Abstract
The basic science literature is replete with descriptions of naturally occurring or experimentally induced pathological grooming behaviors in animals, which are widely considered animal models of obsessive-compulsive disorder (OCD). These animal models rely largely on observed similarities between animal behaviors and human OCD behaviors, and on studies of animal pathological grooming disorders that respond to serotonin enhancing drugs. However, current limitations in assessment of complex cognition and affect in animals precludes the field’s ability to match the driving primary processes behind observable phenomenology in animal “OCD” with human behavioral disorders. We propose that excessive grooming behaviors in animals may eventually prove to be equally, or possibly more relevant to, other conditions in humans that involve pathological grooming or grooming-like behaviors, such as trichotillomania, body dysmorphic disorder, olfactory reference syndrome, compulsive skin-picking, and onychophagia. Research is needed to better understand pathological grooming behaviors in both humans and animals, as animal models have the potential to elucidate pathogenic mechanisms and inform the treatment of these psychiatric conditions in humans.
Introduction
Everyone seems to know someone who has a pet who supposedly has “OCD.” Usually, this refers to a dog that licks its paws excessively, i.e., has acral lick dermatitis, or to a cat that excessively grooms. Is this really the animal equivalent of obsessive-compulsive disorder (OCD)?
Excessive grooming behaviors in animals, either naturally occurring (veterinary) or experimentally induced, appear extensively in the basic science literature and are considered by many to be animal models of OCD1. For example, a study published in Nature described a genetically-engineered mouse line deficient for the SAPAP3 protein that exhibited pathological grooming behaviors2. These behaviors were reversed when the gene coding for the protein was replaced, and when the mice were treated with a serotonin-reuptake inhibitor. The authors suggested this as an animal model of OCD. Other naturally occurring examples of pathological grooming in animals, such as acral-lick dermatitis in canines, have been described as models of human OCD3. These excessive grooming behaviors in animals, which can cause hair loss and skin lesions and are associated with anxiety-like behaviors, do indeed have similarities to OCD symptoms such as repetitive hand washing. Such ethological models may aid in elucidating the etiology of human behavioral disorders.
One limitation of such models, however, is that the field currently lacks sophisticated means to assess thought patterns in animals, and we have only rudimentary ways of assessing affect. More specifically, evidence is lacking that these animals experience the equivalent of OCD-like obsessive thoughts that drive grooming behaviors. The presence and content of obsessional thinking is difficult to assess and therefore model in an animal. For example, it is difficult to know whether acral lick syndrome in dogs is driven by intent to clean the paws and remove germs, as is often the case when humans with OCD compulsively wash.
Alternatively, animal models have similar features to, and may possibly prove to be more analogous to, other human psychiatric conditions that more consistently manifest pathological grooming or grooming-like behaviors such as trichotillomania, onychophagia (compulsive nail biting), compulsive skin-picking, olfactory reference syndrome (ORS), or body dysmorphic disorder (BDD). These conditions appear to have a relationship to OCD, as variably evidenced by high rates of comorbidity, shared familial heredity, and shared phenomenological features of compulsive behaviors and (for some conditions) obsessive thoughts4–6. Unlike most cases of OCD, however, the primary behaviors of trichotillomania, onychophagia, and compulsive skin picking are excessive or distorted grooming rituals. In BDD, which consists of a distressing or impairing preoccupation with an imagined or slight defect in physical appearance, a majority of patients excessively groom – for example, excessively comb, pull, tweeze, or shave hair; or pick their skin. Similarly in ORS, a distressing or impairing preoccupation with the belief that one emits a foul or offensive body odor, “grooming” behaviors such as excessive showering and tooth brushing are common.
This paper provides a brief survey of pathological grooming from the human and animal literature. It also describes putative animal models of human pathological grooming, as these models, if they prove applicable, may help advance the neurobiological understanding of these often-disabling human conditions. We propose that many animal models of OCD should also be considered as possible models of pathological grooming and grooming-type behaviors in humans, which can occur in a number of psychiatric conditions other than OCD. Because these psychiatric conditions can be conceptualized as having a component of pathological grooming, we hope this article will stimulate the development and application of animal models that are more specific to understanding and treating these human conditions.
An Ethological Perspective
Psychiatry’s current diagnostic classification systems, DSM-IV-TR and ICD-10, are largely atheoretical. They are based primarily on the phenomenology of behavior, thought, and emotions rather than on disorders’ etiology or pathophysiology. As psychiatric conditions are complex, deriving etiological models is a difficult task. Attempts during the last century have tended to be too biologically reductionistic, too environmentally determined, or too individual-specific and therefore difficult to test or prove (e.g. psychodynamic theories). Yet advancing understanding of etiology may be necessary to develop more effective treatments and preventive measures. An ethological approach – one that aims to understand human behavior, emotion, and thought in the context of adaptation and survival of animals in general – has the potential to bridge biological and social models.
Psychiatric conditions characterized by pathological grooming may be particularly well suited to ethological models, due to their obvious derivation from adaptive behaviors7. Pathological grooming may be the result of a) excessive degrees of normal adaptive behavior or b) distortion of adaptive behaviors by some pathological process, (i.e., a quantitative or qualitative difference from normal behavior, or both).
Animal models have the potential to improve understanding of human behavior by providing opportunities to test hypotheses using techniques not feasible with humans. They may also help isolate and simplify observable behaviors. However, such simplification exposes a weakness of animal models, which is that emotions or thoughts behind these behaviors cannot be adequately assessed. For example, we infer fear from behaviors such as freezing or startle responses in rodents, but lack an understanding of whether there are anticipatory thoughts or emotions about future events, which are more analogous to human anxiety. Arguably, human behaviors may serve as better models for veterinary conditions. Such has been the case for pathological grooming in pets such as acral lick dermatitis, for which serotonin-reuptake inhibitors have been successfully tested and used in dogs, under the premise that the condition is analogous to human OCD8. Although difficult to assess, it is becoming increasingly possible, however, to demonstrate at least some forms of affect, thought, and even consciousness in animals9,10. Research that leads to better integration of affect and thought with behaviors will create better animal models of human conditions.
Purposes of grooming behaviors in animals
Grooming is highly ubiquitous in mammals, birds, and insects11 and thus is likely evolutionarily ancient. Grooming can serve various functions for the animal (Table 1). Overall, perhaps the most common purpose of grooming across species is to clean in order to maintain health, e.g., by removing detritus and disease-carrying parasites12. It may also help to attract mates, improve predation or avoidance of predators (via removal of odors), and improve mobility (e.g., grooming of feathers for flight)12. Other functions of grooming include thermoregulation, stimulation of pheromone release, self-stimulation, increasing or decreasing arousal, and decreasing irritation13. Many of these functions overlap within the same behavioral action.
Table 1.
