Abstract
Objective
To estimate the effects of using depot medroxyprogesterone acetate (DMPA) or oral contraceptives (OC) containing 20 micrograms ethinyl estradiol and 0.15 mg desogestrel on serum lipid levels.
Methods
Serum lipids were measured at baseline and every 6 months thereafter for 3 years on 703 white, black, and Hispanic women using DMPA, OC, or nonhormonal (NH) birth control. DMPA discontinuers were followed for up to 2 additional years.
Participants completed questionnaires containing demographic and behavioral measures every 6 months and underwent 24 hour dietary recalls annually. Mixed model regression analyses and general estimating equations procedures were used to estimate changes over time in lipids by method, along with their predictors.
Results
OC users experienced significantly greater increases in levels of triglycerides (TG), total cholesterol (TC), very low density lipoprotein cholesterol (VLDL-C), and high density lipoprotein cholesterol (HDL-C) than NH users (P<.001). However, no difference was noted in the LDL-C to HDL-C ratio between OC and NH users. Among DMPA users, HDL-C levels initially decreased for 6 months, but then returned to baseline. The LDL-C to HDL-C ratio rose during the first 6 months of DMPA use, but then dropped back to baseline over the next 24 months. After DMPA was discontinued, women who used OC increased their TG, TC, VLDL-C, and HDL-C levels significantly more than those who chose NH (P< .05).
Conclusion
Use of very low dose OC containing desogestrel can elevate lipid levels. DMPA users were at increased risk of developing an abnormally low HDL-C level as well as an abnormally high LDL level and an increase in the LDL to HDL cholesterol ratio, although these effects appeared to be temporary.
Although a number of investigations have been conducted on the relationship between depot medroxyprogesterone acetate (DMPA) and lipid levels, results are not consistent between studies. For example, some have demonstrated that DMPA does not effect serum lipids while others have shown an adverse relationship.1-4 Two others reported a beneficial effect.5,6 Findings from all these studies are limited because they are cross-sectional in design3,6-8 or have very small sample sizes.1,4,5,9-12 Only three large trials have been published which followed women for an extended period and they do not agree in their findings.13-15 Furthermore, none of these three studies took into account the effect of diet on lipid levels.
Moreover, data are limited on the effects of oral contraceptives (OC) containing only 20 micrograms ethinyl estradiol (EE) and 0.15 mg desogestrel on the lipid profile. This third generation progestin has been used in newer birth control pills because it is less androgenic and thus should have less effect on carbohydrate metabolism and lipid levels. It is not certain, however, whether this actually occurs with use of OC containing only 20 micrograms ethinyl estradiol (EE) and 0.15 mg desogestrel because only three longitudinal studies have included more than 30 women using this formulation 16-18 and none included a control group not using hormonal contraception. Furthermore, these studies are limited in their generalizability to non-white populations as they included very few of these women or did not conduct analyses by race/ethnicity.
The purpose of this study was to fill these gaps in the literature by estimating the effect of DMPA as well as OC containing 20 micrograms ethinyl estradiol (EE) and 0.15 mg desogestrel on serum lipid levels over 3 years of contraceptive use in non-Hispanic white, non-Hispanic black, and Hispanic women. In addition, we measured serum lipids after DMPA discontinuation to estimate if any observed changes were reversible after discontinuation of this method.
Methods
As part of a larger study, 805 non-Hispanic black, non-Hispanic white, and Hispanic women between 16 and 33 years of age were recruited between October 9, 2001, and September 14, 2004.19 Recruitment was conducted to achieve a sample that was balanced by age group (16–24 y and 25–33 y), race/ethnicity (white, black, Hispanic), and contraceptive method (OC, DMPA, nonhormonal). This 3 × 2 × 3 grid depicted target sample sizes within each of 18 “cells” that were based on the overall sample size. Once the targeted number was achieved in a given cell, the cell was essentially closed to recruitment. All women underwent eligibility screening including a medical interview, anthropometry, and fasting phlebotomy during the follicular phase of their menstrual cycle. Criteria for exclusion included current pregnancy or breastfeeding; pregnancy planned within the next 3 years; use of DMPA within the past 6 months; use of OCs within the past 3 months; current use of hormonal intrauterine device; contraindication to hormonal contraception; lack of menses for >3 months within the past year; bilateral oopherectomy; use of over-the-counter phytoestrogen supplements; dietary isoflavone intake exceeding 84 mg/d; use of glucocorticoid; illness or medication known to affect BMD; eating disorder; and strict vegetarian diet. No participants were using statins.
