Abstract
Apolipoprotein E4 (apoE4) and female sex are risk factors for developing Alzheimer's disease. It is unclear whether apoE4 contributes to behavioral function at younger ages. Standard neuropsychological assessments (IQ, attention, executive function) and a test developed in this laboratory (Memory Island test of spatial learning and memory) were used to determine whether E4 and sex affect neuropsychological performance in healthy primary school children (age 7-10). A medical history was also obtained from the mother to determine if negative birth outcomes were associated with apoE4. Mothers of apoE4+ children were more likely to report that their newborn was placed in an Intensive Care Unit. A sex difference in birth weight was noted among apoE4- (males > females), but not apoE4+, offspring. Conversely, among apoE4+, but not apoE4- children, there was a sex difference in the Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary score favoring boys. ApoE4- girls had better visual recall than apoE4+ girls or apoE4- boys on the Family Pictures test. Finally, apoE4+, unlike ApoE4-, children did not show spatial memory retention during the Memory Island probe trial. Thus, apoE4 may affect neurobehavioral performance, particularly spatial memory, as well as antenatal health, decades before any clinical expression of neurodegenerative processes.
Apolipoprotein E (apoE), a lipid transport protein implicated in artherosclerosis and neurodegeneration (1,2), is widely distributed throughout the brain (3-5). ApoE is important for neuron migration, axon guidance, microtubule stability, dendritic spine density, synaptic plasticity, and regeneration following injury (1). The three major human apoE isoforms, apoE2, apoE3, and apoE4, differ in binding affinity to members of the low density lipoprotein family of receptors (6). ApoE4-carrying individuals have a shorter lifespan and age less successfully (7,8). ApoE4 has been associated with an earlier onset of Alzheimer's disease (AD) (9,10) and interacts with female sex to increase AD risk (11). In addition to AD, apoE4 has been associated with age-related cognitive decline in the absence of dementia (12-14). A fundamental issue is whether apoE4 alters the neurobiology of the brain in ways that only become more evident during aging or following environmental challenges later in life.
The neurobehavioral consequences of apoE4 depend on the cognitive domain measured and at what age it is assessed (14-17). The apoE4 allele was more common among Czech university graduates relative to those that did not complete secondary school (18). Similarly, apoE4-carrying young adults showed better recall of a list of words (19). However, apoE4 did not modify California Achievement Test performance among adolescents (20) and apoE4-carrying high-school students with a family history of AD did exhibit reduced performance on the reading subtest of the California Achievement Test and in visual-spatial memory (21). Relatively little is known about the potential effects of apoE4 on cognition in children. There were no effects of apoE4 on verbal and non-verbal reasoning in eleven year olds but there were effects of apoE4 on this measure when the same study participants were retested as nondemented octogenarians (22). Similarly, overall IQ was unaltered by apoE4 in children (23-25). However, Mental Development Index scores were higher among Mexican apoE4 carrying two-year olds (26). The primary objective of the present study was to examine whether apoE4 affects neuropsychological performance in 7 to 10 year old children. As prior research has implicated apoE in fetal health (27-29), it was also determined if apoE4 was associated with adverse birth outcomes. Based on an earlier investigation (13), we hypothesized that reduced spatial memory would be observed among children with at least one apoE4 allele.
Methods
Study Participants
Flyers were posted at Oregon Health & Science University (OHSU) to recruit healthy 7-10 year old boys and girls. This age range was selected because language skills are sufficiently developed to readily assess relatively complex functions and prepubescent children might be expected to show less evidence of sex differences than at older ages. Exclusion criteria were children with severe visual impairments, born more than 5 weeks premature, epilepsy, cerebral palsy, congenital abnormalities, severe brain trauma, or any other medical condition that could interfere with cognitive assessments. The parent completed an informed consent and a disclosure form so that the OHSU medical record database was examined for each child to verify the exclusion criteria. For study participation, there was a $50 Toys-R-Us® gift certificate. Saliva samples were collected at the beginning of the session using the Oragene self-collection methodology (DNA Genotek Inc., Ottawa, ON, Canada) and genotypes were determined at the General Clinical Research Center of OHSU as described (13). All procedures were approved by the Institutional Review Board of OHSU.
Behavioral Assessments
The children completed a session that averaged about 1.5 hours. The neurobehavioral assessments included several general domains (attention, intelligence, and executive function), spatial learning and memory, and instruments sensitive to effects of apoE4 in the elderly (13). The sequence of tests was: 1) Dot location (30); 2) Conner's Continuous Performance Test (31); 3) Memory Island spatial navigation (13,32); 4) Family Pictures (30); 5) Wechsler Abbreviated Scale of Intelligence (WASI): Vocabulary and Block Design; 6) Forward and Backward Spatial Span. A single assessor (S.F.A.), blinded to the genotypes, administered all tests to the children. In addition, the mother filled out a questionnaire to determine demographics and pregnancy outcomes (e.g. use of an Intensive Care Unit after birth, whether the birth occurred vaginally or by Cesarean), as well as the Behavior Rating Inventory of Executive Function (BRIEF) (32). Each of these assessments is described in further detail below.