Normal animal grooming behaviors and their purposes*
| Animal grooming behaviors | Purposes of behavior |
|---|---|
| Avian preening cleaning the feathers, applying oil from the preen gland, removing protective sheaths |
Maintain hygiene, thermoregulation, maintain organs of flight, attract mates12, 92 |
| Feline oral grooming stroking the tongue through the skin and hair, licking limbs and applying saliva to head |
Removal of parasites, dirt and excess oil; thermoregulation and maintaining insulating capacity of fur93 |
| Rodent oral grooming rhythmic paw and body licking |
Thermoregulation, chemocommunication, antibacterial (saliva), dearousal12 |
| Canine oral grooming rhythmic paw and body licking and chewing |
Removal of dirt and parasites, thermoregulation, dearousal, antibacterial (saliva)12, 94 |
Grooming behaviors are therefore clearly important for adaptation and survival. As such, they are preserved and may be based in phylogenetically ancient parts of the brain14. Gardner (1988) described grooming behaviors as examples of “fixed action patterns.”14 These are highly stereotyped and extremely similar between individuals. They may be elicited by outside stimulation but continue even after the stimulus is removed. They may even start spontaneously, without external stimulation. They are not learned, but at the same time are not as basic as reflexes15. Decerebrate animals can produce all the component actions involved in grooming, which provides evidence that the brainstem is necessary and sufficient to generate fixed action-patterns16, 17. Another region that may be involved in grooming is the cerebellum; Lurcher mice, which are characterized by degeneration of cerebellar Purkinje and granule cells, demonstrate decreased bouts of grooming yet maintain normal grooming sequences18. The neostriatum, specifically the anterior dorsolateral portion, appears necessary for the implementation of the serial order pattern of grooming, as evidenced by lesion studies in rodents16.
Pathological grooming in humans
Several psychiatric conditions in humans prominently feature pathological grooming or grooming-like behaviors. Pathological grooming may be: 1) One symptom of a DSM-IV-TR psychiatric disorder – i.e., BDD, OCD, or ORS; 2) Classified by DSM-IV-TR as its own disorder – i.e., trichotillomania; or 3) An “orphan” condition – i.e., compulsive skin-picking or onychophagia, which may be diagnosed as an impulse-control disorder not otherwise specified or perhaps as a type of stereotypic movement disorder19, 20. The nature of the pathological grooming behaviors in these disorders and conditions can sometimes be discriminated by the driving purpose underlying them (Table 2). These include avoidance of contamination, improvement of appearance or body odor, modulation of emotion or stress, or simply habit.
Table 2.
Human disorders or syndromes involving pathological grooming, and the putative driving forces behind the behaviors
| Human disorder or syndrome involving pathological grooming | Putative driving force(s) behind behaviors |
|---|---|
| obsessive-compulsive disorder (OCD) | obsessive thoughts Compulsive behaviors, such as hand- washing, are attempt to reduce distress or prevent some dreaded event or situation such as contamination21, 22 |
| body dysmorphic disorder (BDD) | appearance preoccupations and perceived appearance flaws30, 33 Excessive grooming behaviors in BDD are done in attempt to improve appearance |
| olfactory reference syndrome | perceived body odor41 Excessive showering, washing, or brushing of teeth are performed in attempt to diminish “odor” |
| trichotillomania | tension and aversive affective states45 |
| compulsive skin-picking and onychophagia | tension or aversive affective states49 |
Obsessive-compulsive disorder
OCD is characterized by recurrent obsessions and/or compulsions that cause marked distress, are time consuming, and/or interfere with daily functioning20. Obsessions are recurrent and persistent thoughts, impulses, or images that are experienced as intrusive and inappropriate and cause marked anxiety or distress. Compulsions are repetitive behaviors such as hand washing, ordering, checking, or mental acts that the person feels driven to perform usually in response to the obsession, which are aimed at preventing or reducing distress or preventing some dreaded event or situation. Most factor analytic studies have identified four primary subtypes or dimensions, of OCD: contamination obsessions with cleaning compulsions, aggressive/sexual/religious/somatic obsessions with checking compulsions, symmetry obsessions with ordering compulsions, and hoarding obsessions with collecting compulsions21, 22.
OCD is likely a heterogenous condition23 as the four subtypes suggest, and as neuroimaging studies have demonstrated24–27. Contamination fears with cleaning compulsions may be most analogous to pathological grooming in animals, in that the most common purpose of grooming in animals is to clean (e.g., remove detritus and parasites). Individuals with contamination fears may excessively wash their hands or other parts of their body as an attempt to relieve anxiety caused by conscious, explicit cognitions (obsessive in form) about contracting illness through dirt or germs. Occasionally, an intense feeling of disgust drives the behavior. The cleaning behaviors are usually repeated because of obsessional doubt or uncertainty that the action was complete and effective, or, alternatively, by a feeling of “incompleteness.” When extreme, repetitive washing may cause skin damage such as dermatitis or fissures. Whether animal models involving pathological grooming apply best to the OCD subtype of contamination fears with cleaning compulsions remains to be tested directly, however.
Thus, pathological grooming in animals may prove to be a reasonable animal model for some, but not all, forms of OCD. In the literature animal models of pathological grooming may be more widely linked to OCD2, 26 than to the other disorders and conditions simply because OCD is a better known disorder. However, these animal models may be additionally suited – or perhaps better suited – to these other human psychiatric disorders and conditions, all of which involve pathological grooming or grooming-like behaviors; for several, such behavior is the core feature of the disorder. It is worth noting that several lines of evidence suggest that these other disorders may be related to OCD [e.g., 33–35], supporting the concept of “obsessive-compulsive spectrum disorders.”
Body dysmorphic disorder (BDD)
Patients with BDD are preoccupied with perceived defects or flaws in their appearance, incorrectly believing they are disfigured or ugly, which causes significant suffering and functional impairment19. BDD affects 0.7%–2.4% of the general population27–29 and is associated with high rates of psychiatric hospitalization (48%)30 and suicide attempts (22–28%)30–32.
Pathological grooming behaviors in BDD are driven by appearance preoccupations and are attempts to improve perceived appearance flaws. 44% to 64% of individuals with BDD perform excessive compulsive grooming behaviors30, 33. Examples include excessive face washing to remediate “oily” skin, combing or cutting hair, brushing or whitening teeth, plucking hairs (e.g., to even-out eyebrows or remove “excessive” facial hair), and shaving. In addition, compulsive skin-picking occurs in 28%–35% of individuals with BDD and is done to try to improve the appearance or texture of the skin. In some cases, skin picking as a symptom of BDD causes severe tissue damage34. Occasionally, it is life-threatening35. Because BDD can involve any body part, the possibilities for grooming behaviors are numerous and protean.
