously. During AF, electrograms were recorded for 5 min on a physiologic recorder (Bard, Billerica, MA), filtered at 0.05 – 100 Hz (ECG) and 30-500 Hz (intracardiac) and digitized at 1 kHz. Data was exported at 16-bit resolution to custom software written in Labview (National Instruments, Austin, TX). The research protocol was completed prior to the ablation procedure.
ECG Estimates of Fibrillatory Cycle Length
AF CL was estimated from each ECG lead, using autocorrelation, as previously described.6, 7
Vectorial ECG Analysis
We defined regional ECG lead groups to represent the intra-atrial AF CL. We reasoned, a priori, that leads I, V5 and aVL reflect LA activity, and used the mean of their autocorrelation derived CL to estimate the LA AF CL. We similarly reasoned that leads II, III and aVF reflect the inferior atrial surface, i.e. CS activity, and used their mean for comparison. Finally, we used the mean of leads V1, V2, and aVR to estimate the RA AF CL.
Intracardiac Fibrillatory cycle length
Intracardiac AF CL was measured manually. We counted 20 consecutive cycles (over 4-5 Sec) and excluded continuous fractionated electrograms and intervals ≤70 ms.7, 8 Measurements were repeated by 2 investigators for a subset of 25 intervals, with an interobserver correlation outcome of R2 = 0.99, slope = 0.99 and intercept (error) of 1.4 ms.
We measured lateral LA AF CL using basket electrodes near the LA appendage. For CS measurements, the mid-CS bipole was selected, and for RA measurements we used the high right atrial electrodes. Electrode positions were verified fluoroscopically referenced to nonfluoroscopic clinical mapping (NavX, St Jude Medical, Sylmar, California).
Statistical Analysis
Continuous data, presented as means ± standard deviation, were compared using the two-tailed t-test. Comparisons of ECG estimates to intra-atrial regional AF CL, and interobserver measurements, were quantified using the Pearson correlation coefficient. The predictive value of the AF CL gradient was examined using the chi-square test. Significance was assessed at a two-tailed alpha level of 0.05.
Results
Baseline characteristics of the patient population are summarized in table I.
Table 1.
Baseline Clinical Characteristics of study subgroups and overall population
| Paroxysmal AF (n = 10) | Persistent AF (n=20) | All Patients (n=30) | |
|---|---|---|---|
| Age, y | 61 ± 8 | 61 ± 9 | 61 ± 9 |
| Men/women | 9/1 | 20/0 | 29/1 |
| Left Atrial Diameter, mm | 41 ± 5† | 48 ± 5 | 45 ± 6 |
| Left ventricular ejection Fraction, % | 58 ± 8 | 54 ± 12 | 55 ± 10 |
| Coronary artery disease, n | 3 | 6 | 9 |
| Hypertension, n | 7 | 12 | 19 |
| Diabetes, n |
5 |
4 |
9 |
| Medication Use, % | |||
| Beta-adrenergic blocker | 50 | 75 | 67 |
| ACE inhibitor/ARB | 60 | 65 | 63 |
| Calcium-channel blocker | 40 | 35 | 37 |
| Digoxin | 40 | 35 | 37 |
| Class I anti-arrhythmic drugs | 10 | 15 | 13 |
| Amiodarone |
10 |
10 |
10 |
| Left Atrial Cycle Length, ms | |||
| Intra-Atrial | 206 ± 41† | 162 ± 16 | 176 ± 34 |
| Surface elecrtrocardiogram | 216 ± 44 | 162 + 19 | 180 ± 38 |
| Coronary Sinus Cycle Length, ms | |||
| Intra-Atrial | 207 ± 43† | 160 ± 14 | 177 ± 35 |
| Surface ECG | 214 ± 37 | 161 + 15 | 180 ± 36 |
| Right Atrium Cycle Length, ms | |||
| Intra-Atrial | 206 ± 42* | 170 ± 19 | 183 ± 33 |
| Surface ECG | 209 ± 43 | 164 + 21 | 179 ± 37 |
Unless specified otherwise, values are means ± SD
ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker
p<0.005
p<0.02 vs. persistent AF
Regional Intra-Atrial AF Cycle Length
By manual counts, the intra-atrial AF CL was 176 ± 34 ms in the lateral LA, 177 ± 35 ms in the CS, and 183 ± 33 ms in the lateral RA. The CL was shorter in patients with persistent than in patients with paroxysmal AF in the lateral LA (162 ± 16 versus 206 ± 41 ms, p<0.001), CS (160 ± 14 versus 207 ± 43 ms, p<0.001) and lateral RA (170 ± 19 versus 206 ± 42 ms, p<0.02). These results are summarized in table I.