The Dot Location test is a spatial memory assessment and a component of the Children's Memory Scale. Dot Location includes age appropriate difficulty levels for children aged 4-8 and 9-17 (30). The primary dependent measures were the learning, short-delay, long-delay, and total correct (expressed as age-corrected scaled scores). Further, the percent of the distracter items recalled was recorded.
The Conner's Continuous Performance Test is a 14 min computerized assessment of attention where respondents press the space bar whenever any letter except the target, an ‘X’, is displayed. The inter-stimulus intervals were 1, 2 and 4 seconds. The primary measures are omission and commission errors, hit reaction time standard error, detectability, and response style (31).
Memory Island is a human equivalent of the Morris water maze and has been used previously with healthy adults, the elderly, and children (13,32). This approach has also been validated in that hippocampal lesions disrupt spatial memory in a virtual reality paradigm (34). The children were first asked to navigate using a joystick to a target location visibly marked with a flag adjacent to the target (visible session). Unique targets in each of the four quadrants are used for visible target training in four trials. The starting orientation of the participant was varied for each trial, and these variations are kept consistent for all participants. After completing training to find the visible targets, the children were trained to navigate to a hidden target (i.e., no flag adjacent to the target) in four trials. The participants had to remember where the hidden target was and how to get there. The location of the hidden target, a sculpture, was constant for all children. In each trial of the visible or hidden session, if the subject was unable to locate the target within two minutes, a directional arrow appears to guide them to the target (Figure 1). Approximately fifteen minutes following the last hidden target trial, the participant received a 30 second probe trial with the target removed to assess spatial memory. In each trial, movement of the children was recorded in time-stamped coordinate files, which were used to calculate cumulative distance to the target and distance traveled (virtual units), velocity (virtual units/second), latency to reach the target, and percentage time spent in each quadrant.
Figure 1.
Screen shot of Memory Island during a visible trial. If the target (insert) is not reached in two-minutes, an arrow appears (shown).
The Family Pictures visual recognition test is part of the Child Memory Scale (30). Children were shown pictures of people in a particular scene and asked to remember everything they could about each scene (4 scenes total). Immediate recall was assessed by asking who was in the scene, where they were in the scene, based on a quadrant division of the scene, and a basic description of what they were doing in the scene (eating, gardening, etc.). After an interval of 30 min, participants were asked the same questions again. All intervals for multi-part tests are approximations due to the variable time demands of children during assessments. For the Immediate and Delayed scores, one point was given for correctly identifying who was in the picture, one point for the location, and two points for the correct description of their actions. This assessment was selected because it was previously shown to be sensitive to the effects of apoE4 in non-demented elderly (13).
The Wechsler Abbreviated Scale of Intelligence (WASI) is an abbreviated version of the Wechsler Intelligence Scale for Children (Third Edition, WISC-III) that provides subtest and composite scores representing intellectual functioning. The Vocabulary and Block Design components were completed to assess performance relative to normative data.
The Spatial Span provides a measure of visual-spatial working memory and is also a subtest of the WISC-III. The child watches an examiner tap a sequence of numbered cubes on the Spatial Span board (numbered side faces examiner) and then is asked to tap out the same sequence. The Spatial Span is discontinued if a subjects scores 0 on each of two trials of the same item. In the first test, the child must repeat the same order (Spatial Span Forward) and, in the second test, the order is reversed (Spatial Span Backward).
The Behavior Rating Inventory of Executive Function (BRIEF) is an 86-item parental questionnaire of executive functioning in the context of the child's everyday activities. Behaviors are rated as never, sometimes, or often a problem (1 to 3 points, respectively) and expressed as a T50 score (32).
Statistical analysis
All analyses were conducted using SPSS, version 16.0 (SPSS Inc., Chicago, IL). A p value of < .05 was considered statistically significant, although, because multiple tests were conducted, statistics that met more conservative thresholds (.01 or .001) were also noted. Likelihood ratios were reported to determine if apoE4 status (E4- versus E4+) was associated with nominal level outcomes. The age corrected (T50 or Scaled Scores) were used for all behavioral tests except where noted. As sex differences favoring males in spatial learning and memory have been observed previously in this research area (13,33), a 2 (ApoE: E4- versus E4+) by 2 (Sex: Boy versus Girl) ANOVA was completed for continuous neurobehavioral measures. For Memory Island, the visible, hidden, and probe trials were analyzed separately. The probe trial data was analyzed with a mixed (Trial: target, left, right) × ApoE × Sex ANOVA. Note that the opposite quadrant could not be included because inclusion of all four quadrants in the model simultaneously violates the ANOVA data requirements. Paired t-tests comparing the percent time in the target relative to each of the other quadrants were also conducted for each ApoE4 group. As prior research in this laboratory with children identified a pronounced improvement in spatial memory on Memory Island between ages nine and ten (unpublished data), age was also entered into the ANOVA model for the probe trial analyses. A post hoc power analysis for the comparison of two groups with alpha = .05 was conducted with G*Power 3.1.0 (35).