Of 2,999 women who responded to advertisements, 1,404 met general inclusion criteria and matched an “open” recruitment cell (age group × race × contraceptive method). Of these, 805 women provided written consent (and parental consent if <18 years of age) to undergo further screening for the larger study. Of these, 5 withdrew prior to completing their first visit and 97 had abnormal laboratory or bone scan results. Thus, 703 women were invited to participate in the longitudinal study. Those excluded (n=102) did not differ from women included in the longitudinal study (n=703) on age, marital status, parity, or education (all P >.05).
Following counseling, women were allowed to select one of three types of birth control: 245 chose OC (0.15 mg desogestrel + 20 micrograms ethinyl estradiol taken for 21 days, followed by 2 days of placebo and 5 days of 10 micrograms ethinyl estradiol); 240 chose DMPA; and 218 chose nonhormonal contraception (NH). Contraception was dispensed every 3 months. At baseline and every 6 months thereafter, women were weighed wearing light indoor clothing with a digital scale accurate to the nearest 0.1 kg, and height was measured using a wall-mounted stadiometer (Heightronic, Snoqualmie, WA) accurate to the nearest 0.001 m. To obtain estimates on daily calorie intake along with amount of protein, fat, and carbohydrate consumed, a registered dietician conducted a 24-hour dietary recall interview with each participant at baseline and every 12 months thereafter at a scheduled visit. Nutrient calculations were performed using the Nutrition Data System for Research (NDS-R) software, version 4.05 (Nutrition Coordinating Center, University of Minnesota, Minneapolis). 20 All participants received free well-woman care and contraception during the study as well as monetary compensation. Those who did not return for scheduled visits were reminded by phone and certified letters.
Serum lipids were measured at baseline and every 6 months thereafter. All blood samples were collected between 7:00 AM and 10:00 AM following an overnight fast. Assays were performed on the VITROS 5,1 FS Chemistry System (Ortho-Clinical Diagnostics, Raritan, NJ) using the manufacturer’s reagents and calibrators. The triglycerides (TG) and total cholesterol (TC) tests were performed using the VITROS TRIG and CHOL slides, respectively, along with the VITROS chemistry products calibrator kit 2. Each slide analyzed 5.5 microliter of serum using a multilayered, analytical element coated on a polyester support with colorimetric detection and analysis based upon an enzymatic method. Direct high density lipoprotein cholesterol (HDL-C) was measured utilizing the VITROS dHDL slide with the VITROS chemistry products calibrator kit 25. Each slide utilized 10 microliter of serum and employed a colorimetric test on a multilayered analytical element coated on a polyester support. Analyses are based upon a non HDL-C precipitation method followed by enzymatic detection. Coefficients of variation for TG, TC, and direct HDL-C were 0.9%, 1.5%, and 3%, respectively, and were determined using a single lot of reagents calibrated weekly. Indirect lipid measures for low density lipoprotein cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) were calculated based upon values obtained for TC and direct HDL-C. Samples were assayed in batches (total of 37) at the University of Texas Medical Branch Laboratory between December 2007 and June 2008 after the study was completed.
Participants also completed a written questionnaire containing demographic and behavioral measures. Demographic information obtained included age, race/ethnicity, marital status, education, income, and parity. Behavioral measures included prior breastfeeding and hormonal contraceptive use, smoking, alcohol use, and physical activity. Tobacco use was measured with questions from the MONICA Smoking Assessment.21 For analytic purposes, current smokers were those who reported regular or occasional smoking while nonsmokers were those who currently did not smoke. Alcohol use was calculated from questions on the Diet History Questionnaire regarding how often subjects drank alcohol (either beer, wine or wine coolers, or liquor or mixed drinks) during the past 12 months and the amount usually consumed when drinking.22 Weight bearing physical activity was taken from a measure that included a list of 56 common activities, and questions on the frequency and duration of up to two physical activities performed during the past month.
Data were available on 186 women for the entire 36 months of study duration. Overall, 137 women discontinued the study because they desired a different contraceptive method, 257 women were lost to follow up, and 123 women did not complete the study due to other reasons. Overall, study discontinuation after 36 months was not statistically different between the contraceptive methods (77% in NH, 76% in DMPA and 68% in OC users).
We examined whether abnormal lipid levels at baseline were related to attrition over the 36 months of study duration. OC users who declined to participate in the continuation portion of the study at 18 and 24 months had higher abnormal triglyceride levels (triglyceride >170 mg/dL) at baseline compared to those who did continue (11% vs. 4% at both occasions, P<.05). Similarly, NH users who discontinued this portion of the study at 12 and 18 months had higher abnormal baseline LDL levels (≥ 160 mg/dL) compared to study continuers, 11% vs. 4% (P<.05), and 10% vs. 4% (P=.066), respectively. However, the opposite was true for DMPA users with regards to levels of HDL-C (≤ 35 mg/dL) and the LDL-C to HDL-C ratio (>3.5). DMPA users who discontinued the follow up portion at 18 months had lower abnormal HDL levels at baseline (12% vs. 22%, P<.05) while those who did so at 18 and 30 months had lower abnormal LDL-C to HDL-C ratios (18% vs. 8% and 21% vs.10% respectively; P<.05).