Regional AF Cycle Length Estimates from the Surface ECG
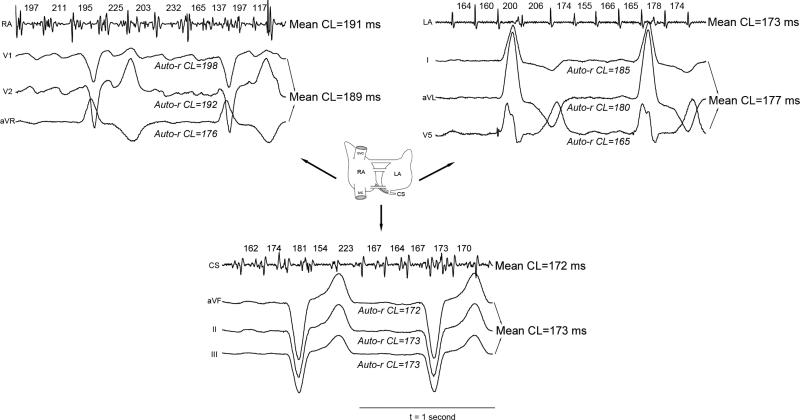
Our ECG method provided accurate estimates of regional intra-atrial AF CL. This is illustrated in figure 1, in a patient with a left-to-right atrial rate gradient (i.e. right atrial AF CL – LA AF CL = 18 ms). Referenced to the gold standard intracardiac CL the left-ECG leads (X-vector) differed by 4 ms (177 vs. 173 ms), inferior-ECG (Y-vector) leads by 1 ms (173 ms vs. 172 ms), and the right-ECG leads (Z-vector) CL by 2 ms (189 ms vs. 191 ms).
Figure 1. Accurate ECG AF CL Estimates in a Patient with Left-to-Right AF Rate Gradient.
Intracardiac electrograms and measured AF CL are shown for the LA, CS and RA. Corresponding ECG lead-sets are shown (i.e. V5, I and aVL for the LA; aVF, II and III to represent the CS; V1, V2 and aVR for the RA) with mean regional CL estimates. ECG estimates differ from the measured intra-atrial AF CL by 1-4 ms.
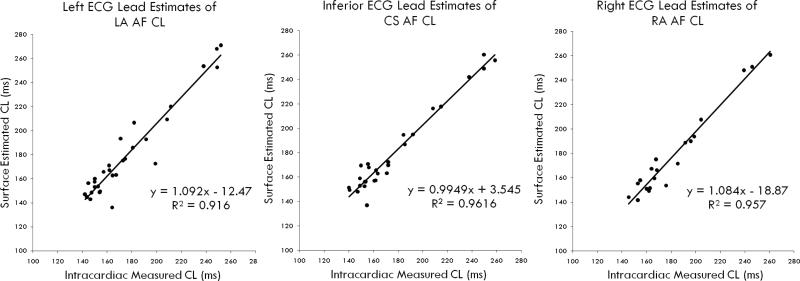
For the entire population, the ECG estimates closely approximated the instantaneous AF CL in LA (figure 2: slope 1.10, intercept −12.5 ms, p<0.001), CS (figure 2: slope = 0.99, intercept = 3.5 ms, p<0.001) and RA (figure 2: slope = 1.08, intercept = −18.9 ms, p<0.001).
Figure 2.
Correlation Between ECG Estimates and Measured Intracardiac AF CL for (A) Left Atrium; (B) Coronary sinus; and (C) Right atrium.
Relationship between ECG Regional AF Cycle Length Estimates and Type of AF and Left Atrial Diameter
There was no difference in the accuracy of ECG CL estimates for patients with paroxysmal AF (all chamber error 8 ± 4 ms) or persistent AF (all chamber error 7 ± 6 ms; p=0.76). Regional ECG AF CL estimates were similarly accurate, whether the LA diameter was ≤40 mm (all chamber error 5±3 ms) or > 40 mm (8±6 ms, all chambers; p=0.32).
Vectorial ECG Analysis Reflects the AF Rate Gradients
ECG AF CL indices predicted the polarity (i.e. left-to-right, or right-to-left) of CL gradients 62% of the time (p <0.05). When the gradient was >10 ms, the direction was correctly predicted in 8 of 11 patients (73%). However, due to the small magnitude of the intra-atrial gradients in the series, ECG estimates were overall less successful at identifying the magnitude of these gradients (measured left-to-right gradient: 7.3 ± 11 ms, ECG estimate: −0.1 ± 8 ms).