As in OCD, these BDD behaviors are “compulsive” in that they are performed in response to a strong urge to relieve negative feelings (usually shame, dysphoria, or anxiety) triggered by preoccupations about perceived appearance flaws. The individual feels driven to perform the behaviors, which are repetitive and usually difficult to control.
BDD has many similarities to OCD, and BDD is often conceptualized as an “obsessive-compulsive spectrum disorder”36, 37. Perfectionism also appears to be a shared trait in OCD38 and BDD39, which may have relevance to pathological grooming. For example, individuals with BDD may be driven to repeat grooming procedures in attempts to achieve perfection in their appearance.
Animal models involving pathological grooming clearly seem relevant to BDD, especially when the purpose is to improve appearance and attract mates. Similar behaviors in BDD may be driven by the same evolutionarily-based desire. However, applying animal models to BDD has the same limitations as for OCD – namely, that we do not understand the cognitions and emotional states that drive compulsive grooming in animals and to what extent they map onto preoccupations in these disorders.
Olfactory Reference Syndrome
Individuals with ORS are preoccupied with the belief that they emit an unpleasant or offensive body odor, which they falsely believe other people can perceive. In DSM-IV-TR, ORS is a type of delusional disorder, somatic type, although it appears to have similarities with OCD and BDD40, 41. Commonly, preoccupations focus on perceived odors such as halitosis, sweat, and flatulence. Nearly all patients perform at least one excessive compulsive behavior in an attempt to diminish the perceived odor, and several could be considered grooming behaviors41. For example, many patients excessively shower, wash, or brush their teeth. Given that one function of grooming in animals is to remove odors to improve predation or avoid predators, animal models of pathological grooming behaviors may be relevant to ORS, although the same limitations apply to ORS as to OCD and BDD.
Trichotillomania
Trichotillomania is defined in DSM-IV-TR as recurrent pulling out of hair that results in noticeable hair loss20. The definition also includes a sense of tension immediately before pulling out the hair or when trying to resist it, and then pleasure, gratification, or release after pulling out the hair. DSM-IV-TR categorizes trichotillomania as an impulse control disorder. It has an estimated prevalence rate of 1–3% in community samples42. In some cases, hair pulling or plucking is a symptom of BDD (when hair is removed to try to improve appearance).
What drives this hair-pulling behavior in trichotillomania can vary between individuals and even within the same individual at different moments. Many (although not all43) patients feel tension before and gratification afterwards, which fits with the phenomenology of impulse control disorders and has lent itself to conceptualization of the disorder as involving an addictive process44. Unlike OCD, BDD, or ORS, the hair pulling is not driven by a distressing cognition. In general, trichotillomania appears to be maintained by virtue of its ability to effectively reduce aversive affective states45 such as boredom or under-stimulation, anxiety, or dysphoria. About 80–90% of individuals with trichotillomania have a comorbid psychiatric disorder, commonly anxiety or depressive disorders, BDD, or OCD5. This makes it difficult to ascertain if the trichotillomania is a means to modulate negative emotional states caused by these disorders or if they are otherwise connected indirectly, e.g., through shared endophenotypes and/or genetics.
While animal models might seem to best fit trichotillomania, it is unclear if this is actually the case. Trichotillomania is generally considered a problematic “habit,” whereas pathological grooming in animals appears to subserve various functions that may map more closely onto OCD, BDD, or ORS (i.e., cleansing/maintaining health, attracting a mate, or odor removal, respectively).
Compulsive Skin-picking and Onychophagia
Compulsive skin-picking (also known as dermatillomania or psychogenic excoriation) has appeared extensively in the dermatologic literature but is not classified as a separate disorder in DSM-IV-TR. It is estimated to occur in 2–4% of the population46. It usually involves attempts to remove irregularities in the skin’s surface5. Compulsive skin-picking can result in severe tissue damage47, and many individuals find the behavior hard to control. In some cases, skin picking is a symptom of BDD, as discussed above. Skin picking secondary to OCD has also been reported, in attempts to remove contaminants48. Alternatively, skin picking may serve to release tension or modulate negative affective states49, as in trichotillomania50.
Onychophagia, or nail-biting, is similar to skin-picking in that it is a repetitive behavior that can result in considerable tissue damage. In its nonpathological form it is a common grooming behavior that controls the length of the fingernails with the teeth. It becomes problematic when it is excessive and the sufferer has difficulty controlling it. It can cause significant damage to the skin of the fingers and sometimes the nail bed51. This behavior has notable similarities to acral lick dermatitis in dogs, in which the paws can be severely damaged.
Pathological grooming in animals
Examples of veterinary pathological grooming have been described in several species: acral-lick dermatitis in dogs, psychogenic alopecia in cats, and psychogenic feather picking in birds are most commonly compared to human disorders. Others include wool pulling and eating in sheep and self-mutilation in the horse and rabbit52. Similar to pathological grooming behaviors in humans, most veterinary cases are rooted in normal, healthy grooming practices which have become compulsive and excessive, often resulting in physical damage such as hair, wool, or feather loss and skin lesions.
Canine acral-lick dermatitis
Canine acral-lick dermatitis is a psychogenic disorder of dogs characterized by continuous licking and/or chewing of the legs and paws. In severe cases, it presents with alopecia, skin ulcers, and granulomatous lesions in affected regions13, 53. The compulsive licking may be initiated by medical conditions such as allergies or foreign bodies, but dogs susceptible to the disorder may continue to lick after the primary cause has been removed54. Stressors such as social isolation and confinement may contribute to the onset of the condition and to an increase in its intensity and frequency. It has been compared to OCD in humans because both conditions present with similar behaviors (paw-licking in canines, repetitive hand-washing in humans) and both respond to drugs that increase serotonin levels in the brain52. BDD has also been shown to respond to serotonin-reuptake inhibitors, whereas trichotillomania responds less consistently55, 56.
Feline psychogenic alopecia
Feline psychogenic alopecia is characterized by excessive self-grooming in the absence of a dermatological or medical cause57. Self-grooming in cats serves basic purposes such as cleansing, removal of parasites, and thermoregulation, but grooming can also occur as a displacement behavior or in response to social or environmental stressors. Psychogenic alopecia has been diagnostically conceptualized as “OCD,” along with other feline compulsive behaviors such as self-mutilation, tail-chasing, and wool or fabric sucking or chewing58.
Avian psychogenic feather picking
Psychogenic feather picking has been described mostly in psittacine birds (the family including parrots and macaws) and compared to trichotillomania in humans13. Similar to psychogenic grooming disorders in humans, it involves an excessive and extreme version of normal avian preening, which can include chewing or plucking and lead to skin damage or prevention of normal growth of feathers59. Clinical observation indicates that feather picking in captive birds may be a response to understimulation, and it has been linked to many of the stressors that may be involved in the onset of trichotillomania, such as stress, boredom, frustration, and being alone60. The SRI clomipramine has been demonstrated to be effective in the treatment of excessive feather picking in psitticine birds61, suggesting the involvement of serotonin. Phenomenologically, feather picking in birds has similarities to compulsive skin picking in humans.