Of the 240 initial DMPA users, 182 discontinued this method, 68 of whom remained in the study for up to 2 additional years to participate in the reversibility portion of the study. There were no differences in baseline characteristics between DMPA users who remained in the study (n=68) and those who did not (n=114) with regard to age, race/ethnicity, height, weight, age at menarche, lifestyle variables, and calcium intake (P>.05). Of the 68 women who were followed after DMPA discontinuation, 44 began OC and were given the same formulation used in the study, while the remaining 24 chose NH. All procedures were approved by the Institutional Review Board of The University of Texas Medical Branch.
Statistical Analysis
One-way analysis of variance with Bonferroni correction for continuous variables and chi-square test for categorical variables were performed to compare the three contraceptive groups at baseline. We used longitudinal analyses to compare changes in serum lipid levels for each contraceptive method, along with their predictors over time. To accommodate the repeated measurements, the data were modeled with the use of a mixed effects regression procedure (xtmixed module; Stata Corporation, College Station, TX), which allowed us to obtain regression coefficients for various predictors while adjusting for the estimated errors for the repeated measurements. This class of model also allows inclusion of time-dependent covariates and accommodates subjects with incomplete data because of variation in number and spacing in observations over the period of follow-up, which frequently occurs in longitudinal studies. The primary outcomes were serum lipid levels and the LDL-C to HDL-C ratio. To examine the overall effect of method, race, and time, our models included contraceptive method (OC/DMPA/NH), race/ethnicity, and duration of contraceptive use (time) as main effects after adjusting for other covariates. Interaction terms (method x race/ethnicity; method x time) were then included in the model. Age, age at menarche, parity, previous use of pills and DMPA, and lifestyle variables (smoking, alcohol use and physical activity) were included as fixed covariates. The effect of socioeconomic variables (such as income, education, marital status, and previous breastfeeding) was also examined and retained if found to be statistically significant. Similar mixed models were also constructed to estimate the changes in serum lipids and the LDL-C to HDL-C ratio after discontinuation of DMPA. Separate mixed models were constructed to examine the effect of appetite change, and daily intake of protein, fat, carbohydrate, and total calories (based on 12-month follow-up data) on serum lipid levels and the LDL-C to HDL-C ratio.
Generalized estimating equations models 23 were also constructed to examine the risk of abnormal serum lipid levels and the LDL-C to HDL-C ratio by contraceptive method after adjusting for baseline status. This method can be considered a linear regression technique, which models repeated effects for individual study subjects over time. Each model allowed us to estimate odds ratios (OR) for the predictors while adjusting for the estimated errors due to repeated measurements. All analyses were performed using Stata 10 (Stata Corporation).
Results
The mean age of the entire sample was 24 ± 5 years. Twenty-nine percent of the sample (n = 200) was non-Hispanic black, 36% (n = 256) were Hispanic (predominately Mexican American), and 35% (n = 247) were non-Hispanic white. The number of women in each racial/ethnic group and in each age category (16-24 y and 25-33 y) did not significantly differ by contraceptive method (Table 1). Furthermore, there were no significant differences between contraceptive groups in baseline height, weight, body mass index, total fat mass, percent body fat, age at menarche, previous use of birth control pills, or amount of alcohol use or weight bearing exercise (Table 1). NH users did have a higher mean parity, OC users were less likely to have used DMPA in the past, and DMPA users were more likely to smoke. Furthermore, DMPA users had higher serum levels of TC and LDL-C than NH users at baseline. The LDL-C to HDL-C ratio was lower among women who used hormonal contraception than NH users as well. These baseline differences were controlled in the multivariable models.
Table 1.