Temporal Variability
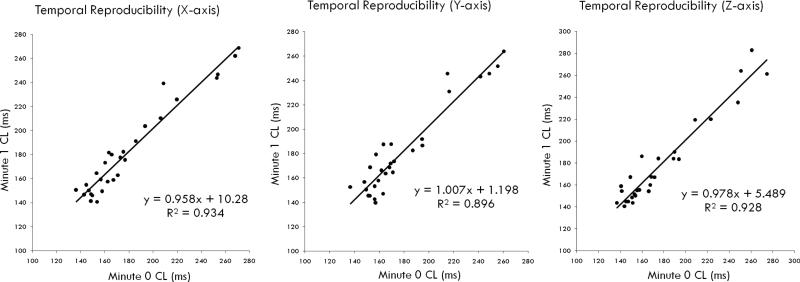
The ECG estimates of AF CL were highly stable temporally, and correlated very closely among periods separated by 1 min (correlation coefficients, r = 0.97, 0.95, and 0.96, for LA, CS and RA estimates, respectively, n=29) and 5 min (r = 0.98, 0.98, and 0.99, n=15, figure 3).
Figure 3. Temporal Reproducibility of ECG AF CL Estimates.
Regional estimates at time zero agreed very closely to the estimates at 1 and 5 min (not shown) for LA, CS and RA.
Discussion
This study demonstrates that the ECG can be used to precisely estimate regional CL, and therefore instantaneous rate-gradients during AF. Our time-domain autocorrelation, applied to a priori vectorially-selected ECG leads, accurately estimated simultaneous intracardiac LA, RA and CS AF CL. In contrast, prior studies of the spectral dominant frequency of QRS-subtracted ECG leads estimated accurately the right atrial AF CL only. Moreover, our studies suggest that the regional AF CL were temporally stable on the short term. Further studies should examine whether the outcome of ablation procedures is improved when guided by measures such as AF CL gradients instead of the clinical classification of “paroxysmal” versus “persistent” AF.
Significance of Intra-Atrial AF Cycle Length Gradients
Numerous studies in animals9 and humans10 suggest that AF may be perpetuated by rapid sources, resulting in a gradient from this ‘driver’ (short CL) to other sites (longer CL). Using simultaneous bi-atrial mapping, Lazar et al. showed a left-to-right AF rate gradient (i.e. shorter CL in LA than RA) in patients with paroxysmal AF, consistent with a LA or PV driver, but not in patients with persistent AF who are more resistant to PV isolation.1 Although Sanders et al.11 subsequently showed a similar gradient in persistent AF, these authors used sequential point-by-point rather than simultaneous mapping, and therefore did not analyze instantaneous gradients.
Comparison of Methods to assess AF Cycle length
To the best of our knowledge, there are no prior reports using the ECG to estimate LA, CS and RA CL during AF. Although prior studies have reported ECG indices of RA AF CL, AF is typically driven from the LA, such that RA CL alone is likely to be less useful in monitoring therapy and cannot be used to estimate regional CL gradients. Most prior ECG studies began by subtracting an averaged QRS waveform to reveal f-waves, and then analyzed the CL in the frequency domain.4, 12 It is unclear why ECG spectral analyses of LA and CS AF CL have not been reported. Perhaps because known pitfalls13 like sensitivity to noise interference, instability of the signal over time, variable results when the same CL intervals are presented in variable orders, and potential overestimation of rate by double counting.7 In addition, QRS subtraction may leave a residual signal, and may eliminate signal components if transiently synchronized to the QRS (e.g. atrial flutter). These effects may be exaggerated if the QRS duration is prolonged. Autocorrelation analysis, on the other hand, obviates the need for QRS subtraction and accurately estimates atrial CL in many arrhythmias, from the surface ECG6, 14 and intracardiac electrograms.15 Another strength of our method is that averaging vectorially-selected leads corrects in part for varying lead placement in relation to anatomic variability.
Clinical Implications
Non-invasive ECG monitoring of regional AF CL has several potential applications. A CL difference between LA and RA may indicate a favorable response to PV isolation alone, as suggested by intracardiac analysis,2 although this hypothesis should be tested prospectively. As a corollary, the absence of such a gradient may indicate that more extensive ablation will be necessary to eliminate AF. Another testable hypothesis is whether a lengthening of the AF CL by pharmacologic therapy, in patients with or without prior ablation, predicts maintenance of sinus rhythm.