Barbering in laboratory mice
Barbering is a phenomenon occasionally seen in caged mice in which a “barber” mouse systematically removes the hairs or whiskers of other mice with its incisors; the other mice accept this passively and often seek it out62. In certain strains of mice it is common, suggesting a genetic basis63. Barbering may be a useful model of trichotillomania64, although it requires a pair for it to occur. The barbering mouse and the barbered mouse may each model a component of the phenomenology of trichotillomania, since the behavioral act of plucking and the sensation of the hair being plucked may each or both be an important driving force in any given individual with trichotillomania. Thus, it may be important to study both members of the mouse pair. In addition, cases have been reported in which patients with BDD who compulsively picked their own skin also compulsively picked the skin of other people32.
Neurobiology of pathological grooming
Studies in animals and humans have begun to provide clues to understanding the underlying neurobiology of pathological grooming. Thus far, animal models have provided the most compelling genetic, lesion, and neurochemistry data, while data in humans have come primarily from a limited number of neuroimaging studies.
Serotonin may play a role in pathological grooming. In animal models such as acral-lick dermatitis in canines, compulsive behaviors are reduced with SRIs8, 65. Response to SRIs has also been reported for avian psychogenic feather plucking66, 67. Similarly, SRIs often reduce compulsive cleaning and washing behaviors in OCD and compulsive skin picking and grooming behaviors in BDD55. The reduction of compulsive behaviors with medications that enhance serotonin neurotransmission in animals or humans suggests, but does not prove, that serotonin dysregulation plays a role in their pathophysiology68, 69.
Dopamine agonists can cause stereotyped behaviors in animals and can exacerbate OCD symptoms and tics70. Medications that block dopamine can reduce grooming behaviors in animals71 and may be effective in OCD72 and trichotillomania73. There is also recent evidence of glutamatergic involvement in pathological grooming behaviors in animals, including the possibility that serotonergic medications may mediate their effects on pathological grooming via glutamate systems2. The neuropeptide oxytocin may also have a role in grooming, as Marroni et al (2007) found that rats exhibited increased grooming behaviors when oxytocin was injected into the central nucleus of the amygdala74. Consistent with this finding, grooming behaviors may also serve the purpose of enhancing bonding and establishing and maintaining social relationships75, 76.
Mouse models have provided early evidence for genetic contributors to pathological grooming. Greer and Capecchi (2002) produced a mutant mouse strain deficient in expression of the Hoxb8 homeodomain gene. These mice exhibited excessive grooming behavior, to the point of hair loss and skin lesions, and excessively groomed cagemates77. They found that Hoxb8 is normally expressed in multiple areas of the brain, including but not limited to the caudate-putamen (areas relevant to OCD) and cerebellum. The authors proposed this mutant mouse strain as an animal model for trichotillomania, although it may prove relevant to other human grooming conditions.
As mentioned earlier, Welch et al. (2007) used genetically engineered mice to produce a behavioral phenotype of increased anxiety-like and compulsive grooming behaviors that resulted in hair loss and skin lesions2. The mouse line contained a deletion in the gene encoding for SAPAP3, a protein highly expressed in the striatum that regulates the trafficking and targeting of neurotransmitter receptors and signaling molecules at postsynaptic excitatory synapses. Subsequently, Züchner et al. (2009) examined the human orthologue of SAPAP3 in three clinical groups: trichotillomania, trichotillomania with OCD, and OCD without trichotillomania, and in healthy controls78. They found heterozygous variants in 4.2% of the trichotillomania/OCD patients but only 1.1% of healthy controls and concluded that SAPAP3 may increase susceptibility for OCD and trichotillomania. These findings need to be replicated. In the first association study of SAPAP3 in humans, Bienvenu et al. (2008) conducted a family-based association analysis of patients with OCD or grooming conditions (onychophagia, skin picking, and/or trichotillomania)79 and found that four of the six single nucleotide polymorphisms (SNPs) were associated with at least one grooming condition, and all three haplotypes were associated with at least one grooming condition. None of the SNPs or haplotypes was associated with OCD specifically. However, as this sample was selected based on an OCD diagnosis as part of a larger study, most of the individuals with grooming conditions also had OCD, and about one third of those with OCD had a grooming condition. The authors therefore conclude that although SAPAP3 seems to be associated more with grooming conditions than OCD, they cannot rule out the possibility that the association is specifically with a subtype of OCD with comorbid grooming conditions. These intriguing findings, too, require replication.
Neuroanatomically, the basal ganglia and limbic systems appear to be involved in grooming behaviors in animals. Several animal studies have implicated these regions in rodents2, 16 and primates80. Circuits within the neostriatum, in particular, may be important in balancing sensory-driven and central pattern-generating circuits16.
The neurobiology of OCD, in large part elucidated from neuroimaging studies, implicates similar neuroanatomic areas – specifically, cortico-striatal-thalamic circuit dysfunction81. This circuit is thought to be responsible for habit learning, routine performance of these habits, and acquisition of stereotyped behaviors82. The orbitofrontal cortex and subregions of the anterior cingulate are considered major paralimbic inputs to these circuits. A model of dysfunction in this circuit involves over-activity in the direct, relative to the indirect, pathway, setting up a loop of recurrent, difficult-to-control thoughts and behavioral sequences81.
In BDD83, a similar phenomenon could explain the recurrent obsessive thoughts and repetitive behaviors that often involve grooming. There have only been a few neuroimaging studies in BDD. One morphometric MRI study demonstrated greater total white matter compared to controls and a leftward shift in caudate asymmetry83, suggesting striatal involvement in BDD. A more recent morphometric MRI study, however, did not find significant abnormalities in these regions84. A functional magnetic resonance imaging (fMRI) study of visual processing in BDD demonstrated greater activity relative to healthy controls in fronto-striatal systems including the orbitofrontal cortex and caudate when viewing their own face85. In addition, activity in these regions varied with BDD symptom severity. This suggests that a pathophysiological process similar to that in OCD may be operating in BDD when viewing their face, and may reflect obsessive thoughts and urges to engage in repetitive grooming or other compulsive behaviors.
There have likewise been only a few neuroimaging studies in trichotillomania. O’Sullivan et al. (1997) found smaller volume of the left putamen relative to controls86, and another morphometric MRI study found smaller cerebellar volumes, with an inverse correlation between a primary sensorimotor subregion and symptom severity. These findings, along with a positron emission tomography (PET) study showing increased cerebellar metabolism87, suggests abnormalities of the cerebellum, which plays a role in complex, coordinated motor sequences. Chamberlain et al (2008) found increased grey matter densities in fronto-striatal and limbic systems, relative to controls88. However, a functional MRI study probing striatal function with the implicit learning task did not find evidence of abnormal striatal activation89.
