Abstract
Background & objectives
Antiretroviral drug concentrations are important determinants of clinical response to a drug accounting for both toxicity and efficacy. Several factors such as age, ethnicity, body weight and patients’ immune status may influence antiretroviral drug concentrations. The aim of the study was to determine the influence of immunological status, sex and body mass index on the steady state pharmacokinetics of lamivudine (3TC) and stavudine (d4T) in HIV-infected adults, who were undergoing treatment with generic fixed dose combinations (FDC) of these drugs in India.
Methods
Twenty seven HIV-1 infected patients receiving antiretroviral treatment (ART) for at least two weeks at the Government ART clinic at Tambaram, Chennai, took part in the study. Serial blood samples were collected predosing and at different time points after drug administration. Plasma 3TC and d4T levels were estimated by HPLC.
Results
The patients’ immune status, sex or body mass index had no impact on the pharmacokinetics of 3TC. In the case of d4T, peak concentration was significantly lower in patients with CD4 cell counts < 200 cells/μl than those with ≥200 cells/μl (P < 0.05), but were within the therapeutic range. The mean CD4 cell counts increased from 101 cells/μl at initiation of ART to 366 cells/μl at 12 months of treatment.
Interpretation & conclusions
Blood levels of 3TC and d4T drugs that are part of generic FDCs commonly used by HIV-infected individuals in India were within the therapeutic range and not influenced by nutritional or immune status. There was a significant improvement in CD4 cell counts over 12 months of treatment. Indian generic FDCs manufactured and used widely in the developing world provide effective concentrations of antiretroviral drugs.
Keywords: Generic FDCs, India, lamivudine, pharmacokinetics, stavudine
Generic fixed dose combinations (FDC) comprising two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-NRTI (NNRTI) are widely used in the scaling-up of antiretroviral treatment (ART) in developing countries. A vast majority of HIV-infected patients in India receive nevirapine (NVP)-based highly active anti-retroviral treatment (HAART), the common companion drugs being lamivudine (3TC) and stavudine (d4T) or zidovudine (AZT)1. Several factors such as drug-drug interactions, drug-food interactions, sex, age, body weight, disease state (renal and hepatic function) and pregnancy can influence drug concentrations2. Drug-drug interactions for drugs metabolized by the hepatic cytochrome P-450 enzymes are relatively common. The bioavailability of antiretroviral drugs can be considerably reduced by food3. Higher antiretroviral drug exposure in women compared to men has been associated with a greater likelihood of virologic success4. Further, higher NRTI-triphosphate concentrations (AZT & 3TC) have been reported in women compared to men5. Advanced HIV disease has been associated with malabsorption, notably of anti-tuberculosis drugs6. It was further reported that malaborption correlated significantly with degree of immune suppression and body mass index (BMI)7. Differences in patients’ body weight appear to cause differences in exposure of certain antiretroviral drugs, notably efavirenz, raising the question of whether efavirenz dose should be increased in people with higher body weight8, 9. In addition to these known variables, genetic differences in HIV-infected patients have been found to account for variations in antiretroviral drug response and toxicity2.
We had earlier shown that patients’ immune status, sex or BMI had no impact on the pharmacokinetics of NVP, and that plasma NVP concentrations were maintained within the therapeutic range of the drug in the majority of adults who received the drug as a generic FDC10. The aim of the present study was to examine the influence of immunological status, sex, BMI and dose of d4T on the steady state pharmacokinetics of 3TC and d4T in HIV-infected patients on treatment with FDCs in India.
Material & Methods
Patients
The study was conducted in 27 HIV - infected adults attending the outpatient clinic of the Tuberculosis Research Centre, Chennai, during October 2005 to March 2006. These patients had participated in a controlled clinical trial and were being followed up at regular intervals. During each follow up visit they underwent a complete medical examination and were tested for biochemical and haematological parameters and CD4 cell counts. The patients were required to meet the following inclusion criteria (i) 18–50 yr age; (ii) body weight not less than 30 kg; (iii) no severe hepatic or renal dysfunction (serum transaminases within two and half times the upper limit of normal range and creatinine < 1.2 mg/dl); (iv) non diabetic (random blood glucose between 80–140 mg/dl); (v) undergoing treatment with generic FDC of antiretroviral drugs (NVP 200 mg/3TC 150 mg/d4T 30/40 mg or AZT 300 mg twice daily) for a minimum antiretroviral period of 2 wk; (vi) not suffering from any serious opportunistic infection that could cause malabsorption of drugs; and (vii) willingness to participate in the study and provide informed written consent. Chronic alcoholics and female patients on hormonal birth control pills were excluded from the study. None of the patients were receiving concomitant medications for tuberculosis. The study was conducted after obtaining clearance from the Institutional Ethics Committee.