Limitations
The main limitation of this study is our small population, in which few patients exhibited significant left-vs.-right atrial CL gradients. A larger population will enable testing of the hypothesis that patients in whom ECG AF CL is longer in the RA than LA may benefit from PV isolation alone without more extensive ablation. Physiologically, the validity of ‘mean’ intraatrial AF CL is unclear given the AF CL irregularity, although we did confirm its temporal reproducibility over minutes. Finally, our population showed a male predominance, reflecting the Veterans Affairs population. Although no clear gender differences have been reported in AF CL, future studies should include larger populations of both genders.
Conclusions
Autocorrelation analysis of vectorially-selected ECG leads accurately reflects regional LA, CS, and RA cycle lengths during AF, and thus instantaneous rate gradients. This was confirmed in patients with persistent and paroxysmal AF, with or without LA dilatation, and was reproducible.
Acknowledgements
We are grateful to Kathleen Mills, BA for assisting in this study.
Footnotes
Disclosures:
Mr. Ravi, Ms. Tran, and Dr. Bullinga have no disclosures. Dr. Krummen has received speaking honoraria from Medtronic Corporation and has served as a consultant for Boston Scientific. Dr. Narayan is a member of the speakers’ bureau for St. Jude Medical, Boston Scientific and Medtronic Corporations.
References
- 1.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004;110:3181–6. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 2.Lazar S, Dixit S, Callans DJ, Lin D, Marchlinski FE, Gerstenfeld EP. Effect of pulmonary vein isolation on the left-to-right atrial dominant frequency gradient in human atrial fibrillation. Heart Rhythm. 2006;3:889–95. doi: 10.1016/j.hrthm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavee C, Takahashi Y, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–13. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 4.Bollmann A, Kanuru NK, McTeague KK, Walter PF, DeLurgio DB, Langberg JJ. Frequency analysis of human atrial fibrillation using the surface electrocardiogram and its response to ibutilide. Am J Cardiol. 1998;81:1439–45. doi: 10.1016/s0002-9149(98)00210-0. [DOI] [PubMed] [Google Scholar]
- 5.Lin YJ, Tai CT, Kao T, Tso HW, Higa S, Tsao HM, Chang SL, et al. Frequency analysis in different types of paroxysmal atrial fibrillation. J Am Coll Cardiol. 2006;47:1401–7. doi: 10.1016/j.jacc.2005.10.071. [DOI] [PubMed] [Google Scholar]
- 6.Brown JP, Krummen DE, Feld GK, Narayan SM. Using electrocardiographic activation time and diastolic intervals to separate focal from macro-re-entrant atrial tachycardias. J Am Coll Cardiol. 2007;49:1965–73. doi: 10.1016/j.jacc.2006.10.080. [DOI] [PubMed] [Google Scholar]
- 7.Narayan SM, Krummen DE, Kahn AM, Karasik PL, Franz MR. Evaluating fluctuations in human atrial fibrillatory cycle length using monophasic action potentials. Pacing Clin Electrophysiol. 2006;29:1209–18. doi: 10.1111/j.1540-8159.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 8.Nademanee K. Is pulmonary vein isolation by segmental ostial ablation a correct approach for treatment of atrial fibrillation? Heart Rhythm. 2006;3:1029–30. doi: 10.1016/j.hrthm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Waldo AL. The interrelationship between atrial fibrillation and atrial flutter. Prog Cardiovasc Dis. 2005;48:41–56. doi: 10.1016/j.pcad.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation. 2004;110:3293–9. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 11.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 12.Xi Q, Sahakian AV, Ng J, Swiryn S. Atrial fibrillatory wave characteristics on surface electrogram: ECG to ECG repeatability over twenty-four hours in clinically stable patients. J Cardiovasc Electrophysiol. 2004;15:911–7. doi: 10.1046/j.1540-8167.2004.03577.x. [DOI] [PubMed] [Google Scholar]
- 13.Ng J, Kadish AH, Goldberger JJ. Effect of electrogram characteristics on the relationship of dominant frequency to atrial activation rate in atrial fibrillation. Heart Rhythm. 2006;3:1295–305. doi: 10.1016/j.hrthm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Hoppe BL, Kahn AM, Feld GK, Hassankhani A, Narayan SM. Separating atrial flutter from atrial fibrillation with apparent electrocardiographic organization using dominant and narrow F-wave spectra. J Am Coll Cardiol. 2005;46:2079–87. doi: 10.1016/j.jacc.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, Paterson DJ, et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;114:536–42. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]