Future studies of disorders involving pathological grooming are needed to clarify their underlying neurobiology. Neuroimaging studies should pay particular attention to the striatal and cerebellar systems, given animal studies indicating their involvement in grooming behaviors2, 16. Genes and genetic polymorphisms that may be relevant to animal models of pathological grooming may be particularly promising avenues of exploration in studies of human conditions involving pathological grooming. The efficacy of SRIs and dopamine antagonists for animal grooming suggests they are worth studying in those human disorders for which little treatment research has been done (e.g., ORS). If future studies substantiate a role for the glutamate system and oxytocin in pathological grooming in animals, investigation of medications that modulate these systems may be warranted.
Conclusions and Future Directions
In general, attempts at discovery or development of animal models for OCD, pathological grooming, or other psychiatric conditions have not adequately matched the primary emotional or cognitive processes in humans with analogous primary processes in animals. Animal models instead have, by and large, matched on the level of secondary, observable behavior. For many animal models, unfortunately, it may be extremely difficult and perhaps impossible to find matches for primary processes such as avoidance of contamination, minimization of odor, improvement of appearance, or modulation of complex emotions and cognitions, which occur in human disorders involving pathological grooming. However, simpler models in animals, such as stress-relief models, can be derived from experiments designed to measure behavioral effects of artificially-induced stress induction. These models may be especially relevant to trichotillomania, compulsive skin-picking, or onychophagia. Animal models of grooming behaviors aimed at improving of appearance for purposes of mating may also be identifiable and relevant to BDD.
Any animal model of a human behavioral condition should be valid on several levels90 (Table 3). This includes criteria based on face validity such as: 1) symptoms are analogous to those of the human condition; 2) observable behavioral changes in both the animal model and the human condition can be measured; 3) independent observers agree on objective criteria for drawing conclusions about the subjective state. In addition, treatments effective for the human condition should also be effective in the animal model (predictive validity), and the model should be reproducible. Another important criterion is construct validity, in which the model is based on similar underlying pathophysiology as the human condition91. With these points as guidance, the criteria for face validity for an animal model of behavioral symptoms of repetitive grooming behaviors appear to fit non-OCD conditions involving pathological grooming in humans at least as well as they fit OCD. Although there is the same difficulty as in OCD of discerning whether the driving, primary process underlying pathological grooming behaviors in humans matches that in animals, at the least on the behavioral level, trichotillomania, compulsive skin-picking, and nail-biting – which are not triggered by complex cognitions (unlike OCD, BDD, and ORS) – are more easily matched between species.
Table 3.
Proposed validation criteria for evaluating animal models of human psychiatric disorders90, 91, 95, 96
| Category of validation criteria | Examples |
|---|---|
| 1. Face validity (isomorphism) |
|
| 2. Predictive validity |
|
| 3. Construct validity (underlying pathophysiological mechanisms) |
|
Until cognitions and affect can better be elucidated in animal models, animal pathological grooming behaviors appear to best parallel human disorders that consistently display pathological grooming and grooming-like behaviors. Already there appear to be links between animal lesion and genetic studies that have identified the brainstem, cerebellum, and neostriatum as important in grooming and early neuroimaging studies in trichotillomania. However, currently the field still lacks data to provide adequate construct validity for the use of these animal models for human conditions involving pathological grooming and research is needed to determine their relevance to these conditions. Yet there are many potential opportunities to further understand and validate these and other animal models, which will help make significant advances in the study of pathological grooming conditions in humans.
FIGURE.
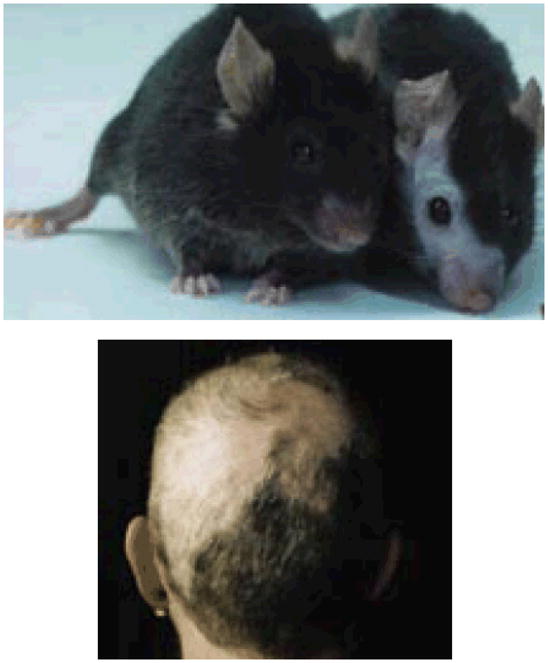
Barbering in laboratory mice (top) 69 and trichotillomania (bottom)
Kurien BT, Gross T, Scofield RH. Barbering in mice: a model for trichotillomania. BMJ. 2005;331:1503–1505. Reprinted with permission, Copyiright 2005.
Acknowledgments
Source of Funding: Supported by the following grants from the National Institute of Mental Health: K23MH079212 to Dr. Feusner and K24MH063975 to Dr. Phillips.
Footnotes
Focus points:
- Pathological grooming in animals has been used as a model for human OCD based largely on observed similarities in behaviors.
- However, current limitations in assessment of complex cognition and affect in animals preclude the field’s ability to infer that any equivalents of OCD-like obsessive thoughts drive these repetitive grooming behaviors.
- Disorders of grooming in animals may be equally or possibly better suited as models for human conditions that involve pathological grooming or grooming-like behaviors, such as trichotillomania, compulsive skin-picking, onychophagia, body dysmorphic disorder, and olfactory reference syndrome. However, research is needed to determine the relevance of such animal models to these human conditions.
Disclosures:
- Dr. Feusner receives research support from the National Institute of Mental Health, the Obsessive-Compulsive Foundation, and the UCLA Academic Senate. He has received speaking honoraria and travel reimbursement from academic institutions.
- Dr. Phillips receives research support from the National Institute of Mental Health, the FDA, the American Foundation for Suicide Prevention, and Forest Laboratories (medication for an NIMH-funded study). She has received publication/speaking honoraria from Merck (for a contribution to the Merck Manual) Oxford University Press, and academic institutions. Future royalties may potentially be received from Guilford Press and The Free Press. She has received travel and expense reimbursement from various academic and professional organizations.
- Emily Hembacher has no disclosures to report.