Sample characteristics according to contraceptive selected at baseline
| Characteristic | OC (n = 245) |
DMPA (n = 240) |
NH (n = 218) |
|---|---|---|---|
| Number of study subjects (%) | |||
| Age, % | |||
| 16–24 y | 54.3 | 56.7 | 45.9 |
| 25–33 y | 45.7 | 43.3 | 54.1 |
| Race % | |||
| Black | 29.8 | 30.0 | 25.2 |
| White | 33.5 | 34.2 | 38.1 |
| Hispanic | 36.7 | 35.8 | 36.7 |
| Current smoker | 57 (23.3)§ | 87 (36.3)† | 48 (22.0) |
| Mean ± SD | |||
| Weight, kg | 73.3 ± 17.7 | 71.8 ± 19.2 | 73.2 ± 18.6 |
| BMI, kg/m2 | 27.9 ± 6.4 | 27.2 ± 6.9 | 28.3 ± 7.0 |
| Fat Mass, kg | 27.0 ± 11.2 | 25.6 ± 12.1 | 27.2 ± 11.7 |
| Fat Mass, % of total | 36.4 ± 7.4 | 34.8 ± 8.4 | 36.7 ± 7.5 |
| Age at menarche, y | 12.2 ± 1.5 | 12.5 ± 1.7 | 12.2 ± 1.6 |
| Previous use of pill (months) | 21.9 ± 31.8 | 16.9 ± 29.6 | 17.7 ± 28.6 |
| Previous use of DMPA injection (#)† | 1.4 ± 3.5‡ | 3.5 ± 6.5§ | 2.6 ± 5.2 |
| Alcohol use, gm/day | 1.6 ± 8.7 | 1.2 ± 6.1 | 2.3 ± 14.5 |
| Physical activity, min/week | 115 ± 119§ | 85 ± 92 | 94 ± 108 |
| Parity | 0.9 ± 1.1‡ | 1.1 ± 1.2† | 1.6 ± 1.5 |
| Total Cholesterol, mg/dl | 173 ± 33 | 169 ± 29† | 177 ± 32 |
| LDL Cholesterol, mg/dl | 107 ± 28 | 104 ± 26 † | 113 ± 29 |
| HDL Cholesterol, mg/dl | 47 ± 11 | 45 ± 11 | 45 ± 12 |
| VLDL Cholesterol, mg/dl | 19 ± 13 | 20 ± 13 | 20 ± 12 |
| LDL to HDL Ratio | 2.4 ± 0.8 | 2.4 ± 0.9 | 2.6 ± 0.9†‡ |
| Serum Triglyceride, mg/dl | 94 ± 65 | 98 ± 64 | 100 ± 60 |
OC = oral contraceptive; DMPA = depot medroxyprogesterone acetate; NH = nonhormonal contraception; BMI = body mass index; SD = standard deviation
One-way analysis of variance with Bonferroni correction was used for continuous variables and chi-square tests were used for categorical variables. To identify specific pairwise differences for categorical variables, we created separate 2×2 tables for each of the pairs and used chi square tests. To adjust for multiple comparison, P<.017 (.05/3) was used to indicate the statistical significance between any two contraceptive groups.
Significant difference was found between DMPA and nonhormonal contraception after Bonferroni adjustment
Significant difference was found between OC and nonhormonal contraception after bonferroni adjustment
Significant difference was found between OC and DMPA after bonferroni adjustment
Over the 3 years of study duration, OC users experienced increases in levels of TG, TC, VLDL-C, and HDL-C which were greater than those experienced by NH users (p < .001; Figure 1). The patterns differed somewhat by the type of assay. TG, VLDL-C, and HDL-C all steadily increased over the 36 months of study duration, although the greatest increase occurred during the first 6 months of OC use. TC levels increased during the first 6 months of OC use, but then leveled off. In contrast, NH users exhibited little change in their TG and TC levels over the 36 months. The LDL-C levels of OC users increased initially from 107 to 112 mg/dl between baseline and 6 months but then fell to 104 mg/dl by 36 months. This change differed from those observed in LDL-C levels among NH users who experienced a large decrease during the same time period (113 to 100 mg/dl; P<.002). Over the 3 years, OC users were more likely than NH users to demonstrate abnormal levels of TG, TC, and LDL-C (Table 2). No differences were observed between DMPA and NH users in TG, TC, LDL-C, or VLDL-C levels.
Figure 1.
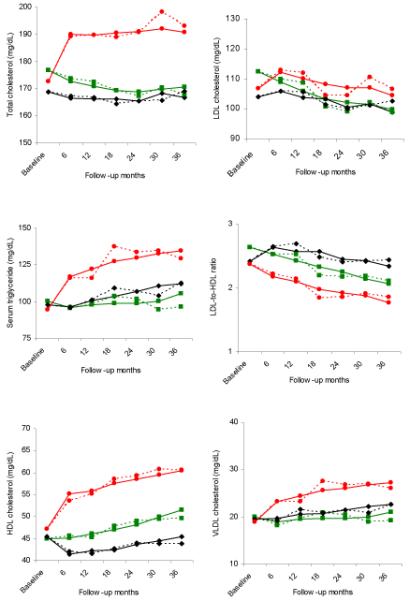
Mean change across 36 months of follow-up by contraceptive method for (A) total cholesterol, (B) serum triglyceride, (C) HDL cholesterol, (D) LDL cholesterol, (E) LDL-to-HDL ratio, and (F) VLDL Cholesterol. Solid lines represent the estimated mean (modeled) and dotted lines represent the unadjusted values. Green squares: nonhormonal contraception; black diamonds: depot medroxyprogesterone acetate; red circles: oral contraceptives.