Conduct of study
The study was conducted at the Government Hospital of Thoracic Medicine, Tambaram, Chennai. Eligible study participants were admitted to the hospital at least a day prior to start of the study. Informed written consent was obtained from all patients. On the day of the study, blood samples (3 ml) were drawn in heparinised containers before dosing and serially at 0.5, 1, 2, 4, 6, 8, and 12 h after administration of the FDC pill with 200 ml water. All the blood samples were centrifuged immediately and plasma stored at −20°C until estimations of 3TC and d4T were undertaken.
Estimation of plasma 3TC and d4T
Estimations of plasma 3TC and d4T were carried out by HPLC (Shimadzu Corporation, Kyoto, Japan) with UV detection according to validated methods11. The limits of detection of 3TC and d4T were 0.01 and 0.001μg/ml respectively. The precision of the assay ranged from 2.9 to 7.1 per cent for 3TC and 3.3 to 8.7 per cent for d4T, the accuracy being 101 and 96 per cent for 3TC and d4T respectively. The per cent recoveries of 3TC and d4T from plasma were 94 and 91 per cent respectively.
Pharmacokinetic analysis
Pharmacokinetic variables such as peak concentration (Cmax), minimum concentration (Cmin), time to attain peak concentration (Tmax), exposure (AUC), and half-life (t1/2) were calculated employing a non compartmental model following first-order kinetics (Version 4.1) (Pharsight Corporation, Mountain View, CA, USA).
Statistical evaluation
Analysis of data was performed using SPSS (version 10) package (SPSS Inc., Chicago, IL, USA). Data were expressed as median and range as the data were not normally distributed. The significance of differences in the pharmacokinetic parameters of 3TC and d4T between various groups of patients, classified based on CD4 cell counts, sex, BMI, concomitant co-trimoxazole (in the case of 3TC) and dose of d4T (in the case of d4T) was tested using Mann-Whitney test. P≤ 0.05 was considered to be statistically significant. Pearson’s correlation test was used to evaluate correlation between Cmax and AUC0–12 of drugs with that of patients’ body weight.
Results
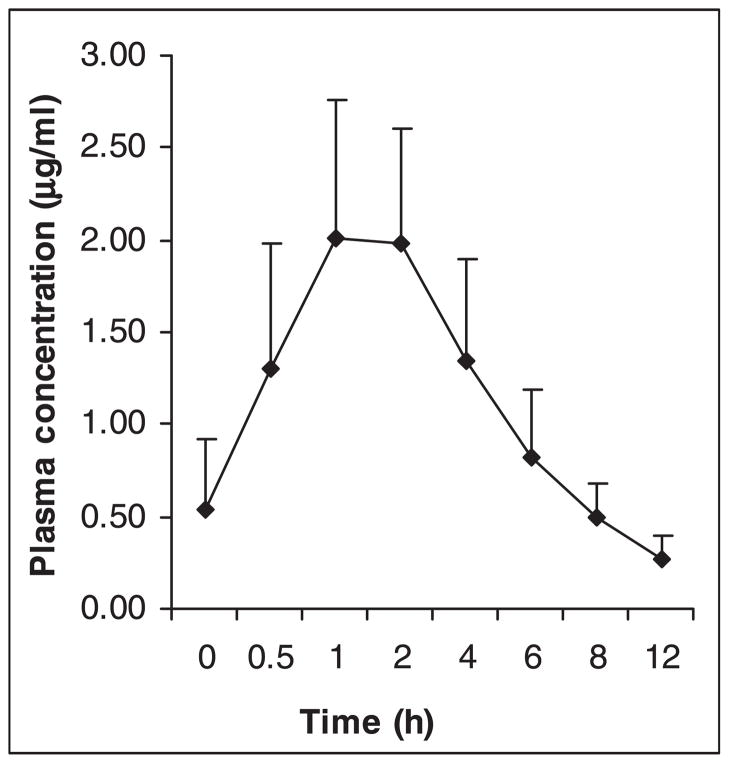
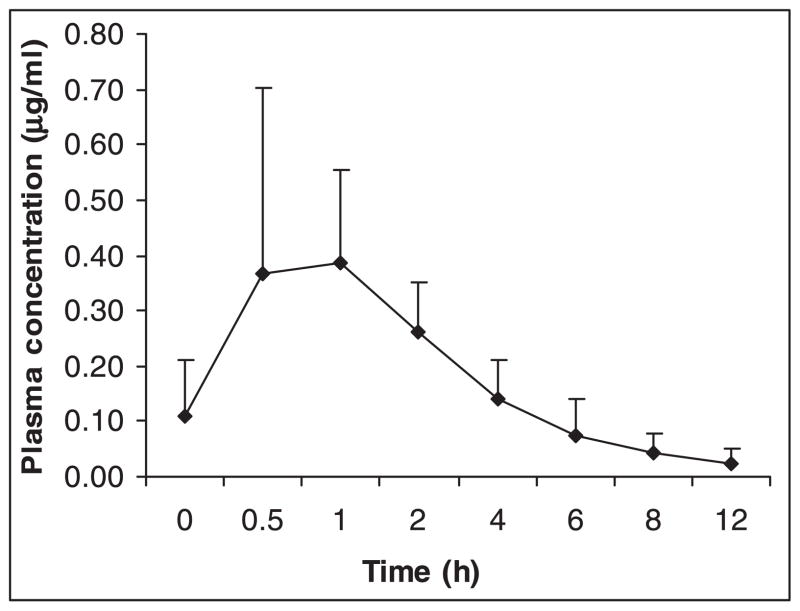