References
- 1.Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology. Psychiatr Clin North Am. 2006 Jun;29(2):371–390. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Welch JM, Lu J, Rodriguiz RM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007 Aug 23;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein DJ, Shoulberg N, Helton K, Hollander E. The neuroethological approach to obsessive-compulsive disorder. Compr Psychiatry. 1992 Jul–Aug;33(4):274–281. doi: 10.1016/0010-440x(92)90053-s. [DOI] [PubMed] [Google Scholar]
- 4.Lenane MC, Swedo SE, Rapoport JL, Leonard H, Sceery W, Guroff JJ. Rates of Obsessive Compulsive Disorder in first degree relatives of patients with trichotillomania: a research note. J Child Psychol Psychiatry. 1992 Jul;33(5):925–933. doi: 10.1111/j.1469-7610.1992.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 5.Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry. 2005 Oct–Dec;17(4):227–232. doi: 10.1080/10401230500295354. [DOI] [PubMed] [Google Scholar]
- 6.Chosak A, Marques L, Greenberg JL, Jenike E, Dougherty DD, Wilhelm S. Body dysmorphic disorder and obsessive-compulsive disorder: similarities, differences and the classification debate. Expert Rev Neurother. 2008 Aug;8(8):1209–1218. doi: 10.1586/14737175.8.8.1209. [DOI] [PubMed] [Google Scholar]
- 7.Jones I, Blackshaw JK. An evolutionary approach to psychiatry. Aust N Z J Psychiatry. 2000 Feb;34(1):8–13. doi: 10.1046/j.1440-1614.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- 8.Hewson CJ, Luescher UA, Parent JM, Conlon PD, Ball RO. Efficacy of clomipramine in the treatment of canine compulsive disorder. J Am Vet Med Assoc. 1998 Dec 15;213(12):1760–1766. [PubMed] [Google Scholar]
- 9.Benson J, Greaves W, O’Donnell M, Taglialatela J. Evidence for symbolic language processing in a Bonobo (Pan paniscus) 2002. [Google Scholar]
- 10.Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Consciousness and Cognition. 2005 Mar;14(1):30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Sachs BD. In: The development of grooming and its expression in adult animals. Colbern DL, Gispen WH, editors. 1988. pp. 1–17. [DOI] [PubMed] [Google Scholar]
- 12.Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992 Jul;72(3):825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- 13.Moon-Fanelli AA, Dodman NH, RLOS . Trichotillomania. Arlington: American Psychiatric Publishing, Inc; 1999. Veterinary models of compulsive self-grooming: parallels with trichotillomania; pp. 63–92. [Google Scholar]
- 14.Gardner R. Psychiatric syndromes as infrastructure for intraspecific communications. In: Chance MRA, editor. Social Fabrics of the Mind. London: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 15.Gould JL. Ethology. The Mechanisms and Evolution of Behavior. New York: W.W. Norton; 1982. [Google Scholar]
- 16.Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996 May 15;16(10):3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berridge KC. Progressive degradation of serial grooming chains by descending decerebration. Behav Brain Res. 1989 Jul 1;33(3):241–253. doi: 10.1016/s0166-4328(89)80119-6. [DOI] [PubMed] [Google Scholar]
- 18.Strazielle C, Lalonde R. Grooming in Lurcher mutant mice. Physiol Behav. 1998 Apr;64(1):57–61. doi: 10.1016/s0031-9384(98)00014-6. [DOI] [PubMed] [Google Scholar]
- 19.Matthews PC. Pathological habit disorder. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie. 1988;33(9):826–829. doi: 10.1177/070674378803300908. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Summerfeldt LJ, Richter MA, Antony MM, Swinson RP. Symptom structure in obsessive-compulsive disorder: a confirmatory factor-analytic study. Behav Res Ther. 1999 Apr;37(4):297–311. doi: 10.1016/s0005-7967(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 22.Leckman JF, Grice DE, Boardman J, et al. Symptoms of obsessive-compulsive disorder. Am J Psychiatry. 1997 Jul;154(7):911–917. doi: 10.1176/ajp.154.7.911. [DOI] [PubMed] [Google Scholar]
- 23.Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005 Feb;162(2):228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert AR, Mataix-Cols D, Almeida JR, et al. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: a voxel-based morphometry study. J Affect Disord. 2008 Jul;109(1–2):117–126. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 25.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004 Jun;61(6):564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 26.Hill RA, McInnes KJ, Gong EC, Jones ME, Simpson ER, Boon WC. Estrogen deficient male mice develop compulsive behavior. Biol Psychiatry. 2007 Feb 1;61(3):359–366. doi: 10.1016/j.biopsych.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Faravelli C, Salvatori S, Galassi F, Aiazzi L, Drei C, Cabras P. Epidemiology of somatoform disorders: a community survey in Florence. Soc Psychiatry Psychiatr Epidemiol. 1997 Jan;32(1):24–29. doi: 10.1007/BF00800664. [DOI] [PubMed] [Google Scholar]
- 28.Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. Am J Psychiatry. 2001 Dec;158(12):2061–2063. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- 29.Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological Medicine. 2006;36(6):877–885. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- 30.Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. J Nerv Ment Dis. 1997 Sep;185(9):570–577. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry. 2005 Jun;66(6):717–725. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- 32.Veale D, Boocock A, Gournay K, et al. Body dysmorphic disorder. A survey of fifty cases. British Journal of Psychiatry. 1996 Aug;169(2):196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- 33.Phillips KA. The Broken Mirror. New York: Oxford University Press; 2005. [Google Scholar]
- 34.Phillips KA, Taub SL. Skin picking as a symptom of body dysmorphic disorder. Psychopharmacol Bull. 1995;31(2):279–288. [PubMed] [Google Scholar]
- 35.O’Sullivan RL, Phillips KA, Keuthen NJ, Wilhelm S. Near-fatal skin picking from delusional body dysmorphic disorder responsive to fluvoxamine. Psychosomatics. 1999 Jan-Feb;40(1):79–81. doi: 10.1016/S0033-3182(99)71276-4. [DOI] [PubMed] [Google Scholar]
- 36.Hollander E. Obsessive compulsive spectrum disorders: an overview. Psychiatric Annals. 1993;23(7):355–358. [Google Scholar]