Table 2.
Odds ratios (95% confidence intervals) of developing abnormal serum lipid level by different contraceptive methods over 3 years: Generalized estimating equations model results (n =703)
| Serum Lipid | Nonhormonal | OC | DMPA |
|---|---|---|---|
| Total Cholesterol >200 mg/dl | 1.00 | 2.69 (1.62-4.47) | 0.60 (0.35-1.04) |
| Serum Triglyceride >170 mg/dl | 1.00 | 3.18 (1.51-6.71) | 1.43 (0.70-2.95) |
| HDL Cholesterol ≤ 35 mg/dl | 1.00 | 0.16 (0.07-0.39) | 2.51 (1.48-4.28) |
| LDL Cholesterol ≥ 160 mg/dl | 1.00 | 4.30 (1.63-11.32) | 2.90 (1.05-8.00) |
| LDL to HDL Ratio >3.5 | 1.00 | 0.66 (0.29-1.52) | 2.41 (1.18-4.92) |
A separate generalized estimating equations model used for each of the serum lipids and ratio listed above
Adjusted by age, race, parity, physical activity, smoking, previous use of DMPA and percent body fat.
With regards to changes noted among DMPA users, mean HDL-C levels dropped from 45 mg/dl at baseline to 41 mg/dl at the 6 month visit but then steadily rose over the remainder of the follow up period. By 36 months, the level had increased to its baseline value. NH users demonstrated an increase of 45 to 51 mg/dl over 36 months, which was different when compared to DMPA and OC users (P < .001). Furthermore, DMPA users were 2.5 times more likely than NH users to demonstrate a level of HDL-C ≤ 35 mg/dl over the 36 month of study period. In contrast, OC use was protective against developing an abnormally low HDL-C level (Table 2)
Calculation of the LDL-C to HDL-C ratio demonstrated a decrease in all three contraceptive groups over 36 months. DMPA users demonstrated an initial increase in this ratio at 6 months (2.4 to 2.6 mg/dl) followed by a drop back to baseline over the next 18-24 months. By the 36 month visit, the ratio had dropped further to 2.3 mg/dl. This decrease was less than that observed among NH users over the 36 month interval (2.6 to 2.1 mg/dl; P < .001). Overall, those who chose DMPA were 2.4 times more likely than NH users to demonstrate an abnormally high ratio of >3.5 over the 36 months. In contrast, OC users experienced a greater decrease in their LDL-C to HDL-C ratio than NH users (P<.001).
Percent body fat and race both affected the lipid profile regardless of contraceptive method. Whites and Hispanics had higher levels of TG, TC, LDL, and VLDL and lower HDL levels than black women. Body fat also had an adverse effect on the lipid profile. Irrespective of method, increased parity had a beneficial effect on TC and LDL, but an adverse effect on HDL. Women with abnormal levels at baseline were not at increased risk of adverse changes to their lipid profile as a result of using these contraceptives. Changes in appetite, total caloric intake and amount of protein, fat, and carbohydrate consumed per day did not predict any changes in serum lipid level or LDL-C to HDL-C ratio.
Multivariable analyses were performed on both the entire sample (n=703) and only those who completed all 36 months of follow-up (n=186). The results did not significantly differ when analyses were conducted using only the 186 completers as compared to the entire sample (data not shown).
After DMPA was discontinued, women who used OC increased their TG, TC, VLDL-C, and HDL-C levels more than those who chose NH contraception (P < .05; Figure 2). The patterns of those who switched from DMPA to OC were very similar to those observed among OC users during the continuation portion of the study. No differences were observed between OC and NH users after DMPA discontinuation in the LDL-C to HDL-C ratio.
Figure 2.
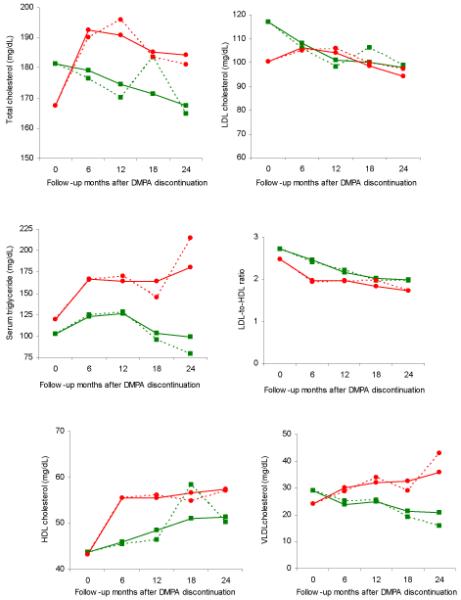
Mean change of lipid profile after depot medroxyprogesterone acetate discontinuation across 24 months for (A) total cholesterol, (B) serum triglyceride, (C) HDL cholesterol, (D) LDL cholesterol, (E) LDL-to-HDL ratio, and (F) VLDL Cholesterol. Solid lines represent the estimated mean (modeled) and dotted lines represent the unadjusted values. Green squares: depot medroxyprogesterone acetate to nonhormonal contraception; red circle: depot medroxyprogesterone acetate to oral contraceptives.
