Abstract
Glass patterns are moirés created from a sparse random dot field paired with its spatially-shifted copy. Because discrimination of these patterns is not based on local features, they have been used extensively to study global integration processes. Here, we investigated whether 4–5.5 month old infants are sensitive to the global structure of Glass patterns by measuring Visual Evoked Potentials (VEPs). Although we found strong responses to the appearance of the constituent dots, we found sensitivity to the global structure of the Glass patterns in the infants only over a very limited range of spatial separation. In contrast, we observed robust responses in the infants when we connected the dot pairs of the Glass pattern with lines. Moreover, both infants and adults showed differential responses to exchanges between line patterns portraying different global structures. A control study varying luminance contrast in adults suggests that infant sensitivity to global structure is not primarily limited by reduced element visibility. Together our results suggest that the insensitivity to structure in conventional Glass patterns is due to inefficiencies in extracting the local orientation cues generated by the dot pairs. Once the local orientations are made unambiguous or when the interpolation span is small, infants can integrate these signals over the image.
Keywords: texture, visual development, VEP, Glass patterns, integration
INTRODUCTION
Early visual mechanisms with spatially localized receptive fields are tuned for features such as orientation, disparity and direction of motion. However, our visual experience is mainly made up of coherent scenes, objects and textures and not local features. The processes by which local features are integrated into perceptual groupings has been a subject of intensive research (Sasaki, 2007). There is a general consensus that the global integration of local features involves specialized mechanisms in extra-striate visual areas such as hMT+/MST and V3A in the case of coherent motion in random-dot displays (Braddick, O’Brien, Wattam-Bell, Atkinson, Hartley & Turner, 2001, Koyama, Sasaki, Andersen, Tootell, Matsuura & Watanabe, 2005, Morrone, Tosetti, Montanaro, Fiorentini, Cioni & Burr, 2000, Rees, Friston & Koch, 2000, Smith, Wall, Williams & Singh, 2006, Tootell, Mendola, Hadjikhani, Ledden, Liu, Reppas, Sereno & Dale, 1997, Tootell, Reppas, Kwong, Malach, Born, Brady, Rosen & Belliveau, 1995) and in occipitotemporal and ventral areas such as LOC and V4 in the case of orientation cues (Altmann, Bulthoff & Kourtzi, 2003, Braddick, O’Brien, Wattam-Bell, Atkinson & Turner, 2000, Ostwald, Lam, Li & Kourtzi, 2008). Because global integration tasks tap higher-level visual processing mechanisms, they have been used as behavioral probes of the development of extra-striate cortical areas in humans (for reviews, see Braddick & Atkinson, 2007, Norcia & Pei, 2007).
Sensitivity to the global coherence of orientation-defined textures (Norcia, Pei, Bonneh, Hou, Sampath & Pettet, 2005, Pei, Pettet & Norcia, 2007), collinear structure of orientation-defined contours (Gerhardstein, Kovacs, Ditre & Feher, 2004, Norcia et al., 2005) and the orientation of luminance-defined Gestalt groupings (Quinn, Bhatt, Brush, Grimes & Sharpnack, 2002) has been found to be present in infants as young as 2–3 months of age, which would suggest that global form integration is present at a very early age. However relative to adults, infants lack the bias for detecting closure cues (Gerhardstein, et al, 2004), and have higher orientation coherence thresholds in textures (Pei et al., 2007).
To evaluate how global sensitivity in infants is dependent of the strength of local orientation cues, we measured their sensitivity to Glass patterns, moirés made from random dots paired with their spatially shifted copies (Glass, 1969, Glass & Switkes, 1976). Glass patterns are useful in that sensitivity to them requires first the detection of the local elements followed by a “local” integration of dots into oriented dipoles and finally a “global” integration of many dipoles into texture flows (Dakin & Bex, 2001, Kurki, Laurinen, Peromaa & Saarinen, 2003). Developmental immaturities in any of these processing stages may limit performance. Although different global organizations of Glass patterns (e.g. the random, rotation or translation fields depicted in Fig. 1) are readily distinguishable by adults, these patterns are locally indistinguishable. Because of their well-controlled hierarchical nature, Glass patterns have been extensively used to study feature integration (see references in Pei, Pettet, Vildavski & Norcia, 2005, Smith, Bair & Movshon, 2002).
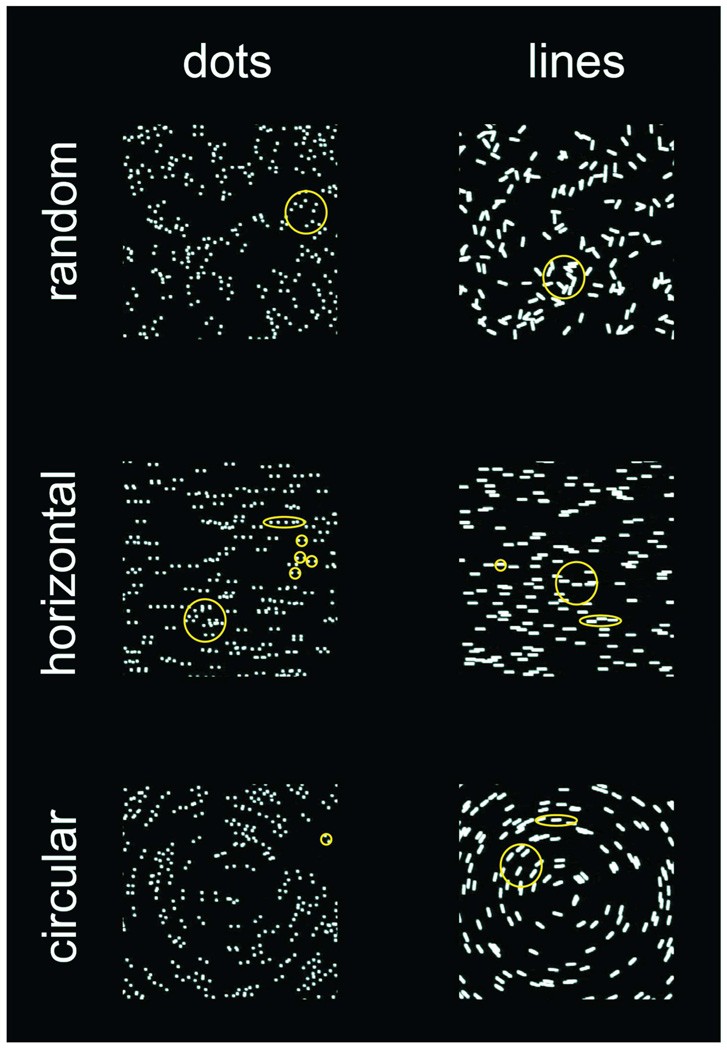
Figure 1.