- 37.Mataix-Cols D, Pertusa A, Leckman JF. Issues for DSM-V: how should obsessive-compulsive and related disorders be classified? Am J Psychiatry. 2007 Sep;164(9):1313–1314. doi: 10.1176/appi.ajp.2007.07040568. [DOI] [PubMed] [Google Scholar]
- 38.Frost RO, Steketee G. Perfectionism in obsessive-compulsive disorder patients. Behaviour Research and Therapy. 1997 Apr;35(4):291–296. doi: 10.1016/s0005-7967(96)00108-8. [DOI] [PubMed] [Google Scholar]
- 39.Buhlmann U, Etcoff NL, Wilhelm S. Facial attractiveness ratings and perfectionism in body dysmorphic disorder and obsessive-compulsive disorder. Journal of Anxiety Disorders. 2008 Apr;22(3):540–547. doi: 10.1016/j.janxdis.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Stein DJ, Le Roux L, Bouwer C, Van Heerden B. Is olfactory reference syndrome an obsessive-compulsive spectrum disorder?: two cases and a discussion. J Neuropsychiatry Clin Neurosci. 1998 Winter;10(1):96–99. doi: 10.1176/jnp.10.1.96. [DOI] [PubMed] [Google Scholar]
- 41.Phillips KA, Gunderson C, Gruber U, Castle D. Delusions of body malodour; the olfactory reference syndrome. In: Brewer W, Castle D, Pantelis C, editors. Olfaction and the Brain. New York: Cambridge University Press; 2006. [Google Scholar]
- 42.Christenson GA, Pyle RL, Mitchell JE. Estimated lifetime prevalence of trichotillomania in college students. J Clin Psychiatry. 1991 Oct;52(10):415–417. [PubMed] [Google Scholar]
- 43.Christenson GA, Mackenzie TB, Mitchell JE. Characteristics of 60 adult chronic hair pullers. Am J Psychiatry. 1991 Mar;148(3):365–370. doi: 10.1176/ajp.148.3.365. [DOI] [PubMed] [Google Scholar]
- 44.Grant JE, Odlaug BL, Potenza MN. Addicted to hair pulling? How an alternate model of trichotillomania may improve treatment outcome. Harv Rev Psychiatry. 2007 Mar–Apr;15(2):80–85. doi: 10.1080/10673220701298407. [DOI] [PubMed] [Google Scholar]
- 45.Diefenbach GJ, Mouton-Odum S, Stanley MA. Affective correlates of trichotillomania. Behav Res Ther. 2002 Nov;40(11):1305–1315. doi: 10.1016/s0005-7967(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 46.Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics. 2000 May–Jun;41(3):210–215. doi: 10.1176/appi.psy.41.3.210. [DOI] [PubMed] [Google Scholar]
- 47.Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry. 1999 Jul;60(7):454–459. doi: 10.4088/jcp.v60n0707. [DOI] [PubMed] [Google Scholar]
- 48.Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs. 2001;15(5):351–359. doi: 10.2165/00023210-200115050-00002. [DOI] [PubMed] [Google Scholar]
- 49.Hayes SL, Storch EA, Berlanga L. Skin picking behaviors: An examination of the prevalence and severity in a community sample. J Anxiety Disord. 2009 Jan 23; doi: 10.1016/j.janxdis.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Diefenbach GJ, Tolin DF, Meunier S, Worhunsky P. Emotion regulation and trichotillomania: a comparison of clinical and nonclinical hair pulling. J Behav Ther Exp Psychiatry. 2008 Mar;39(1):32–41. doi: 10.1016/j.jbtep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Wells JH, Haines J, Williams CL. Severe morbid onychophagia: the classification as self-mutilation and a proposed model of maintenance. Aust N Z J Psychiatry. 1998 Aug;32(4):534–545. doi: 10.3109/00048679809068328. [DOI] [PubMed] [Google Scholar]
- 52.Man J, Hudson AL, Ashton D, Nutt DJ. Animal models for obsessive-compulsive disorder. Current Neuropharmacology. 2004;2:169–181. [Google Scholar]
- 53.Owen LN. Canine lick granuloma treated with radiotherapy. Journal of Small Animal Practice. 2008;8(30):454–456. [Google Scholar]
- 54.Shumaker AK, Angus JC, Coyner KS, Loeffler DG, Rankin SC, Lewis TP. Microbiological and histopathological features of canine acral lick dermatitis. Vet Dermatol. 2008 Oct;19(5):288–298. doi: 10.1111/j.1365-3164.2008.00693.x. [DOI] [PubMed] [Google Scholar]
- 55.Phillips KA, Hollander E. Treating body dysmorphic disorder with medication: evidence, misconceptions, and a suggested approach. Body Image. 2008 Mar;5(1):13–27. doi: 10.1016/j.bodyim.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bloch MH, Landeros-Weisenberger A, Dombrowski P, et al. Systematic review: pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry. 2007 Oct 15;62(8):839–846. doi: 10.1016/j.biopsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Virga V. Behavioral dermatology. Vet Clin North Am Small Anim Pract. 2003 Mar;33(2):231–251. v–vi. doi: 10.1016/s0195-5616(02)00102-x. [DOI] [PubMed] [Google Scholar]
- 58.Overall KL, Dunham AE. Clinical features and outcome in dogs and cats with obsessive-compulsive disorder: 126 cases (1989–2000) J Am Vet Med Assoc. 2002 Nov 15;221(10):1445–1452. doi: 10.2460/javma.2002.221.1445. [DOI] [PubMed] [Google Scholar]
- 59.Rosskopf WJ, Jr, Woerpel RW. Pet avian conditions and syndromes of the most frequently presented species seen in practice. Vet Clin North Am Small Anim Pract. 1991 Nov;21(6):1189–1211. doi: 10.1016/s0195-5616(91)50132-7. [DOI] [PubMed] [Google Scholar]
- 60.Bordnick PS, Thyer BA, Ritchie BW. Feather picking disorder and trichotillomania: an avian model of human psychopathology. J Behav Ther Exp Psychiatry. 1994 Sep;25(3):189–196. doi: 10.1016/0005-7916(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 61.van Hierden YM, de Boer SF, Koolhaas JM, Korte SM. The control of feather pecking by serotonin. Behav Neurosci. 2004 Jun;118(3):575–583. doi: 10.1037/0735-7044.118.3.575. [DOI] [PubMed] [Google Scholar]
- 62.Bresnahan JF, Kitchell BB, Wildman MF. Facial hair barbering in rats. Lab Anim Sci. 1983 Jun;33(3):290–291. [PubMed] [Google Scholar]
- 63.van den Broek FA, Omtzigt CM, Beynen AC. Whisker trimming behaviour in A2G mice is not prevented by offering means of withdrawal from it. Lab Anim. 1993 Jul;27(3):270–272. doi: 10.1258/002367793780745462. [DOI] [PubMed] [Google Scholar]
- 64.Kurien BT, Gross T, Scofield RH. Barbering in mice: a model for trichotillomania. British Medical Journal. 2005 Dec;331(7531):1503–1505. doi: 10.1136/bmj.331.7531.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wynchank D, Berk M. Fluoxetine treatment of acral lick dermatitis in dogs: a placebo-controlled randomized double blind trial. Depress Anxiety. 1998;8(1):21–23. [PubMed] [Google Scholar]
- 66.Seibert LM, Crowell-Davis SL, Wilson GH, Ritchie BW. Placebo-controlled clomipramine trial for the treatment of feather picking disorder in cockatoos. J Am Anim Hosp Assoc. 2004 Jul–Aug;40(4):261–269. doi: 10.5326/0400261. [DOI] [PubMed] [Google Scholar]