Discussion
Third generation birth control pills containing norgestimate, desogestrel or gestodene were designed to minimize adverse effects associated with the androgenic activity of oral contraceptives, including unfavorable effects on lipid metabolism. Since their introduction, a number of studies have confirmed that their effects on lipids are indeed less than those observed with first and second generation birth control pills. 24,25 In fact, a comprehensive review of 23 studies on OC containing desogestrel reported that total cholesterol changes resulting from use of these pills were small.26 Our findings differed somewhat as we observed an increase in TC of 20 mg/dl after 36 months of use. This variation in findings between our study and those published previously may be explained by differences in methodology. For example, prior studies have not controlled for the time of the menstrual cycle in which lipids were measured as we did. This is problematic as LDL-C can decline as much as 10-15 mg/dl during the cycle.27 Also, we required a 3 month wash out period in which no hormonal contraception was used. This is in contrast to prior studies in which the length of time that women were not exposed to hormonal contraception may not have been adequate to assure that the effect measured was not from prior drug use. Finally, prior studies often compared women using these birth control pills to those using another formulation rather than to a control group using non hormonal contraception as we did. These improvements in study methods allowed us to elucidate that use of OC containing desogestrel can elevate total cholesterol levels.
However, this rise in total cholesterol may not indicate an increased risk of atherosclerosis as we also observed some positive changes among OC users. Our finding that LDL-C improved by approximately 2% overall is similar to that reported by the above review article of OCs containing desogestrel. Of interest, we noted an even greater drop in LDL-C among NH users. The reason for this decrease among women using NH contraception is unclear, but we do know that among women using NH contraception, there was more attrition among those with abnormal LDL-C than normal levels at 12 and 18 months. This did not occur among OC and DMPA users with regards to LDL-C levels and thus may explain our findings.
Similar to prior reports on third generation pills, 26,28,29 we observed a steady rise over time in HDL-C levels among OC users. This is due to suppression of hepatic lipase by ethinyl estradiol which slows the transport to the liver of HDL-bound cholesterol. Desogesterel is less androgenic than first and second generation progestins, so it does not counteract the estrogen effects. As a result, the LDL to HDL cholesterol ratio was lower in OC than NH users over the 36 months of study duration. This is an important finding as the LDL-C to HDL-C ratio has been reported to have more prognostic value than either value alone and is especially accurate for predicting CHD risk among those with elevated TG levels.30 Thus, the changes we observed in the lipid profile with use of this OC may not contribute to an increased risk of atherosclerosis.
With regard to triglycerides, we detected a 43% rise over 36 months among OC users. This is consistent with prior reports that ethinyl estradiol, the estrogen component of the OC we studied, increases the secretion of TG by the liver. This is not a concern, however, as it has been shown that a rise in estrogen-induced triglycerides does not increase the risk of CHD when accompanied by a rise in HDL-C.31
In contrast, we observed that DMPA users were at increased risk of developing an abnormally low HDL-C level as well as an abnormally high LDL level and an increase in the LDL to HDL cholesterol ratio. In fact, DMPA users were 2-3 times more likely than NH users to develop abnormal values at some point during the 36 months of study period. However, these adverse effects on serum lipids were temporary and levels improved over time even if DMPA was continued. .
This study has several limitations. First, we did not randomly assign women to a contraceptive method because the three types under study have different efficacies and randomization could have led to unintended pregnancies. Second, data were not available to examine the effects of OC discontinuation on serum lipids. Third, discontinuation rates for all contraceptive methods were high. However, this is common in contraceptive studies as there are many reasons women may choose to change or discontinue their method.32-34 Fourth, too few women were followed after DMPA discontinuation to stratify our analysis by important variables. Finally, we studied only one formulation of OC, so our findings cannot be generalized to other types of birth control pills with different amounts of estrogen or other progestins. Together, these limitations could impact the overall generalizability of our findings, and selection bias cannot be ruled out.
Overall, we concluded that any adverse effects of DMPA we observed on the lipid profile were temporary and reversible. Consistent with our findings, an international, multicenter, case-control study on hormonal contraception conducted by WHO concluded that there was little or no increased risk of CVD among current users of injectable contraceptives.35 However, one small study did observe arterial impairment using magnetic resonance imaging among women using DMPA more than 1 year which suggests that long term use could increase the risk of CVD.36 In addition, a prior study by our research team demonstrated that DMPA may increase the risk by increasing the percent body fat and truncal obesity.37 Additional studies with larger sample sizes are needed to clarify this issue. When counseling women about contraception, efficacy rates and ease of use also must be considered as these may be more critical issues in many cases. In all cases, individualized counseling will determine the best method for each patient.