The baseline characteristics of study participants are provided in Table I. Of the 27 patients, six were receiving AZT and 21 d4T. Stavudine measurements could not be performed in seven patients. This study reports the steady state pharmacokinetics of 3TC and d4T from 27 and 14 patients respectively. Plasma concentrations of 3TC and d4T increased steadily with peak concentrations obtained at 1 h for both the drugs (Figs 1 and 2). The pharmacokinetic variables calculated based on plasma 3TC and d4T levels are shown in Tables II and III respectively. Comparisons of pharmacokinetic variables of 3TC and d4T in patient groups divided based on CD4 cell counts (< and ≥ 200 cells/μl), sex (males and females) and BMI (<18.5 & ≥ 18.5 kg/m2) were made. The normal range of BMI was taken as 18.5–24.9 kg/m2 12. Further, patients receiving 3TC were grouped based on whether they received concomitant co-trimoxazole or not, and patients receiving d4T were divided based on the dose they received (30 & 40 mg). None of the differences in Cmax, Cmin, AUC0–12 and t1/2 of 3TC between the various patient groups was statistically significant. Of the 27 patients analysed for 3TC pharmacokinetics, 15 were receiving concomitant treatment with co-trimoxazole [trimethoprim (TMP) 160 mg/sulphamethoxazole (SMX) 800 mg] once daily. No difference in Cmax, Cmin, AUC0–12 and t½ of 3TC was observed between patients receiving and not receiving co-trimoxazole. With respect to d4T, a significant difference in Cmax between patients with CD4 cell counts < 200 cells/μl (0.33μg/ml) and ≥ 200 cells/μl (0.53 μg/ml) was observed (P < 0.05). Of the 14 patients analysed for d4T pharmacokinetics, 10 were receiving 30 mg dose of the drug, while the remaining 4 were receiving 40 mg dose. The differences in the pharmacokinetic variables of d4T between these two groups of patients were not statistically significant. The correlation between patients’ body weight and Cmax and AUC0–12 of 3TC and d4T was not significant.
Table 1.
Baseline characteristics of study participants
| Characteristics | Value |
|---|---|
| Sex | |
| Males | 17 |
| Females | 10 |
| Age (yr) | |
| Mean | 36 |
| Range | 26–50 |
| Body weight (kg) | |
| Mean | 52 |
| Range | 35–91 |
| Height (cm) | |
| Mean | 159 |
| Range | 140–173 |
| BMI (kg/m2) | |
| Mean | 20.1 |
| Range | 13.6–33.0 |
| Duration of ART (months) | |
| Mean | 4.4 |
| Range | 1–17 |
| CD4 counts (cells/μl) | |
| Mean | 218 |
| Range | 25–684 |
| < 200 cells/μl (No.) | 15 |
| ≥ 200 cells/μl (No.) | 12 |
BMI, body mass index; ART, antiretroviral treatment
Fig. 1.