Examples (to scale) of Glass patterns and corresponding line patterns. Dot pairs and lines were [TOP] randomly oriented, [MIDDLE] aligned horizontally and [BOTTOM] aligned co-circularly. In the first experiment, the patterns alternated between horizontal and random global organizations. In subsequent experiments, the patterns alternated between co-circular and horizontal global organizations. Our model (yellow outlines) proposes that infants have isotropic integration fields (large circles), but lack collinear specific integration fields (ovals) that allow extraction of orientation signals in Glass patterns. Small circles represent receptive fields. Note that dots, but not lines, within isotropic integration fields appear to have random structure.
In order to characterize visual integration of local elements into global wholes during development, we recorded visual-evoked potentials (VEPs) to Glass patterns in infants and adults using a temporal tagging method that independently “labels” responses to local elements and global structure separately. Using this method, we measured sensitivity of infants and adults to conventional Glass patterns whose local orientation features were defined by dot pairs, and to line patterns whose local orientation features were defined explicitly by connecting the dots of an underlying Glass pattern. By this manipulation, we investigated how the ambiguity of local orientation signals affected global integration. Infant and adult sensitivities were measured as a function of dot pair separation in conventional Glass patterns, and as function of line length in connected Glass patterns. Lastly, we evaluated how the visibility of local elements affected global integration. Since infants have lower contrast sensitivity than adults, we varied luminance contrast of Glass patterns and measured VEP sensitivity and coherence thresholds in adults to determine if decreasing luminance contrast decreases sensitivity to the global structure of Glass patterns. If it did, then limitations on global integration in infants could be a consequence of immaturities in low-level contrast processing mechanisms.
We found that 4–5.5 month old infants were sensitive to the presentation of the local dots. However, they were only sensitive to global structure when the dot pairs were physically connected or were in close proximity. Global sensitivity to Glass patterns in adults was largely invariant with luminance contrast, suggesting that the limitations in detecting the global structure of Glass patterns is not likely due to immaturities in the contrast sensitivity of early visual filters. We propose that the poor infant sensitivity to Glass patterns is due to immaturities at several levels of visual processing, including non-classical receptive field properties in early cortical areas.
MATERIALS AND METHODS
Participants
Fourteen adults and 51 full-term infants between the age of 4 and 6 months of age (mean age = 4.8 ±0.6 months) participated in this study. Adult participants had visual acuity of better than 6/6 in each eye, with correction, if needed and stereoacuity of 40 arc sec or better on the Titmus and Randot stereoacuity tests. Acuity was measured using the Bailey-Lovie chart, which has five letters per line, and equal log increments in the letter sizes across lines. Informed consent was obtained prior to experimentation directly from the adults and from a parent of the infant participants under a protocol that was approved by Smith Kettlewell Eye Research Institutional Review Board.
Apparatus and Stimuli
Stimuli were presented by a Power Macintosh G4 computer on a Westinghouse LCD monitor with a resolution of 1024 × 768 and refresh rate of 60 Hz. The mean luminance was 160 cd/m2. The viewing distance was about 70 cm. Two kinds of test patterns were used in this study: Glass patterns comprised of conventional dot pairs and similar patterns comprised of line segments whose length matched the dot separations of the Glass patterns (Fig. 1).
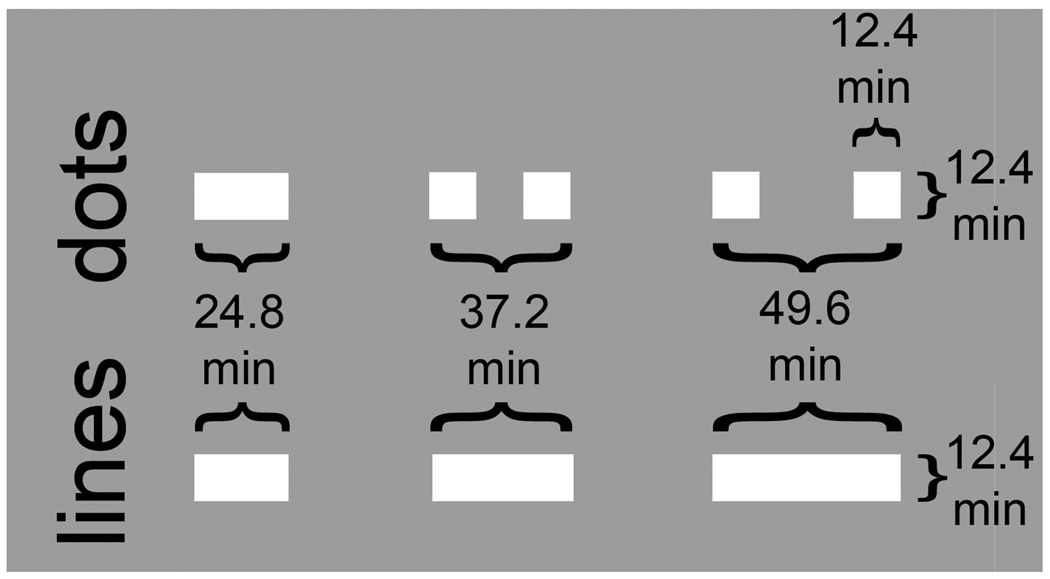
The Glass pattern conditions in Experiments 1, 2 and 4 comprised randomly placed white squares (12.4 × 12.4 min) paired with their copy displaced by 49.6 min (end-to-end; Fig. 2). The corresponding line patterns were comprised of line segments with an aspect ratio of 4:1 (12.4 × 49.6 min; “line pattern” conditions). For Experiment 3, dot pairs and line segments had lengths of 24.8, 37.2 and 49.6 min for infants and 24.8, 49.6, and 124.0 min for adults. The dots of the Glass patterns covered 4% of the screen area of 32 × 32 deg or approximately 0.47 dot pairs per deg2 of visual angle. Luminance contrast (Michelson definition: Lmax− Lmin/ Lmax+ Lmin) was 90% in Experiments 1–3, and varied from 1% to 90% in Experiment 4.