Precis.
Very low dose oral contraceptives containing desogestrel elevate high density and very low density cholesterol values while changes associated with depot medroxyprogesterone acetate are short-lived.
Acknowledgments
This project was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) (R01HD039883, Berenson), a Midcareer Investigator Award In Patient-Oriented Research Award (K24HD043659, Berenson) and General Clinical Research Centers program (M01RR00073), National Center for Research Resources, National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH.
References
- 1.Fahmy K, Khairy M, Allam G, Gobran F, Alloush M. Effect of depo-medroxyprogesterone acetate on coagulation factors and serum lipids in Egyptian women. Contraception. 1991;44:431–44. doi: 10.1016/0010-7824(91)90033-c. [DOI] [PubMed] [Google Scholar]
- 2.Mia AR, Siddiqui NI, Islam MN, Khan MR, Shampa SS, Rukunuzzaman M. Effects of prolonged use of injectable hormonal contraceptive on serum lipid profile. Mymensingh Med J. 2005;14:19–21. [PubMed] [Google Scholar]
- 3.WHO Task Force on Long-acting Systemic Agents for Fertility Regulation A multicentre comparative study of serum lipids and apolipoproteins in long-term users of DMPA and a control group of IUD users. Contraception. 1993;47:177–91. doi: 10.1016/0010-7824(93)90090-t. [DOI] [PubMed] [Google Scholar]
- 4.Oyelola OO. Fasting plasma lipids, lipoproteins and apolipoproteins in Nigerian women using combined oral and progestin-only injectable contraceptives. Contraception. 1993;47:445–54. doi: 10.1016/0010-7824(93)90096-p. [DOI] [PubMed] [Google Scholar]
- 5.Tankeyoon M, Dusitsin N, Poshyachinda V, Larsson-Cohn U. A study of glucose tolerance, serum transaminase and lipids in women using depot-medroxyprogesterone acetate and a combination-type oral contraceptive. Contraception. 1976;14:199–214. doi: 10.1016/0010-7824(76)90088-3. [DOI] [PubMed] [Google Scholar]
- 6.Lizarelli PM, Martins WP, Vieira CS, Soares GM, Franceschini SA, Ferriani RA, Patta MC. Both a combined oral contraceptive and depot medroxyprogesterone acetate impair endothelial function in young women. Contraception. 2009;79:35–40. doi: 10.1016/j.contraception.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Virutamasen P, Wongsrichanalai C, Tangkeo P, Nitichai Y, Rienprayoon D. Metabolic effects of depot-medroxyprogesterone acetate on long-term users: a cross-sectional study. Int J Gynaecol Obstet. 1986;24:291–96. doi: 10.1016/0020-7292(86)90086-x. [DOI] [PubMed] [Google Scholar]
- 8.Fajumi JO. Alterations in blood lipids and side effects induced by DepoProvera in Nigerian women. Contraception. 1983;27:161–75. doi: 10.1016/0010-7824(83)90087-2. [DOI] [PubMed] [Google Scholar]
- 9.Teegarden D, Proulx WR, Martin BR, et al. Peak bone mass in young women. J Bone Miner Res. 1995;10:711–15. doi: 10.1002/jbmr.5650100507. [DOI] [PubMed] [Google Scholar]
- 10.Huovinen K, Tikkanen MJ, Autio S, et al. Serum lipids and lipoproteins during therapeutic amenorrhea induced by lynestrenol and depot-medroxyprogesterone acetate. Acta Obstet Gynecol Scand. 1991;70:349–54. doi: 10.3109/00016349109007886. [DOI] [PubMed] [Google Scholar]
- 11.Garza-Flores J, De la Cruz DL, Valles de Bourges V, et al. Long-term effects of depot medroxyprogesterone acetate on lipoprotein metabolism. Contraception. 1991;44:61–71. doi: 10.1016/0010-7824(91)90106-p. [DOI] [PubMed] [Google Scholar]
- 12.Amatayakul K, Sivassomboon B, Singkamani R. Effects of medroxyprogesterone acetate on serum lipids, protein, glucose tolerance and liver function in Thai women. Contraception. 1980;21:283–97. doi: 10.1016/0010-7824(80)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Grossman RA, Asawasena W, Chalpati S, Taetwong D, Tovanabutra S. Effects of the injectable contraceptive depot medroxyprogesterone acetate in Thai women with liver fluke infestation: final results. Bulletin of the World Health Organization. 1979;57:829–37. [PMC free article] [PubMed] [Google Scholar]
- 14.Liew DFM, Ng CS, Yong YM, Ratnam SS. Long-term effects of Depo-Provera on carbohydrate and lipid metabolism. Contraception. 1985;31:51–64. doi: 10.1016/0010-7824(85)90074-5. [DOI] [PubMed] [Google Scholar]