Mean plasma concentrations of lamivudine at different time points (n = 27). Vertical bars represent standard deviation.
Fig. 2.
Mean plasma concentrations of stavudine at different time points (n = 14). Vertical bars represent standard deviation.
Table 2.
Steady state pharmacokinetics of lamivudine (150 mg bi-daily)
| Cmax (μg/ml) | Cmin (μg/ml) | Tmax (h) | AUC(0-t) (μg/ml-h) | t½ (h) | |
|---|---|---|---|---|---|
| Overall | 2.43 | 0.24 | 1.0 | 12.00 | 4.70 |
| n = 27 | (1.00–3.32) | (0.08–0.51) | (1.0–4.0) | (6.11–18.04) | (1.73–8.77) |
| CD4 counts cell/μl | |||||
| < 200 | 2.44 | 0.32 | 1.0 | 12.60 | 4.70 |
| n = 15 | (1.00–3.24) | (0.08–0.51) | (1.0–4.0) | (6.11–18.04) | (1.73–8.77) |
| ≥200 | 2.36 | 0.22 | 1.0 | 11.74 | 4.45 |
| n = 12 | (1.38–3.32) | (0.13–0.39) | (1.0–4.0) | (7.40–14.02) | (2.26–6.22) |
| Sex | |||||
| Males | 2.37 | 0.24 | 1.0 | 12.02 | 4.70 |
| n = 17 | (1.00–3.10) | (0.11–0.51) | (1.0–4.0) | (6.11–18.04) | (1.73–7.10) |
| Females | 2.57 | 0.23 | 1.0 | 11.47 | 3.93 |
| n = 10 | (1.28–3.32) | (0.08–0.41) | (1.0–2.0) | (7.09–15.33) | (2.22–8.77) |
| BMI (kg/m2) | |||||
| < 18.5 | 2.40 | 0.22 | 1.0 | 11.47 | 3.49 |
| n = 18 | (1.28–3.32) | (0.08–0.51) | (1.0–4.0) | (7.09–18.04) | (1.73–7.06) |
| ≥ 18.5 | 2.44 | 0.38 | 1.0 | 12.48 | 5.60 |
| n = 9 | (1.00–2.89) | (0.11–0.45) | (1.0–2.0) | (6.11–14.02) | (2.40–8.77) |
| Co-trimoxazole | |||||
| Yes | 2.38 | 0.18 | 1.0 | 12.48 | 3.24 |
| n = 15 | (1.00–2.96) | (0.08–0.51) | (1.0–4.0) | (6.11–18.04) | (1.73–8.77) |
| No | 2.46 | 0.23 | 1.0 | 11.46 | 4.90 |
| n = 12 | (1.28–3.32) | (0.13–0.45) | (1.0–2.0) | (7.09–15.41) | (3.00–7.10) |
Cmax, peak concentration; Cmin, minimum concentration; Tmax, time to attain Cmax; AUC, area under the plasma concentration vs. time curve; t½, half life. Values are shown as median and range given in parentheses
Table 3.