Figure 2.
Local structure of the Glass and line pattern elements. [TOP] Dots of conventional Glass patterns were 12.4 × 12.4 min squares that were paired with their shifted copy. Measured end-to-end, the standard dot pair separation was 49.6 min (Expts. 1 and 3). [BOTTOM] Lines of corresponding “connected” Glass patterns matched the length of the corresponding dot pair. In Expt. 2, local elements were 12.4, 37.2 and 49.6 min in separation/length for infants and 12.4, 49.6 and 124 min in separation/length for adults.
In all experiments, the global structure of the Glass and line patterns was updated at 1.0 Hz. In Experiment 1, the elements periodically alternated between globally random and globally horizontal states or between co-circular and horizontal global states every 500 ms. For horizontal patterns, all dot pairs/lines were aligned horizontally (Fig. 1, middle panels). For the random pattern, the dot pairs/lines were randomly oriented (Fig. 1, top panels). For the co-circular pattern, the dot pairs/lines were aligned tangentially along a set of imaginary circles with random radii. In Experiments 2–4, the patterns periodically alternated between co-circular (500 ms) and horizontal (500 ms) patterns (Fig. 1, middle and bottom panels). A new set of dots or lines were presented every 50 ms (20 Hz).
Visual Evoked Potential Recording and Analysis
Cortical electrical activity was recorded with Glass gold-cup surface electrodes placed on the scalp with a conductive gel (10–20; D. O. Weaver) at six sites over occipital areas. Electrode impedance was maintained below approximately 10 kΩ. The EEG was amplified by a factor of 50,000 (adults) or 20,000 (infants) using a Grass Model 12 amplifier with analog filter settings of 0.3–100 Hz, measured at −6 dB points. The EEG was digitized to a nominal 16 bits accuracy at a sampling rate of 776 Hz. The horizontal synch signal from the video card was used to clock the analog-to-digital converter (13 samples per video frame). The display was updated during the vertical blanking interval, and the vertical synch signal was provided via a digital input line to the data acquisition routine for exact synchronization of the data acquisition to the display. If the display was interrupted with a mouse input, both display and data acquisition program loops were reset to a previous point that was at least 1 sec before the mouse press. A spectral analysis was performed for individual observers using an adaptive filter (Tang & Norcia, 1995).
Our experimental design allows the clear separation of responses to local and global aspects of our stimuli. Significant responses (i.e., greater than zero) at the dot update rate (20 Hz) were used to index sensitivity to the presentation of the local elements. Responses to the first 3 harmonics of the global update rate (1–3 Hz) were selected for quantitative analysis to indicate sensitivity to global structure (Fig. 3) to reflect the symmetry in the waveforms. Our stimulation protocol echoes the design of Braddick, et al (1986), who used two temporal frequencies to independently tag phase and orientation of gratings1.
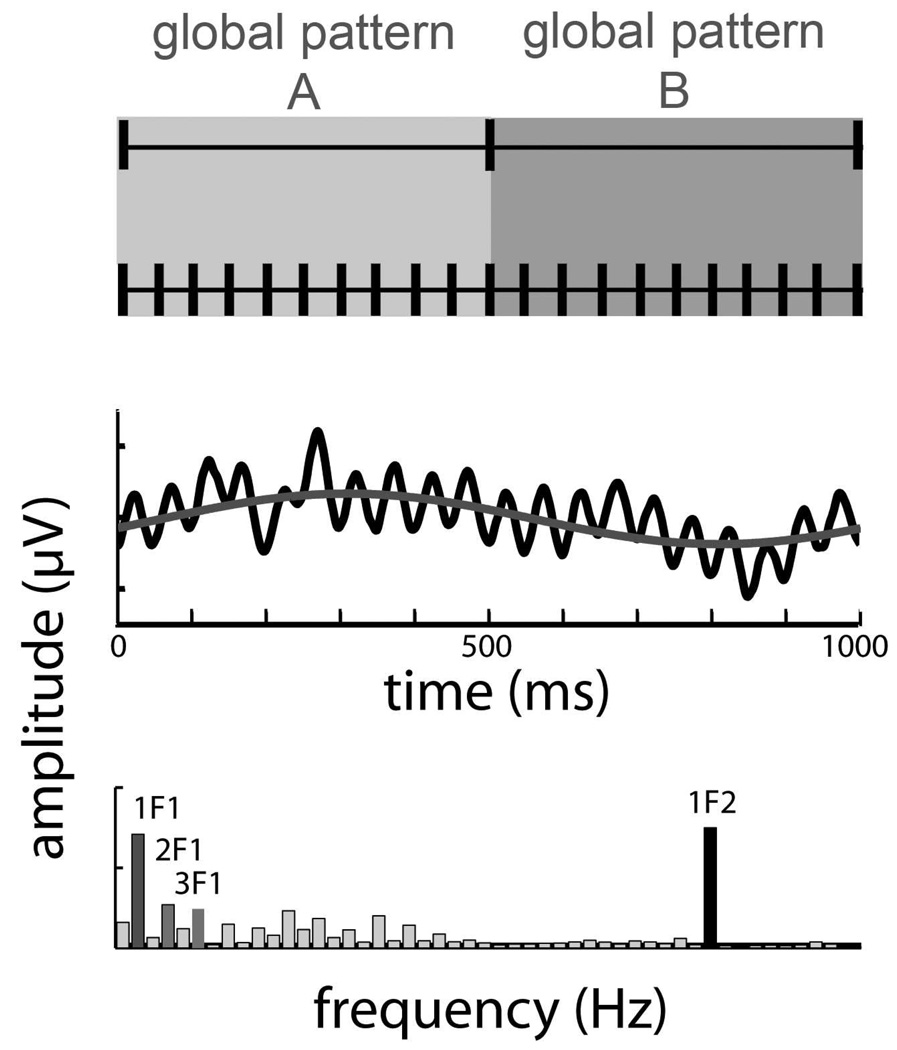
Figure 3.