Results
ApoE Genotype
Among males (N=26), 19 were E3/E3, 2 were E2/E3, 1 was E4/E4, 2 were E3/E4, and 2 were E2/E4. Among females (N=24), 12 were E3/E3, 5 were E2/E3, 1 was E4/E4, 5 were E3/E4, and 1 was E2/E4.
Demographics and Birth History
There were no significant differences between apoE4- and apoE4+ children in terms of age at testing, sex, ethnicity, academic performance, or prenatal exposure to recreational drugs (Table 1). However, apoE4+ offspring were ten times more likely to be placed in an Intensive Care Unit after birth (Likelihood Ratio(1)=5.451, p<.05). A 2 (Sex) × 2 (ApoE4) ANOVA on birth weight revealed a trend for a main effect of Sex (F(1,47)=3.933, p=.053) and an ApoE4 × Sex interaction (p=.096). Apoe4+ boys (3012.1 ± 233.2) and girls (3,254.1 ± 166.5) were equivalent. In contrast, a pronounced sex difference was evident in the apoE4- group (Boys = 3,717.3 ±115.1, Girls = 3,038.6 ±140.5, t(38)=2.248, p<.05). In terms of other pregnancy complications, mothers of apoE4+ offspring were more likely to report cervical cerclage (i.e. a surgical procedure in which a weak cervix is sewn closed to prevent miscarriage, Table 2).
Table 1.
Child and maternal demographics by apoE4 and sex.
| ApoE4 | Sex | |||
|---|---|---|---|---|
| E4- | E4+ | Males | Females | |
| Child | ||||
| Sex (% Female) | 41.5% | 58.3% | NA | NA |
| Genotype (% E4+) | NA | NA | 17.2% | 29.2% |
| Race (% non-white) | 19.5% | 16.7% | 17.2% | 20.8% |
| Result of Cesarean Delivery | 8.1% | 27.3% | 14.8% | 9.5% |
| Birth weight (g) | 3,553.8 (93.6) | 3,166.1 (133.7)* | 3,616.5 (113.1) | 3,292.0 (108.2)* |
| Placed in ICU (% Yes) | 2.7% | 27.3** | 3.7% | 14.3% |
| Hospitalized <1 Day (%) | 24.3% | 0.0%** | 25.9% | 9.5% |
| ADHD | 4.9% | 0.0% | 6.9% | 0.0% |
| Reading (Below age) | 7.3% | 8.3 | 6.9% | 8.3% |
| Overall school (Below age) | 2.4% | 0.0% | 3.4% | 0.0% |
| Age at Testing (years) | 9.0 (0.2) | 9.0 (0.3) | 9.2 (0.2) | 8.9 (0.2) |
| Maternal | ||||
| Age at Pregnancy | 29.2 (1.0) | 31.2 (1.6) | 29.2 (1.0) | 30.2 (1.3) |
| PregnancyWeight Gain (kg) | 15.6 (1.2) | 14.2 (1.4) | 15.6 (1.3) | 14.9 (1.5) |
| Smoking in Pregnancy (%Yes) | 7.5% | 8.3% | 3.4% | 12.5% |
| Alcohol in Pregnancy (%Yes) | 12.2% | 16.7% | 10.3% | 17.4% |
t-test p<.05;
Likelihood ratio p < .05.
ApoE4 = apolipoprotein E, ICU = Intensive Care Unit, ADHD = Attention Deficit Hyperactivity Disorder, NA = Not Applicable.
Table 2.
Pregnancy complications by apoE4 and sex.
| ApoE4 | Sex | |||
|---|---|---|---|---|
| E4- | E4+ | Males | Females | |
| Cervical Cerclage | 0.0% | 16.7%* | 3.4% | 4.3% |
| Vaginal Bleeding | 17.5% | 25.0% | 24.1% | 13.0% |
| Urinary Tract Infection | 7.5% | 0.0% | 10.3% | 0.0% |
| High Blood Pressure | 5.0% | 8.3% | 6.9% | 4.3% |
| Placenta Previa | 0.0% | 8.3% | 0.0% | 4.3% |
| Premature Rupture of Membranes | 5.0% | 8.3% | 6.9% | 4.3% |
Likelihood ratio p < .05
Behavior
Performance on WASI Vocabulary (T50= 54.6 ±1.6) and Block Design (T50=57.7±1.7) for the entire sample was above average. ANOVA revealed a trend for an ApoE4 × Sex interaction (F(1,45)=4.013, p=.051) in the Vocabulary subtest (Table 3). ApoE4+ boys (T50=63.0±4.2) performed better than apoE4+ girls (49.0±2.4, t(10) = 3.11, p<.05). No sex difference was evident among the apoE4- group (boys = 54.0 ±3.1, girls =55.2 ±2.4). There were no differences on the Block Design, Conner's Continuous Performance, Spatial Span tests, or BRIEF.