- 67.Grindlinger HM, Ramsay E. Compulsive feather picking in birds. Arch Gen Psychiatry. 1991 Sep;48(9):857. doi: 10.1001/archpsyc.1991.01810330081012. [DOI] [PubMed] [Google Scholar]
- 68.Aouizerate B, Guehl D, Cuny E, et al. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004 Feb;72(3):195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 69.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000 Nov;28(2):343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 70.Goodman WK, McDougle CJ, Price LH, Riddle MA, Pauls DL, Leckman JF. Beyond the serotonin hypothesis: a role for dopamine in some forms of obsessive compulsive disorder? J Clin Psychiatry. 1990 Aug;51(Suppl):36–43. discussion 55–38. [PubMed] [Google Scholar]
- 71.Green EJ, Isaacson RL, Dunn AJ, Lanthorn TH. Naloxone and haloperidol reduce grooming occurring as an aftereffect of novelty. Behav Neural Biol. 1979 Dec;27(4):546–551. doi: 10.1016/s0163-1047(79)92208-8. [DOI] [PubMed] [Google Scholar]
- 72.Skapinakis P, Papatheodorou T, Mavreas V. Antipsychotic augmentation of serotonergic antidepressants in treatment-resistant obsessive-compulsive disorder: a meta-analysis of the randomized controlled trials. Eur Neuropsychopharmacol. 2007 Jan 15;17(2):79–93. doi: 10.1016/j.euroneuro.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Stewart RS, Nejtek VA. An open-label, flexible-dose study of olanzapine in the treatment of trichotillomania. J Clin Psychiatry. 2003 Jan;64(1):49–52. doi: 10.4088/jcp.v64n0110. [DOI] [PubMed] [Google Scholar]
- 74.Marroni SS, Nakano FN, Gati CD, Oliveira JA, Antunes-Rodrigues J, Garcia-Cairasco N. Neuroanatomical and cellular substrates of hypergrooming induced by microinjection of oxytocin in central nucleus of amygdala, an experimental model of compulsive behavior. Mol Psychiatry. 2007 Dec;12(12):1103–1117. doi: 10.1038/sj.mp.4002015. [DOI] [PubMed] [Google Scholar]
- 75.Gouzoules S, Gouzoules H, Smuts BB, et al. Kinship. Primate societies. 1987:299–305. [Google Scholar]
- 76.Sade DS. Some Aspects of Parent-Offspring and Sibling Relations in a Group of Rhesus Monkeys, with a Discussion of Grooming. Am J Phys Anthropol. 1965 Mar;23:1–17. doi: 10.1002/ajpa.1330230115. [DOI] [PubMed] [Google Scholar]
- 77.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002 Jan 3;33(1):23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 78.Zuchner S, Wendland JR, Ashley-Koch AE, et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol Psychiatry. 2009 Jan;14(1):6–9. doi: 10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bienvenu OJ, Wang Y, Shugart YY, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet. 2009 Jul 5;150B(5):710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacLean PD. Effects of lesions of globus pallidus on species-typical display behavior of squirrel monkeys. Brain Res. 1978 Jun 23;149(1):175–196. doi: 10.1016/0006-8993(78)90597-8. [DOI] [PubMed] [Google Scholar]
- 81.Saxena S, Brody A, Schwartz J, Baxter L. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. British Journal of Psychiatry. 1998;173 (suppl 35):26–38. [PubMed] [Google Scholar]
- 82.Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophr Bull. 1997;23(3):459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- 83.Rauch SL, Phillips KA, Segal E, et al. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Research. 2003 Jan 20;122(1):13–19. doi: 10.1016/s0925-4927(02)00117-8. [DOI] [PubMed] [Google Scholar]
- 84.Feusner JD, Townsend J, Bystritsky A, McKinley M, Moller H, Bookheimer S. Regional brain volumes and symptom severity in body dysmorphic disorder. Psychiatry Res. 2009 May 15;172(2):161–167. doi: 10.1016/j.pscychresns.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feusner JD, Moody T, Townsend J, et al. Abnormalities of Visual Processing and Fronto-Striatal Systems in Body Dysmorphic Disorder. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2009.190. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Sullivan RL, Rauch SL, Breiter HC, et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol Psychiatry. 1997 Jul 1;42(1):39–45. doi: 10.1016/S0006-3223(96)00297-1. [DOI] [PubMed] [Google Scholar]
- 87.Swedo SE, Rapoport JL, Leonard HL, Schapiro MB, Rapoport SI, Grady CL. Regional cerebral glucose metabolism of women with trichotillomania. Arch Gen Psychiatry. 1991 Sep;48(9):828–833. doi: 10.1001/archpsyc.1991.01810330052008. [DOI] [PubMed] [Google Scholar]
- 88.Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005 May;29(3):399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Rauch SL, Wright CI, Savage CR, et al. Brain activation during implicit sequence learning in individuals with trichotillomania. Psychiatry Res. 2007 Apr 15;154(3):233–240. doi: 10.1016/j.pscychresns.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 90.McKinney WT, Jr, Bunney WE., Jr Animal model of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry. 1969 Aug;21(2):240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- 91.Overall KL. Natural animal models of human psychiatric conditions: assessment of mechanism and validity. Prog Neuropsychopharmacol Biol Psychiatry. 2000 Jul;24(5):727–776. doi: 10.1016/s0278-5846(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 92.Zampiga E, Hoi H, Pilastro A. Preening, plumage reflectance and female choice in budgerigars. Ethology Ecology & Evolution. 2004 Dec;16(4):339–349. [Google Scholar]
- 93.Eckstein RA, Hart BL. The organization and control of grooming in cats. Appl Anim Behav Sci. 2000 May 10;68(2):131–140. doi: 10.1016/s0168-1591(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 94.Hart BL, Powell KL. Antibacterial properties of saliva: role in maternal periparturient grooming and in licking wounds. Physiol Behav. 1990 Sep;48(3):383–386. doi: 10.1016/0031-9384(90)90332-x. [DOI] [PubMed] [Google Scholar]
- 95.Wilner P. Behavioral models in psychopharmacology. In: Wilner P, editor. Behavioral models in psychopharmacology: theoretical, industrial, and clinical perspectives. Cambridge: Cambridge University Press; 1991. pp. 3–18. [Google Scholar]
- 96.Fossella JA, Casey BJ. Genes, brain, and behavior: bridging disciplines. Cogn Affect Behav Neurosci. 2006 Mar;6(1):1–8. doi: 10.3758/cabn.6.1.1. [DOI] [PubMed] [Google Scholar]