Steady state pharmacokinetics of stavudine (30/40 mg bi-daily)
| Cmax (μg/ml) | Cmin (μg/ml) | Tmax (h) | AUC(0-t) (μg/ml-h) | t½ (h) | |
|---|---|---|---|---|---|
| Overall | 0.37 | 0.015 | 1.0 | 1.38 | 3.32 |
| n = 14 | (0.20–0.76) | (0.001–0.11) | (0.5–2.0) | (0.59–2.20) | (0.50–6.58) |
| CD4 counts (cell/μl) | |||||
| < 200 | 0.33 | 0.020 | 1.0 | 1.37 | 2.71 |
| n = 8 | (0.20–0.57) | (0.001–0.11) | (1.0–2.0) | (0.59–2.20) | (0.50–6.58) |
| ≥ 200 | 0.53* | 0.025 | 1.0 | 1.47 | 3.32 |
| n = 6 | (0.31–0.76) | (0.01–0.03) | (1.0) | (1.29–2.17) | (2.35–5.71) |
| Sex | |||||
| Males | 0.37 | 0.015 | 1.0 | 1.36 | 2.82 |
| n = 12 | (0.20–0.76) | (0.001–0.11) | (0.5–2.0) | (0.59–2.20) | (0.50–6.58) |
| Females | 0.39 | 0.03 | 0.75 | 1.62 | 3.58 |
| n = 2 | (0.21–0.56) | (0.01–0.05) | (0.5–1.0) | (1.58–1.67) | (3.53–3.63) |
| BMI (kg/m2) | |||||
| < 18.5 | 0.38 | 0.030 | 0.75 | 1.51 | 3.36 |
| n = 8 | (0.21–0.76) | (0.004–0.11) | (0.5–1.0) | (0.92–2.20) | (1.79–6.58) |
| ≥ 18.5 | 0.37 | 0.01 | 1.0 | 1.37 | 2.32 |
| n = 6 | (0.20–0.57) | (0.001–0.03) | (0.5–2.0) | (0.59–1.58) | (0.50–5.64) |
| Dose | |||||
| 30 mg | 0.35 | 0.01 | 1.0 | 1.38 | 2.94 |
| n = 10 | (0.20–0.57) | (0.001–0.11) | (0.5–2.0) | (0.59–2.20) | (0.50–6.58) |
| 40 mg | 0.38 | 0.03 | 0.75 | 1.35 | 3.02 |
| n = 4 | (0.31–0.76) | (0.02–0.03) | (0.5–1.0) | (1.29–2.17) | (2.53–5.71) |
Cmax, peak concentration; Cmin, minimum concentration; Tmax, time to attain C-max; AUC, area under the plasma concentration vs. time curve; t½, half life;
P < 0.05 compared to the group with CD4 counts < 200 cells/μl. Values are shown as median and range given in parentheses
The mean (range) CD4 cell counts at baseline, 6 and 12 months after initiation of antiretroviral treatment (ART) were 101 (25–209), 322 (127–775) and 386 (112–733) cells/μl respectively.
Discussion
This study presents the pharmacokinetic profile of 3TC and d4T delivered as a generic FDC to HIV- infected patients receiving ART from a Government ART clinic at Tambaram, Chennai.
The pharmacokinetic profile of 3TC observed in this study was similar to that reported in Indian and other populations13–16 (plasma peak and trough concentrations ranging from 1.59 to 2.72μg/ml and 0.078 to 0.33μg/ml respectively). Neither the degree of immune suppression, sex nor BMI had any impact on the pharmacokinetics of 3TC as also reported by others17. Trimethoprim lowers the renal clearance of 3TC by competitive inhibition of tubular secretion by TMP18. Our study data, however, showed that concomitant treatment with co-trimoxazole did not cause any significant change in the pharmacokinetics of 3TC. Our data differ from previous reports which found an increase in the average steady state 3TC serum concentrations when co-administered with co-trimoxazole18,19. Although the reason for this difference is not clear, our patients were supplied co-trimoxazole for self-administration and their adherence to this drug was not monitored. Further studies are needed to study the effect of higher dosages of TMP/SMX on 3TC pharmacokinetics.
In the case of d4T, mean Cmax of 0.42μg/ml and t½ of 3.23 h were lower and higher respectively than that reported by others16, 20–22. These studies reported peak concentration ranging from 0.60 to 1.2 μg/ml and half-lives ranging from 1.0 to 1.6 h. Even though Cmax of d4T was lower in patients with CD4 counts < 200 cells/μl than those with CD4 counts ≥ 200 cells/μl, it was within the therapeutic range based on plasma levels. However, the number of patients studied in both these groups was small (8 and 6 patients respectively).
Antiretroviral drug concentrations are among the most important determinants of clinical response to a drug accounting for both toxicity and efficacy. Our finding of adequate plasma concentrations of 3TC and d4T that are not influenced by the stage of immune suppression, sex and BMI among Indian patients is encouraging. The adequacy of blood levels also correlated with the clinical and immunological improvement noted in this patient group, though virological monitoring was not done. The NRTI class of drugs such as 3TC, d4T and AZT become biologically activated after intracellular conversion to phosphorylated metabolites. Even though a relationship between plasma concentrations of NRTIs and outcome has been found in some studies, the usefulness of NRTI plasma concentrations in predicting treatment response remains uncertain23. Also, the relationship between the concentration of active intracellular triphosphates of the NRTIs and sex, BMI and stage of immune suppression has not been reported. Further studies are warranted to examine these relationships and to establish the pharmacokinetic profile of the intracellular metabolites of 3TC and d4T.