Table 3.
Neuropsychological performance by apoE4 and sex.
| ApoE4 | Sex | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| E4- (N=38) | E4+ (N=12) | Males (N=26) | Females (N=24) | ApoE4 | Sex | ApoE4 × Sex | |
| WASI | |||||||
| Vocabulary | 54.5 (2.0) | 54.8 (3.0) | 53.4 (1.9) | 55.8 (2.7) | .097 | .051 | |
| Block Design | 56.5 (1.9) | 62.2 (3.5) | 57.1 (2.2) | 58.7 (2.6) | |||
| BRIEF | |||||||
| Global Executive Composite | 49.1 (1.5) | 47.8 (2.1) | 46.8 (1.8) | 51.0 (1.6) | .057 | ||
| Metacognition Index | 49.7 (1.6) | 48.4 (2.5) | 47.0 (1.8) | 52.0 (1.8) | .079 | ||
| Initiate | 50.5 (1.6) | 48.3 (2.2) | 47.9 (1.7) | 52.3 (1.9) | .061 | ||
| Working Memory | 49.6 (1.6) | 47.9 (2.5) | 48.5 (1.8) | 50.0 (2.1) | |||
| Planning | 49.8 (1.5) | 49.4 (2.9) | 48.3 (2.0) | 51.2 (1.8) | |||
| Organize | 51.8 (1.6) | 53.0 (2.3) | 49.0 (1.8) | 55.4 (1.8) | |||
| Monitor | 47.6 (1.9) | 45.0 (2.3) | 43.4 (2.0) | 50.7 (2.2) | <.05 | ||
| Behavioral Regulation Index | 47.8 (1.4) | 47.0 (2.4) | 46.3 (1.9) | 49.0 (1.6) | .071 | ||
| Inhibit | 47.7 (1.3) | 47.1 (1.7) | 46.1 (1.7) | 49.1 (1.3) | .083 | ||
| Shift | 48.8 (1.7) | 48.4 (2.5) | 48.3 (2.1) | 49.2 (1.9) | |||
| Emotional Control | 48.0 (1.5) | 46.6 (2.7) | 46.6 (2.0) | 48.8 (1.7) | |||
| Negativity | 0.2 (0.1) | 0.3 (0.2) | 0.2 (0.1) | 0.2 (0.1) | |||
| Inconsistency | 2.7 (0.2) | 2.5 (0.3) | 2.7 (0.3) | 2.5 (0.2) | |||
| Dot Location | |||||||
| Total | 11.1 (0.5) | 12.7 (0.6) | 12.3 (0.6) | 10.7 (0.6) | .089 | ||
| Learning | 10.5 (0.6) | 11.8 (0.7) | 11.5 (0.6) | 10.0 (0.7) | |||
| Short-Delay | 12.0 (0.4) | 12.8 (0.3) | 12.6 (0.3) | 11.7 (0.5) | |||
| Long-Delay | 11.8 (0.3) | 11.9 (0.6) | 11.9 (0.4) | 11.7 (0.4) | |||
| Distracter (%) | 72.5 (3.5) | 82.3 (7.1) | 81.7 (3.7) | 67.4 (5.0) | <.05 | ||
| Family Pictures | |||||||
| Total | 23.7 (1.1) | 21.5 (1.5) | 21.1 (1.1) | 25.4 (1.3) | .051 | ||
| Immediate | 11.7 (0.5) | 10.8 (0.7) | 10.4 (0.5) | 12.7 (0.6) | .098 | .081 | |
| Delayed | 11.9 (0.6) | 10.8 (0.8) | 10.7 (0.6) | 12.7 (0.7) | <.05 | ||
WASI: Wechsler Abbreviated Scale of Intelligence, BRIEF: Behavioral Rating Inventory of Executive Function.
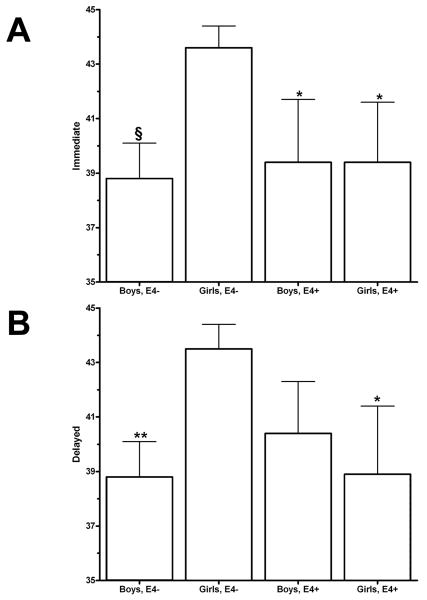
The Family Pictures total score (Immediate + Delayed) was analyzed with an ApoE4 × Sex ANOVA which revealed a trend towards an ApoE4 × Sex interaction (F(1,46)=3.99, p=.051, Table 3). ApoE4- girls exhibited higher immediate recall relative to apoE4+ girls as well as apoE4- boys (Figure 2, Top). Similarly, apoE4- girls showed greater Delayed recall than apoE4+ girls or apoE4- boys (Figure 2, Bottom).