Response component analysis. [TOP] The global structure of the Glass pattern stimuli alternated at a rate of 1 Hz (i.e. changed every 500 ms), while their local elements were updated at a rate of 20 Hz (i.e., changed every 50 ms). [MIDDLE] The event-related waveforms reflect the structure of the stimulus presentation, which exhibits high frequency modulation (black) riding on top of a low frequency component (red). [BOTTOM] Decomposition of the waveform into its spectral components isolates the responses to the global (1–3 Hz) and local (20 Hz) structure of the Glass pattern stimuli in specific frequency bins that are tightly tied to the local and global update rates. Statistical analyses were performed on the spectral components of the responses.
Odd harmonic responses (1, 3, 5 Hz etc) indicate that the evoked response differs after transitions between the two texture flows, while even harmonic responses (e.g. 2, 4, 6 Hz, etc) indicate the responses are equivalent. Responses that are dominated by low frequency components (1–2 Hz) are more sustained than responses where the largest peaks are at the higher harmonics (e.g. 3 Hz and higher). Since the noise spectra of the background EEG has an approximately 1/f profile, responses at higher harmonics may sometimes be a better indicator of the presence of significant responses than the lower harmonic components due to better signal-to-noise ratio.
For each participant and condition, the real and imaginary spectral coefficients were averaged separately across trials, and then the amplitude and phase of the response was calculated (vector average). Similarly, we computed vector averages across participants for each condition by coherently averaging the spectral coefficients. The vector average approaches zero in the limit for EEG noise (VEP absent), and therefore zero amplitude is the appropriate theoretical and empirical floor against which we test for significant responses.
We then converted the complex values of our responses to scalar values for statistical analysis. This was done by computing projected amplitudes (Hou, Pettet & Norcia, 2008), which are the projection of the amplitudes of individual responses onto the average vector. Projected amplitudes were analyzed using planned two-tailed one sample t-tests and repeated measure ANOVAs. Our planned t-tests (Expt. 1–4) provided a direct test of what response components are reliably different from zero. We computed mean vector amplitudes, which consider the reliability of the amplitude and the phase of the response component (Victor & Mast, 1991). If the response components had random phases across participants due to non-evoked responses, then its vector mean would be zero. Complementary, the ANOVAs provide a comparison between conditions, which we used to determine whether our parameters (distance/length in Expt. 3 or contrast Expt. 4) changed sensitivities to the local and global structure of our patterns.
Procedure
Adults were instructed to avoid eye blinks and to fixate at the center of the screen. Infants sat on a parent’s lap in front of the computer screen. To control infant fixation and accommodation, we suspended a small noisy toy on a string at the center of the screen. By observing the centration of reflection of the video monitor in the infant’s pupil, we were able to control fixation to lie within approximately 4 deg of the 32 deg × 32 deg display area (Allen, Tyler & Norcia, 1996). Trials were interrupted if the infants lost fixation. Data were rejected from 1 second prior to interruption, and recording resumed 1 second after the experimenter indicated that the infant had regained fixation. For each stimulus condition, we collected 10 trials for adults and 4–7 trials for the infants. Each trial is 10 seconds buffered by an extra second at the beginning and end of the trial, in which the stimulus was present but responses were not recorded. Conditions were presented in random order, with 1–3 trials of a given condition run as a block.