The main limitation of our study was the small size, particularly for d4T, which was further reduced when patients were grouped based on sex, CD4 cell counts, BMI and dose of the drug. The study findings however, have clinical implications for treatment of HIV-infected patients in India and other developing countries. The National AIDS Control Organisation of India recommends the use of NVP and 3TC with either d4T or AZT24. In India, about 50–60 per cent of patients, initiated on ART receive a stavudine-based regimen25. Similarly, many resource-poor countries use stavudine-based regimen as first-line of treatment, even though this is used to a relatively lesser extent in the developed countries. The dosage of d4T has recently been reduced to 30 mg even for patients of body weight above 60 kg; our data support this new recommendation. Our findings provide some evidence to suggest that dosage recommendations could be uniform across sex, disease stage and nutritional status for 3TC and d4T; similar findings were observed for NVP10. In summary, the study has shown that blood levels of 3TC and d4T – drugs that are part of generic FDCs commonly used by HIV-infected individuals in India are maintained within the therapeutic range among patients with varying nutritional status and at different stages of HIV disease. Programmes that can ensure high rates of adherence can hope for a sustained therapeutic response to these drugs.
Acknowledgments
The authors thank Dr P.R. Narayanan, Director, Tuberculosis Research Centre, Chennai, India, for his encouragement and support, Astra Zeneca Research Foundation, Bangalore, India for pharmacokinetic analyses using WinNonlin software, Ms. Komathi and Ms. Vijayalakshmi for blood collections and the patients who took part in the study. The authors acknowledge the help rendered by the medical officers of the Government Hospital of Thoracic Medicine, Tambaram, Chennai, India. The training provided to Geetha Ramachandran under the Fogarty AIDS International Training and Research Program 5D437W000237, Lifespan/Tufts/Brown Center for AIDS Research (Dr Kenneth Mayer, Program Director) at Tufts University School of Medicine, Boston is gratefully acknowledged.
Contributor Information
A.K. Hemanth Kumar, Department of Clinical Research, Tuberculosis Research Centre (ICMR), Chennai, India.
Geetha Ramachandran, Department of Clinical Research, Tuberculosis Research Centre (ICMR), Chennai, India.
S. Rajasekaran, Government Hospital of Thoracic Medicine, Chennai, India
C. Padmapriyadarsini, Department of Clinical Research, Tuberculosis Research Centre (ICMR), Chennai, India
G. Narendran, Department of Clinical Research, Tuberculosis Research Centre (ICMR), Chennai, India
S. Anitha Sudha Subramanyam, Department of Clinical Research, Tuberculosis Research Centre (ICMR), Chennai, India.
V. Kumaraswami, Department of Clinical Research, Tuberculosis Research Centre (ICMR), Chennai, India
Soumya Swaminathan, Department of Clinical Research, Tuberculosis Research Centre (ICMR), Chennai, India.
References
- 1.Kumarasamy N, Solomon S, Chaguturu SK, Cecelia AJ, Vallabhaneni S, Flanigan TP, et al. The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41:1525–8. doi: 10.1086/497267. [DOI] [PubMed] [Google Scholar]
- 2.Cressey TR, Lallemant M. Pharmacogenetics of antiretroviral drugs for the treatment of HIV-infected patients: an update. Infect Genet Evol. 2007;7:333–42. doi: 10.1016/j.meegid.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Yeh KC, Deutsch PJ, Haddix H, Hesney M, Hoagland V, Ju WD, et al. Single-dose pharmacokinetics of indinavir and the effect of food. Antimicrob Agents Chemother. 1998;42:332–8. doi: 10.1128/aac.42.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher CV, Jiang H, Brundage RC, Acosta EP, Haubrich R, Katzenstein D, et al. Sex-based differences in saquinavir pharmacology and virologic response in AIDS Clinical Trials Group Study 359. J Infect Dis. 2004;189:1176–84. doi: 10.1086/382754. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PL, Lamba J, Aquilante CL, Schuetz E, Fletcher CV. Pharmacogenetic characterisitics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr. 2006;42:441–9. doi: 10.1097/01.qai.0000225013.53568.69. [DOI] [PubMed] [Google Scholar]