Figure 2.
Performance of apoE4- and apoE4+ children on the Immediate (A) and Delayed (B) Family Pictures tests. *p<.05, **p<.01, or §p<.001versus apoE4- females.
There were no effects of apoE4 or sex on the Total scores of Dot Location assessment (Table 3). However, the percentage of total correct items on the distracter trial was higher in boys (81.7 ±3.7%) than girls (67.4 ±5.0%, t(42.89) = 2.32, p < .05).
Memory Island performance was examined separately for the visible and hidden trials. The cumulative distance to the target during the visible trials was analyzed with a 4 (Trial) × 2 (ApoE4) × 2 (Sex) ANOVA. There was an effect of Trial (F(1.7,74.2)=40.45, p≤.0005) and an ApoE4 × Sex interaction (F(1,44)=4.55, p<.05). Further analyses of the sexes separately again revealed a significant effect of Trial, and, for girls, a trend towards an effect of ApoE4 (p=.09). The latency to find the target showed only an effect of Trial (F(1.3,57.2) =38.2, p≤.0005). Similarly, speed showed only a main effect of trial (F(2.3,100.7)=27.4, p<.005).
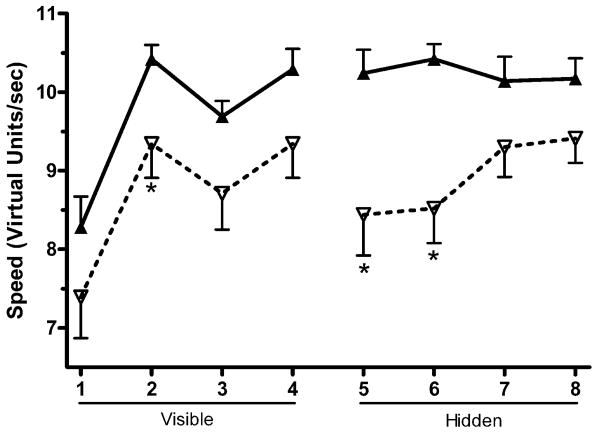
During the hidden trials, there was an effect of Trial on distance traveled (F(2.3,102.4)=4.72, p<.01). For latency during the hidden trials, there was an effect of Trial (F(2.3,101.9) = 6.16, p≤.001). For velocity, there was an effect of Trial (F(3,141)=3.34, p<.05), Sex (F(1,47)=5.74, p<.05), and a Trial × Sex interaction (F(3,141)=2.86, p<.05). Figure 3 shows that boys moved significantly more quickly in the first and second hidden trials with a trend towards a difference observed in the third and fourth trials.
Figure 3.
Speed (virtual units/sec) on the visible and hidden trials of Memory Island. ▲:males; ∇females. (*p<.05 versus males).
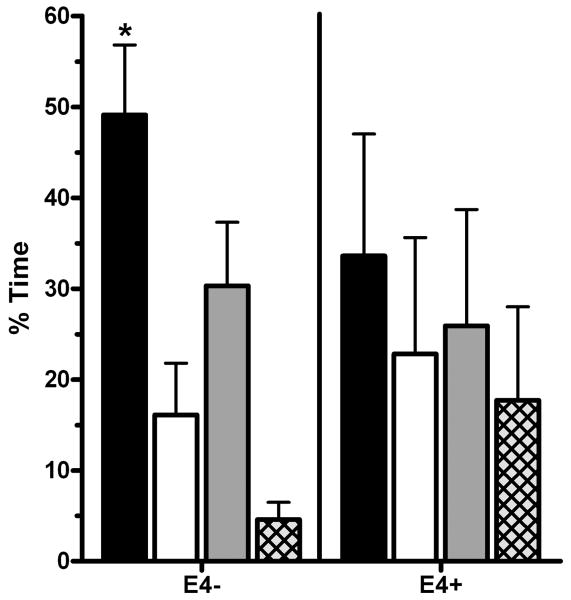
The probe trials were first analyzed with a mixed Quadrant × ApoE4 × Sex × Age (above or below age 10) ANOVA. This analysis revealed an effect of Age (F(1,47)=5.95, p<.05), Quadrant (F(1.4,94)=46.0, p≤.0005), and Age × Quadrant interaction (F(1.4,94)=7.25, p<.005). Examination of the percent-time spent in the target quadrant by age revealed that ten year-olds had significantly higher scores relative to younger children (Ten-Year Olds = 73.9 ±6.2%, Seven to Nine=45.5±6.7%, t(33.6)=3.1, p<.005). Therefore, the quadrant data were further analyzed with the ten-year olds (4 apoE4+ and 7 apoE4-) removed. This analysis (Quadrant × ApoE4 × Sex) revealed a main effect of ApoE4 (F(1,31)=5.20, p<.05), Sex (F(1,31)=9.38, p≤.005), and an ApoE4 × Sex interaction (F(1,31)=6.14, p<.05). Figure 4 shows that while apoE4- children spent more time in the target relative to the right (t(26)=2.78, p≤.01) or opposite quadrants (t(26)=5.91, p≤.0005), apoE4+ children did not show a target preference (p>.40 for all comparisons).