RESULTS
Experiment 1: Sensitivity to organized vs. random Glass and line patterns
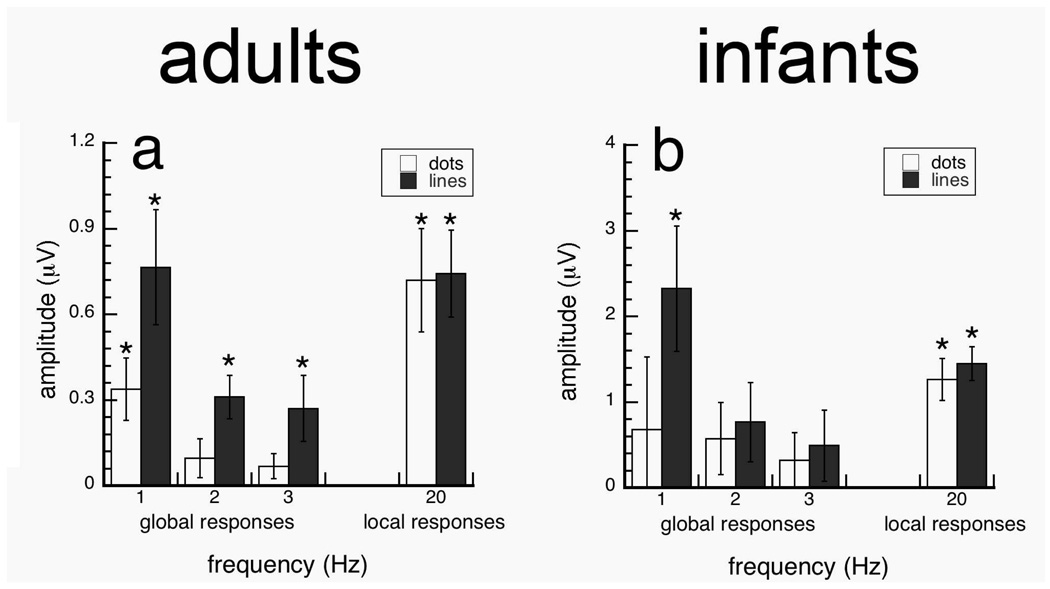
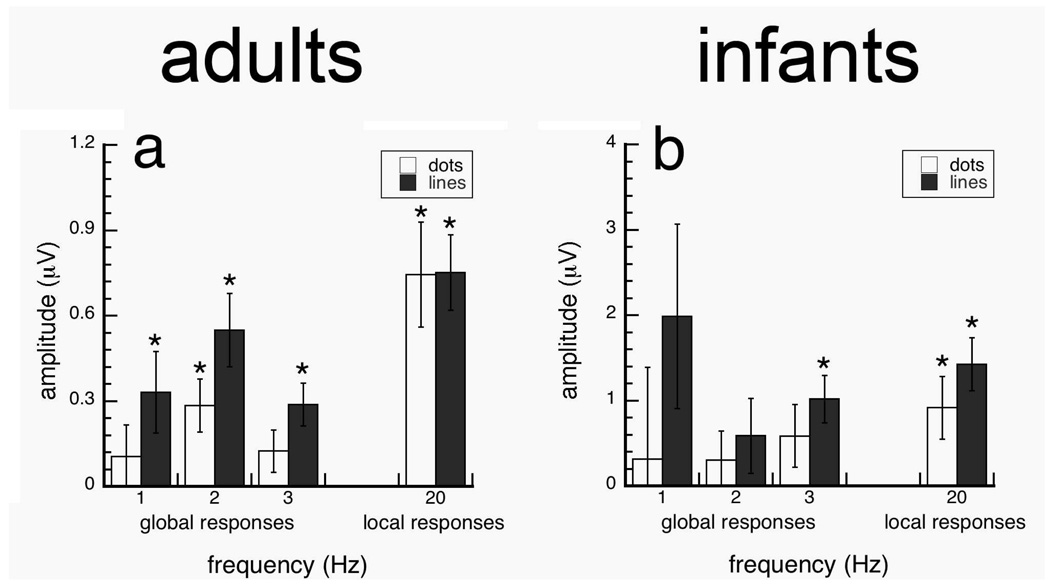
In the first experiment, we measured sensitivity of adults and infants to the local (at 20 Hz) and global (at 1–3 Hz) structure of Glass and line patterns using stimuli whose global structure alternated between globally horizontal and random (Fig. 4a–b). Data are presented from the Oz-Cz derivation because responses were maximal for both the 20 Hz and 1–3 Hz response components. We found that while infants were sensitive to the local update of both Glass and line patterns, they were sensitive to the changes in global structure of line pattern, but not Glass patterns. Meanwhile, adult responses showed sensitivity to changes in the local and global structure of both Glass and line patterns.
Figure 4.
Projected amplitudes of local (20 Hz) and global responses (1–3 Hz) to Glass and line patterns with a 49.6 separation/length for texture flows that alternated between horizontal to random global organizations. Adults and infants showed significant responses to the global structure of line patterns, but only adults showed significant responses to the global structure of Glass patterns. Local responses to the dot and line elements were also significant in infants and adults. Error bars represent ±SEM. *p-value<0.05, **p-value<0.001 two-tailed two-sample t-test.
Adult and infant responses for the local element update rate (20 Hz) were significantly different from zero (two-tailed one sample t-tests; p-values<0.05) for both dot and line patterns (Fig. 4). This indicates that the infants had at least encoded the presentation of local dots in our conditions.
Figure 4 shows that infant responses were only significant at the global update rate (1 Hz) for line patterns [t(17)=3.187, p=0.005], but not for conventional Glass patterns; no other responses were significantly different from zero (p-values > 0.30). Adult responses were reliably different from zero at 1–3 Hz (p>0.05) for line patterns, and were reliably different from zero only at 1 Hz [t(12)=3.093, p=0.009] for Glass patterns. These results suggest that although 4–5.5 month old infants are sensitive to global structure, but only if the local element orientation is made explicit (Fig 4b).
Experiment 2: Sensitivity to different texture flows in Glass and line patterns
Before concluding that responses at the global update reflect truly reflect global processing, we also considered the possible role of occasional junctions from overlapping line segments in random line patterns that could serve as a local cue between the coherent and incoherent textures (Fig. 1, top-middle panels). To eliminate these junctions and to test explicitly for the differential encoding to two different global organizations, we presented dot and line patterns that alternated between a horizontal global pattern and a co-circular one (Fig. 1, middle-bottom panels). Beyond controlling for local junction cues, these patterns test for mechanisms that can discriminate two different global configurations.
We found that both infant and adult evoked responses discriminated organized flows that differed only their global structure (Fig. 5). These results suggest that infants are truly sensitive to global structure -- if line patterns are used. Secondly, the selective responses to line patterns in Experiment 1 were not due to the incidental presence of local junctions formed by overlapping line segments. Not surprisingly, infants once more failed to show sensitivity to global structure in the dot based patterns.