- 6.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, et al. Decreased bioavailability of rifampin and other anti-tuberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob Agents Chemother. 2004;48:4473–5. doi: 10.1128/AAC.48.11.4473-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating J, Bjarnason I, Somasundaram S, Macpherson A, Francis N, Price AB, et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995;37:623–9. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–90. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 9.Matteelli A, Regazzi M, Villani P, De Iaco G, Cusato M, Carvalho AC, et al. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr HIV Res. 2007;5:349–53. doi: 10.2174/157016207780636588. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran G, Hemanthkumar AK, Rajasekaran S, Padmapriyadarsini C, Narendran G, Anitha S, et al. Steady-state pharmacokinetics of nevirapine in HIV-1 infected adults in India. J Int Assoc Physicians AIDS Care (Chic 111) 2007;6:251–4. doi: 10.1177/1545109707301344. [DOI] [PubMed] [Google Scholar]
- 11.Moyer TP, Temesgen Z, Enger R, Estes L, Charlson J, Oliver L, et al. Drug monitoring of antiretroviral therapy for HIV-1 infection: method validation and results of a pilot study. Clin Chem. 1999;45:1465–76. [PubMed] [Google Scholar]
- 12.Dietary guidelines for Indians – a manual. National Institute of Nutrition (Indian Council of Medical Research); Hyderabad, India: 2003. [accessed on April 15, 2008]. pp. 43–44. Available at: http://www.ninindia.org. [Google Scholar]
- 13.Narang VS, Lulla A, Malhotra G, Purandare S. Pharmacokinetic profiling and bioequivalence evaluation of 2 lamivudine tablet formulations after single oral administration in healthy human Indian volunteers. J Acquir Immune Defic Syndr. 2005;38:566–9. doi: 10.1097/01.qai.0000155202.51232.f5. [DOI] [PubMed] [Google Scholar]
- 14.Bruno R, Regazzi MB, Ciappina V, Villani P, Sacchi P, Montagna M, et al. Comparison of the plasma pharmacokinetics of lamivudine during twice and once daily administration in patients with HIV. Clin Pharmacokinet. 2001;40:695–700. doi: 10.2165/00003088-200140090-00005. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41–66. doi: 10.2165/00003088-199936010-00004. [DOI] [PubMed] [Google Scholar]
- 16.van Praag RM, van Weert EC, van Heeswijk RP, Zhou XJ, Sommadossi JP, Jurriaans S, et al. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob Agents Chemother. 2002;46:896–9. doi: 10.1128/AAC.46.3.896-899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore KH, Yuen GJ, Hussey EK. Analysis of potential gender differences in lamivudine disposition using population pharmacokinetics from two phase III trials in HIV-infected patients. Abstract book of the National Conference on Women and HIV; Los Angeles. 1997. (Abstract 1298) [Google Scholar]
- 18.Sabo JP, Lamson MJ, Leitz G, Yong C, MacGregor TR. [accessed on May 22, 2008];AAPS PharmSci. 2002 :2. doi: 10.1208/ps020101. Available at: http://www.aapspharmsci.org. [DOI] [PMC free article] [PubMed]
- 19.Moore KH, Yuen GJ, Raasch RH, Eron JJ, Martin D, Mydlow PK, et al. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin Pharmacol Ther. 1996;59:550–8. doi: 10.1016/S0009-9236(96)90183-6. [DOI] [PubMed] [Google Scholar]
- 20.Kaul S, Christofalo B, Raymond RH, Stewart MB, Macleod CM. Effect of food on the bioavailability of stavudine in subjects with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1998;42:2295–8. doi: 10.1128/aac.42.9.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piscitelli SC, Kelly G, Walker RE, Kovacs J, Falloon J, Davey RT, Jr, et al. A multiple drug interaction study of stavudine with agents for opportunistic infections in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1999;43:647–50. doi: 10.1128/aac.43.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rana KZ, Dudley MN. Clinical pharmacokinetics of stavudine. Clin Pharmacokinet. 1997;33:276–84. doi: 10.2165/00003088-199733040-00003. [DOI] [PubMed] [Google Scholar]
- 23.Back D, Gatti G, Fletcher C, Garraffo R, Haubrich R, Hoetelmans R, et al. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS. 2002;16(Suppl 1):S5–37. doi: 10.1097/00002030-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 24. [accessed on April 24, 2008.];NACO guidelines on ART for adults and adolescents. 2007 Available from: www.nacoonline.org.
- 25.World Health Organization. [accessed on April 15, 2008];Antiretroviral therapy for HIV infection in adults and adolescents: recommendation for a public health approach. 2006 Available at http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]