Figure 4.
Spatial memory retention of apoE4- and apoE4+ children in the probe trial of Memory Island. *p<.05 versus other quadrants, ■: target, □: left,  : right, or
: right, or  : opposite quadrant (for additional details, see text).
: opposite quadrant (for additional details, see text).
Discussion
The present report determined that apoE4+ children, unlike apoE4-, do not show a target preference in the Memory Island paradigm. The hippocampus and adjacent entorhinal cortex are key structures in the neural network responsible for spatial function (36). ApoE is important for several neurodevelopmental processes (1) which may account for the finding of apoE4 carrying children having a thinner entorhinal cortex relative to apoE2 or apoE3 (24). The current results complement those from two other reports (Table 4) showing effects of apoE on spatial learning and memory in young people (17,20).
Table 4.
Neurobehavioral findings in infants, children, adolescents, and young adults comparing apoE4+ versus apoE4-.
| Age | Outcome | Reference |
|---|---|---|
| 2 | apoE4+ > apoE4- on Bayley Scale of Infant Development | 26 |
| 6-15 | apoE4+ = apoE4- on IQ | 6 |
| 7-9 | apoE4+ < apoE4- on spatial memory of Memory Island | present study |
| 8-20 | apoE4+ < apoE4- on entorhinal cortical thickness | 24 |
| 8-16 | apoE4 × health interaction on visual memory | 15 |
| 11 | apoE4+ = apoE4- on verbal and non-verbal reasoning | 22 |
| 11-16 | apoE4 < apoE2 on Rey- Osterrieth Complex Figure Test (ROCFT) | 20 |
| 11-16 | apoE4 × Alzheimer's family history interaction on and ROCFT | 21 |
| 11-16 | apoE4 × Alzheimer's family history interaction on reading and language | 21 |
| 16-30 | apoE4 < apoE3 on navigating through a computerized grid maze | 17 |
| 19-21 | apoE4+ > apoE4- on performance IQ | 37 |
| 20-35 | apoE4+ > apoE4- on hippocampal activity during memory encoding | 47 |
| 22 | apoE4+ > apoE4- on verbal delayed recall | 19 |
>: better performance (higher percent correct, faster reaction time, fewer trials to criterion), <: worse performance (lower percent correct, slower reaction time, more trials to criterion).
Geriatric women performed better than men on Family Pictures (13). Using the same paradigm, and unlike the elderly (13), there was a apoE4 by sex interaction among children. Girls without apoE4 performed better than those with apoE4 at both the immediate condition and after a short delay. Most investigations (23,24,25) have noted that overall IQ was unaffected by apoE4. However, the presence of apoE4 has been associated with improved WAIS performance IQ (37). The effect of sex in mediating the susceptibility to apoE4-induced neuropsychological alterations is consistent with apoE4+ positive females exhibiting greater age-related declines then men on Wechsler Adult Intelligence Scale Performance IQ (12). In addition, during the visible learning trials of Memory Island, there was a genotype × sex interaction. With the heightened sensitivities of females to the consequences of apoE4 (11,38), these findings highlight the importance for future investigations to carefully monitor for apoE by sex interactions or to continue examining females separately (37,39). Sex differences, independent of apoE4, were also identified in the spatial learning trials of Memory Island with boys showing greater velocity and reaching the target sooner than girls, consistent with faster and more accurate performance by males than females in virtual water mazes across the lifespan (13,33,40,41).
ApoE4+ infants were more likely to require ICU and apoE4+ neonates weighed less at birth overall. In addition, a sex difference in birth weights favoring males was observed among the apoE4-, but not apoE4+, offspring. Epidemiological data noted subtle (i.e. 100 gram) sex differences favoring males in birth weight (J.A. Martin, personal communication), so the current finding of a large (400 g) sex difference was unanticipated. While birth weight has been associated with cognition (42), these sequelae are most pronounced for babies that qualify as at least low birth weight (<2,500 g). As only one apoE4+ subject met this criterion, albeit barely (2,495 g), compared to two in the apoE4- group, it is unlikely that the present neurobehavioral findings are an indirect consequence of apoE4 acting simply on birth weight. However, due to the relatively low sample size, the present observations on birth outcomes, cognition, and apoE4 should be regarded as preliminary. The power for the ApoE4 differences in body weight was only moderate (0.57), especially relative to other outcomes (Figure 2 (Top), Power=0.85). Although the veracity of maternal recall over a decade, particularly for mothers that have given birth to several children, may be suspect, events like an ICU visit are unlikely to be forgotten. The body mass and medical resource utilization findings, if replicated based on the medical records, would extend upon prior reports of adverse gynecological and birth outcomes being influenced by apoE (27-29,43-45).