Figure 5.
Projected amplitudes of local (20 Hz) and global responses (1–3 Hz) to Glass and line patterns with a 49.6 separation/length for texture flows that alternated between horizontal and co-circular organizations. Adults and infants showed significant responses to the global structure of line patterns, but only adults showed significant responses to the global structure of Glass patterns. Local responses to the dot and line elements were also significant in infants and adults. Error bars represent ±SEM. *p-value<0.05, **p-value<0.001 two-tailed two-sample t-test.
In infants, responses at the global update were significant for line patterns at 3 Hz [t(11)=3.691, p=0.004], but not at any other harmonic (p-values>0.05). Infant global responses to Glass patterns were not reliably different from zero (p-values>0.10). In adults, responses to the global structure of line-based patterns were reliable across the first three harmonics (1–3 Hz; Fig. 5a) of the global update rate (p-values <0.05). Adult responses to dot-based patterns were reliable at the second harmonic (2Hz) [t(12)=3.070, p=0.010].
The response spectra differed in detail for stimuli that had a horizontal to random transition compared to stimuli that had a horizontal to co-circular transition. In the adults, the responses peaked at 1 Hz in the former case (Fig. 4a–b), but peaked at 2 Hz in the latter (Fig. 5a–b). This pattern implies that responses to transitions between two global organizations are more similar than those between transitions between organized and random stimuli. Infant responses (to line patterns) by contrast were highest and most significant at odd harmonics (i.e., 1 or 3 Hz).
Experiment 3: Effect of local orientation strength
The only distinction between our dot and line stimuli is the presence of two additional pixels in the line patterns that connect the dots. Given this, it is likely that impoverished local orientation cues limit infants in their ability to globally integrate local features in to textures. Infants seem to fail in an intermediate integration stage: a failure to locally integrate dots into appropriate local orientations despite a capacity to globally integrate line segments into globally coherent structures. The ability to interpolate an orientation signal may depend on separation. In the following experiment, we, therefore, determined how dot-pair separation or line segment length affected global integration of Glass patterns. We expected that global responses to conventional Glass patterns would decrease as a function of dot pair length based on previous studies of psychophysical sensitivity (Dakin, 1997, Jenkins, 1983, Kurki et al., 2003), while global responses to connected line patterns might increase as a function of line segment length due to stronger orientation cues (Dakin, 1997).
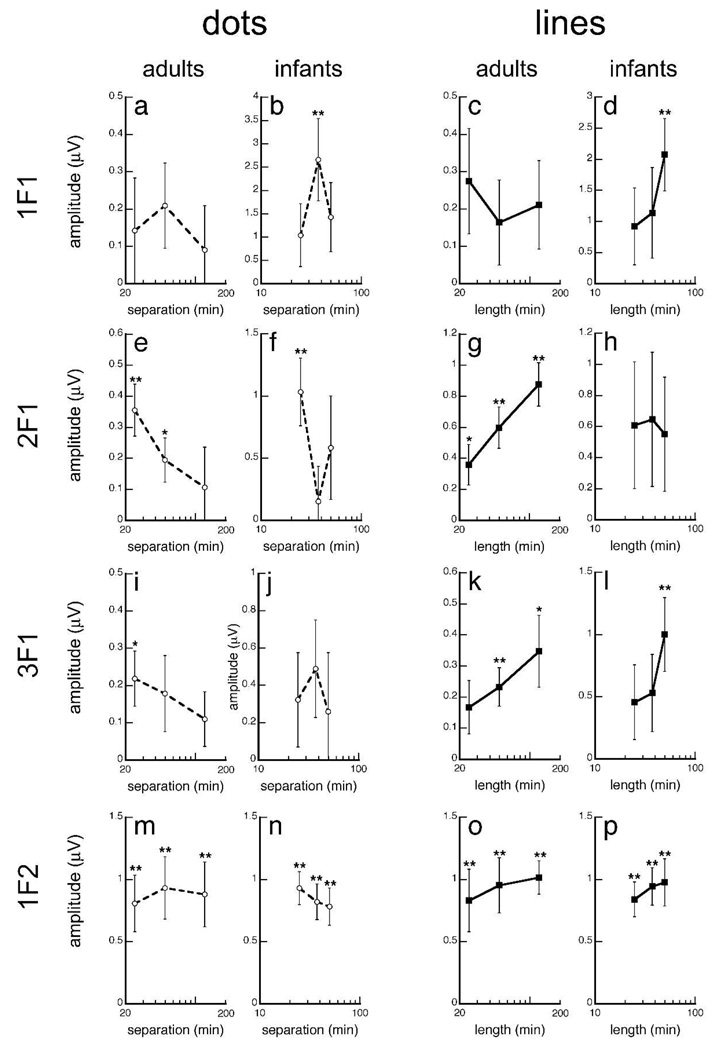
We presented the infants with Glass patterns where dot pair separations were smaller than in the first two experiments (24.8, 37.2 and 49.6 min end-to-end; Fig. 2), reasoning that interpolation might be easier at shorter, rather than larger, dot separations. Line segments of the same length were also presented (Fig. 2). For adults, we presented Glass and line patterns that had local element separations/lengths of 24.8, 49.6, and 124.0 min in an effort to find the maximal interpolation span for Glass patterns. Planned two-tailed t-tests comparing mean responses to zero were used to detect significant responses. For either infant or adult data, four separate 3 (length/separation) X 2 (stimulus type) ANOVAs were carried out to test for separation/line length effects at the different response frequencies (1, 2, 3 and 20 Hz).
We found that infants were sensitive to transitions between two global flows for line patterns and for dot patterns at smaller separations. Infants and adults show a similar trend of decreasing global responses with dot pair separation (Fig. 6e–f) in the face of increasing global responses with line length (Fig. 6k–l). Our data also show that element separation/length had no effect on local responses in infants and adults (Fig.6m–p).