- 15.Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception. 2006;74:90–9. doi: 10.1016/j.contraception.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Berga SL. Metabolic and endocrine effects of the desogestrel-containing oral contraceptive Mircette. Am J Obstet Gynecol. 1998;179:S9–17. doi: 10.1016/s0002-9378(98)70291-1. [DOI] [PubMed] [Google Scholar]
- 17.Akerlund M. Clinical experience of a combined oral contraceptive with very low dose ethinyl estradiol. Acta Obstet Gynecol Scand Suppl. 1997;164:63–5. [PubMed] [Google Scholar]
- 18.Teichmann A. Metabolic profile of six oral contraceptives containing norgestimate, gestodene, and desogestrel. Int J Fertil Menopausal Stud. 1995;40:98–104. [PubMed] [Google Scholar]
- 19.Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–99. doi: 10.1097/AOG.0b013e3181875b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–71. [PubMed] [Google Scholar]
- 21.World Health Organization . Smoking Questionnaire. MONICA Manual (1998-1999), Part III, Section 1. The WHO MONICA (Multinational Monitoring Trends in Cardiovascular Disease) Project; 1999. [Google Scholar]
- 22.National Cancer Institute . Diet History Questionnaire (DHQ) National Institutes of Health; 2000. [Google Scholar]
- 23.Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 24.London RS. The new era in oral contraception: pills containing gestodene, norgestimate, and desogestrel. Obstet Gynecol Surv. 1992;47:777–82. doi: 10.1097/00006254-199211000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan B. Desogestrel, norgestimate, and gestodene: the newer progestins. Ann Pharmacother. 1995;29:736–42. doi: 10.1177/106002809502907-817. [DOI] [PubMed] [Google Scholar]
- 26.Speroff L, DeCherney A. Evaluation of a new generation of oral contraceptives. Obstet Gynecol. 1993;81:1034–47. [PubMed] [Google Scholar]
- 27.Knopp RH. Cardiovascular effects of endogenous and exogenous sex hormones over a woman’s lifetime. Am J Obstet Gynecol. 1988;158(suppl):1630–43. doi: 10.1016/0002-9378(88)90201-3. [DOI] [PubMed] [Google Scholar]
- 28.Cagnacci A, Ferrari S, Tirelli A, Zanin R, Volpe A. Insulin sensitivity and lipid metabolism with oral contraceptives containing chlormadinone acetate or desogestrel: a randomized trial. Contraception. 2009;79:111–6. doi: 10.1016/j.contraception.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Klipping C, Marr J. Effects of two combined oral contraceptives containing ethinyl estradiol 20 microg combined with either drospirenone or desogestrel on lipids, hemostatic parameters and carbohydrate metabolism. Contraception. 2005;71:409–16. doi: 10.1016/j.contraception.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez ML, Webb D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J Am Coll Nutr. 2008;27:1–5. doi: 10.1080/07315724.2008.10719668. [DOI] [PubMed] [Google Scholar]
- 31.NIH Consensus Development Panel on Triglyceride, High-Density Lipoprotein, and Coronary Heart Disease NIH Consensus conference. Triglyceride, high-density lipoprotein, and coronary heart disease. JAMA. 1993;269:505–10. [PubMed] [Google Scholar]
- 32.Berenson AB, Odom SD, Radecki Breitkopf C, Rahman M. Physiologic and psychologic symptoms associated with use of injectable contraception and 20 microgram oral contraceptive pills. Am J Obstet Gynecol. 2008;199:351.e1–e12. doi: 10.1016/j.ajog.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy PA, Brixner D. Hormonal contraceptive discontinuation patterns according to formulation: investigation of associations in an administrative claims database. Contraception. 2008;77:257–63. doi: 10.1016/j.contraception.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577–582. doi: 10.1016/s0002-9378(98)70047-x. [DOI] [PubMed] [Google Scholar]
- 35.WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives. Results of an international, multicenter, case-control study. Contraception. 1998;57:315–24. [PubMed] [Google Scholar]
- 36.Sorensen MB, Collins P, Ong PJL, Webb CM, Hayward CS, Asbury EA, et al. Long term use of contraceptive depot medroxyprogesterone acetate in young women impairs arterial endothelial function assessed by cardiovascular magnetic resonance. Circulation. 2002;106:1646–51. doi: 10.1161/01.cir.0000030940.73167.4e. [DOI] [PubMed] [Google Scholar]
- 37.Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009;200:329.e1–8. doi: 10.1016/j.ajog.2008.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