The present results showing effects of apoE4 in children are in conjunction with several other investigations in children and young-adults and indicate that apoE may modulate neurocognitive function. However, Table 4 indicates that the direction of the effects appears to depend on the domain and age assessed (16,22). Environmental challenges might also modulate the direction of the apoE4 effects in children (24). ApoE4-carrying children were more resistant to the detrimental effects of diarrhea on cognitive function (15). Similarly, among neonates that underwent cardiac corrective surgery, those with apoE2 scored lower on gross and fine motor function when assessed on the Bayley Scale of Infant Development (43). In addition, apoE2 infants, relative to apoE3 homozygotes, had lower Mental Development Index scores, a broad measure that includes memory, problem solving, early number concepts, language, and social skills, following heart surgery (45). Finally, the risk of developing Cerebral Palsy was strongly elevated by the presence of at least one apoE2 allele (44), although see (46).
In conclusion, second to fifth grade children exhibit sex- and apoE4-dependent behavioral differences, typically favoring apoE4- participants. ApoE4, acting either alone or in conjunction with sex modified spatial learning and memory on Memory Island, the Vocabulary Score on the WASI, and immediate and delayed visual recall on the Family Pictures assessment. These early effects of apoE4 might contribute to the enhanced risk of apoE4 carriers to age-related cognitive decline and cognitive impairments following environmental challenges.
Acknowledgments
We would like to thank Dean Inman, Ph.D., and Aaron Cram at the Oregon Research Institute for their contributions and support of the Memory Island software. Robert Butler, Ph.D., Sig-Linda Jacobson, M.D., Jessica Ezzell Hunter, Ph.D., and members of the Raber lab for careful reading of an earlier version of this manuscript and helpful comments, Jean O'Malley for statistical advice, and Anthony Bader and Patrick Murray for technical assistance.
Financial Support: Public Health Service Grant (1 UL1 RR024120-01), National Institute of Drug Abuse (T32 DA07262 and 1P50DA018165), National Center for Research Resources (UL1 RR024140 01), Clinical Research Enhancement Fund (90120298), Ellison Medical Foundation (AG-NS-0201), and the Oregon Clinical and Translational Research Institute (OCTRI).
Abbreviations
- AD
Alzheimer's Disease
- apoE
Apolipoprotein E
- BRIEF
Behavior Rating Inventory of Executive Function
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herz J, Beffert U. Apolipoprotein E receptor: Linking brain development and Alzheimer's disease. Nat Rev Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- 2.Mahley RW, Weisgraber KH, Innerarity TL, Rall SC., Jr Genetic defects in lipoprotein metabolism. Elevation of artherogenic lipoproteins caused by impaired catabolism. JAMA. 1991;265:78–83. doi: 10.1001/jama.265.1.78. [DOI] [PubMed] [Google Scholar]
- 3.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao Y, Gearing M, Mirra SS. Astrocyte-apolipoprotein E associations in senile plaques in Alzheimer disease and vascular lesions: a regional immunohistochemical study. J Neuropathol Exp Neurol. 1997;56:376–381. doi: 10.1097/00005072-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Strittmatter WJ, Bova Hill C. Molecular biology of apolipoprotein E. Curr Opin Lipidol. 2002;13:119–123. doi: 10.1097/00041433-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Glatt SJ, Chayavichitsilp P, Depp C, Schork NJ, Jeste DV. Successful aging: from phenotype to genotype. Biol Psychiatry. 2007;62:282–293. doi: 10.1016/j.biopsych.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Novelli V, Viviani Anselmi C, Roncarti R, Guffanti G, Malovini A, Piluso G, Puca AA. Lack of replication of genetic associations with human longevity. Biogerontology. 2008;9:85–92. doi: 10.1007/s10522-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apoliprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 10.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 11.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Periak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 12.Mortensen EL, Høgh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001;57:89–95. doi: 10.1212/wnl.57.1.89. [DOI] [PubMed] [Google Scholar]
- 13.Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE ε4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007;147:6–17. doi: 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Pardo LM, Schuur M, Sanchez-Juan P, Isaacs A, Sleegers K, de Koning I, Zorkoltseva IV, Axenovich TI, Witteman JC, Janssens AC, van Swieten JC, Aulchenko YS, Oostra BA, van Duijn CM. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.015. in press. [DOI] [PubMed] [Google Scholar]
- 15.Oria RB, Patrick PD, Zhang H, Lorntz B, de Costro CM, Brito GA, Barrett LJ, Lima AA, Guerrant RL. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005;57:310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- 16.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.02.003. in press. [DOI] [PubMed] [Google Scholar]