Figure 6.
Projected amplitudes as a function of [LEFT] dot pair separation and [RIGHT] line length. Infants and adults show a similar trend -- global responses decreased with dot pair separation and increased with line length. However, infants have shorter interpolation spans than adults such that global responses were only significant when dot pair separation is <49.6 min. Error bars represent ±SEM. *p-value<0.05, **p-value<0.001 two-tailed two-sample t-test.
Infants have significant global responses for Glass patterns at separations smaller than the standard of 49.2 min used in the first two experiments, at the 37.2 min separation at 1 Hz [t(20)=3.008; p=0.007], and at 24.8 separation at 2 Hz [t(20)=3.792; p=0.001]. Infant responses to line patterns at corresponding lengths were only significant at 49.6 min at 1Hz [t(20)=3.558; p=0.002] and 3 Hz [t(20)=3.377; p=0.003]. In adults, we found that global responses were significantly different from zero for Glass patterns with separations smaller than 124 min, and for line patterns at all line lengths (see Fig. 6 for details).
In infants, the ANOVAs showed no significant main effects or interactions (p-values>0.10). In adults, the ANOVAs showed no significant main effects of element type, element length or interactions between them at 1 or 3 Hz (p-values>0.05). At 2 Hz, there was a significant effect of element type [F(1,9)=32.825; p<0.001], no significant effect of element length [F(2, 18)=2.057; p=0.157], and a significant interaction between them [F(2, 18)=7.498; p=0.004]. The non-significant effects in infants may reflect the limitations of the short element lengths chosen. It is possible that we would find a reliable interaction of these factors in infants if we extended the line element lengths beyond 49.6 min as in adults. If we restrict analyses of our adult data to element lengths less than 49.6 min, results would also show non-significant main effects and interactions (p-values>0.05). Local responses (20 Hz) had no significant effect of element type, no significant effect of element length and no significant interaction between them in either infants or adults (p-values>0.05; Fig.6m–p).
Experiment 4: Effect of element visibility in adults
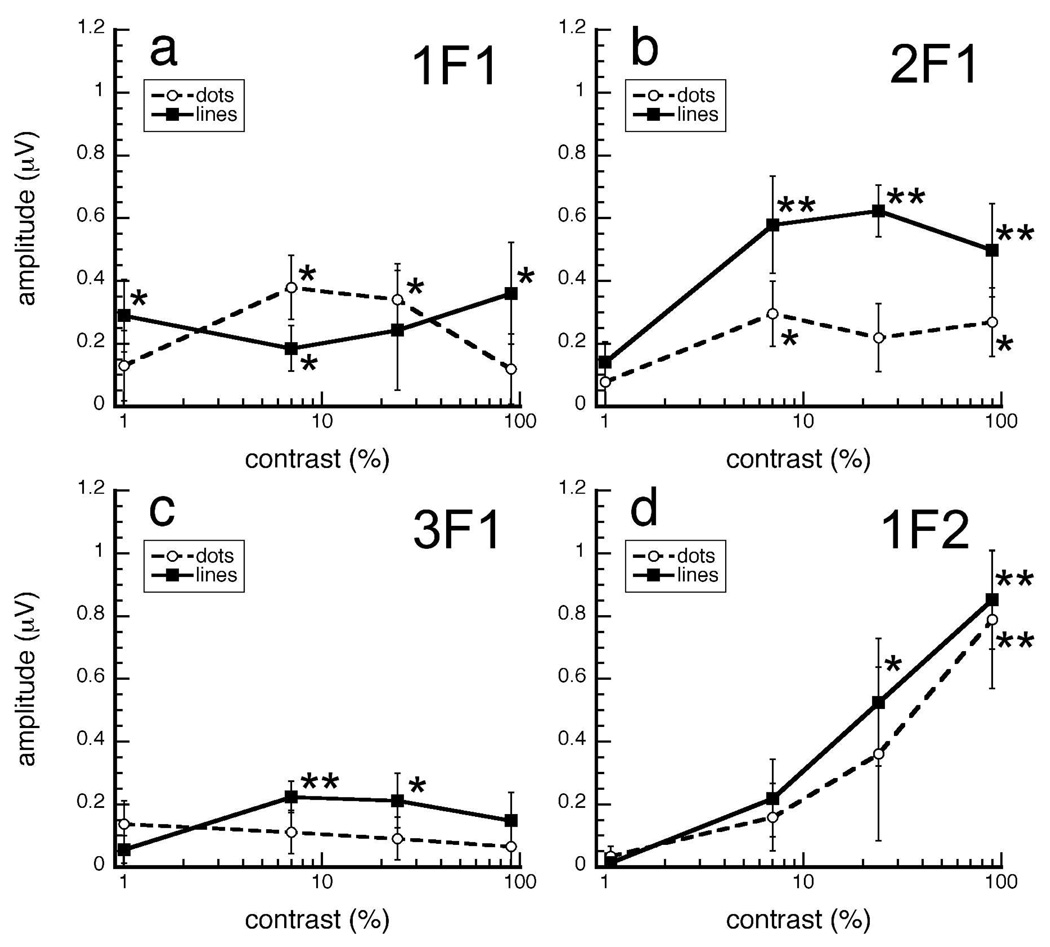
We have taken the presence of robust responses at the local update rate as indicating that the infants have successfully encoded the local pattern elements. However, visibility of local elements might still contribute to infant insensitivity to global patterns. Contrast sensitivity of 4-month old infants is about three times lower than contrast sensitivity of adults (Norcia, Tyler & Hamer, 1990) for 2 c/deg gratings, whose wavelength approximately matches the width of the elements in our Glass pattern stimuli. If limitations in contrast sensitivity simply determines coherence sensitivity to Glass patterns, then we would expect that matching the contrast sensitivity of infants by decreasing luminance contrast by a factor of three would eliminate coherence sensitivity in adults. Since we cannot make infant responses more adult-like by increasing our already maximal stimulus contrast, we sought to make adult responses more infant-like by decreasing stimulus contrast. We found that responses to the local update rate (20 Hz) depend strongly on the luminance contrast of the stimuli, but the responses to the harmonics of the global update rate (1–3 Hz) do not. Furthermore, local responses showed no difference between dots and lines, whereas global responses did.