- 17.Alexander DM, Williams LM, Gatt JM, Dobson-Stone C, Kuan SA, Todd EG, Schofield PR, Cooper NJ, Gordon E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol. 2007;75:229–238. doi: 10.1016/j.biopsycho.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Hubacek JA, Pitha J, Skodová Z, Adámková V, Lánská V, Poledne R. A possible role of Apolipoprotein E polymorphism in predisposition to higher education. Neuropsychobiology. 2001;43:200–203. doi: 10.1159/000054890. [DOI] [PubMed] [Google Scholar]
- 19.Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropolous A, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2006;17:1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- 20.Bloss CS, Delis DC, Salmon DP, Bondi MW. APOE genotype is associated with left-handedness and visuospatial skills in children. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased cognition in children with risk factors for Alzheimer's disease. Biol Psychiatry. 2008;64:904–906. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ. Cognitive change and the APOE 4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- 23.Plomin R, McClearn GE, Smith DL, Skuder P, Vignetti S, Chorney MJ, Chorney K, Kasarda S, Thompson LA, Detterman DK, Petrill SA, Daniels J, Owen MJ, McGuffin P. Allelic associations between 100 DNA markers and high versus low IQ. Intelligence. 1995;21:31–48. [Google Scholar]
- 24.Shaw P, Lerch J, Prussner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: An observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 25.Turic D, Fisher PJ, Plomin R, Owen MJ. No association between apolipoprotein E polymorphisms and general cognitive ability in children. Neurosci Lett. 2001;299:97–100. doi: 10.1016/s0304-3940(00)01789-4. [DOI] [PubMed] [Google Scholar]
- 26.Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandez-Avila M. Apolipoprotein E genotype predicts 24-month Bayley scales infant development score. Pediatr Res. 2003;54:819–825. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- 27.Becher JC, Keeling JW, McIntosh N, Wyatt B, Bell J. 2006 The distribution of apoliprotein E alleles in Scottish perinatal deaths. J Med Genet. 2006;43:414–418. doi: 10.1136/jmg.2005.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman C, Goodman CS, Hur J, Jeyendran RS, Coulam C. The association of Apolipoprotein E polymorphisms with recurrent pregnancy loss. Am J Reprod Immunol. 2009;61:34–38. doi: 10.1111/j.1600-0897.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 29.Infante-Rivard C, Lévy E, Rivard GE, Guiguet M, Feoli-Fonseca JC. Small babies receive the cardiovascular protective apolipoprotein E2 allele less frequently than expected. J Med Genet. 2003;40:626–629. doi: 10.1136/jmg.40.8.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen CJ. Children's Memory Scale: Stimulus Book One. Psychological Corporation; San Antonio: 1997. pp. 1–140. [Google Scholar]
- 31.Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 2003;31:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- 32.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavioral rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 33.Rizk-Jackson AM, Acevedo SF, Inman D, Howieson D, Benice TS, Raber J. Effects of sex on object recognition and spatial navigation in humans. Behav Brain Res. 2006;173:181–190. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 35.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program of the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 36.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 37.Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neurosci Lett. 2000;294:179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]
- 38.Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- 39.Irimajiri R, Golub EJ, Starr A. ApoE genotype and abnormal auditory cortical potentials in healthy older females. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.005. in press. in press. [DOI] [PubMed] [Google Scholar]
- 40.Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Lundgren EM, Tuvemo T. 2008 Effects of being born small for gestational age on long-term intellectual performance. Best Pract Res Clin Endocrinol Metab. 2008;22:477–488. doi: 10.1016/j.beem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, Nord AS, Clancy RR, Nicolson SC, Spray TL. Patient characteristics are more important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–1353. doi: 10.1016/j.jtcvs.2006.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroda MM, Weck ME, Sarwark JF, Hamidullah A, Wainwright MS. Association of Apolipoprotein E genotype and cerebral palsy in children. Pediatrics. 2007;119:306–313. doi: 10.1542/peds.2006-1083. [DOI] [PubMed] [Google Scholar]
- 45.Gaynor JW, Gerdes M, Zackai EH, Bernbaum J, Wernovsky G, Clancy RR, Newman MF, Saunders AM, Heagerty PJ, D'Agostuns JA, McDonald-McGinn D, Nicolson SC, Sprya TL, Jarvik GP. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–1745. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 46.McMichael GL, Gibson CS, Goldwater PN, Haan EA, Priest K, Dekker GA, MacLennan AH. Association between Apolipoprotein E genotype and cerebral palsy is not confirmed in a Caucasian population. Hum Genet. 2008;124:411–416. doi: 10.1007/s00439-008-0564-y. [DOI] [PubMed] [Google Scholar]
- 47.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]