We measured VEPs to dot and line patterns at 90, 24, 7 and 1% contrast in fourteen adults. We carried out a 4 (contrast) X 2 (stimulus type) ANOVA at four temporal frequencies (1, 2, 3 and 20 Hz). As expected, responses at the local update rate (20 Hz) decreased with contrast [F(3,39)=7.126, p=0.001] (Fig. 7d). Although the local responses to dots tended to be lower than responses to lines, they were not reliably different [F(1,13)=0.68, p=0.424]. The interaction of contrast and element type was also non-significant [F(3,39)=0.269, p=0.847], suggesting that decreasing luminance contrast affected the local update of dots and lines similarly.
Figure 7.
Projected amplitudes as a function of luminance contrast in adults. Global responses were primarily unaffected by changes in luminance contrast, whereas local responses were greatly affected. If infant insensitivity to global structure of Glass patterns is primarily caused by poorer contrast sensitivity, then adult sensitivity should be eliminated at 30% (arrow), the luminance contrast used in Expts. 1–2 reduced by a factor of 3. Error bars represent ±SEM. *p-value<0.05, **p-value<0.001 two-tailed two-sample t-test.
In contradistinction, we found the responses at the global update rate and its harmonics (1–3 Hz), were principally resilient to the effects of luminance contrast. Similar to Experiments 1 and 2, global responses at 2 Hz were more reliable. Main effects and interactions were absent at 1 or at 3 Hz (p-values>0.10). At 2 Hz, there was a main effect of element type [F(1,13)=11.873, p=0.004] confirming that observers had better global sensitivity for lines than for dots. The interaction between these factors was not significant [F(3,39)=1.844, p=0.155]. There was an effect of contrast [F(3,39)=3.574, p=0.022], but responses at this harmonic were constant between 7 and 90% (Fig. 7b).
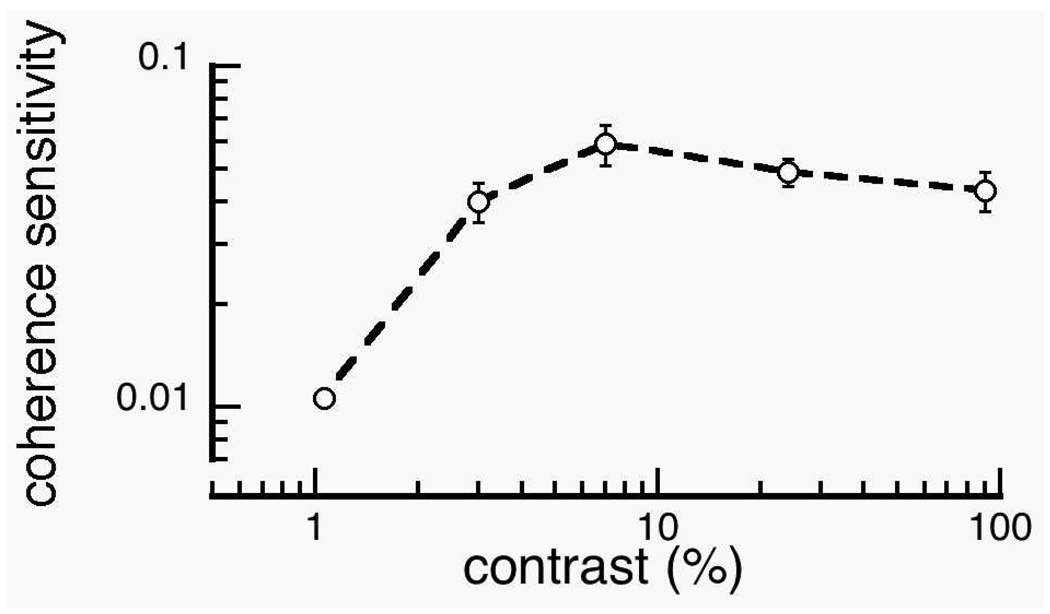
We also asked six of our observers to perform a two-alternative forced choice discrimination between coherent and random patterns. In this task, a fraction of the dots or lines adhered to the global rule and the rest were randomized. The fraction (percent coherence) of organized elements was placed on a staircase that converged on 82% correct responses for each contrast level. Threshold visibility of coherent Glass patterns, expressed as a sensitivity measure (Fig. 8), was largely invariant over contrast, similar to what we observed in the VEP. These data suggest that the global form of Glass patterns can be detected once the local elements are detected. A similar contrast insensitivity has been found in global motion integration (Burr & Santoro, 2001, Eagle & Rogers, 1997) and contour detection in noise tasks (Hess, Dakin & Field, 1998). Because the detectability of the global structure of Glass patterns was mostly invariant to the contrast of local elements in adults, the inability of 4- to 5.5- month old infants to detect the global structure of the dot Glass patterns does not likely stem solely from limitations in detecting local elements.
Figure 8.
Geometric mean of psychophysical coherence sensitivity as a function of luminance contrast in adults. Thresholds are plotted as the inverse of the percentage of coherent elements. Once the dots are visible, sensitivity is invariant to luminance contrast. Error bars represent ±SEM.
DISCUSSION
We investigated how infants extract global image structure in patterns that require at least three stages of processing: detection of the local elements, extraction of an orientation signal from the local elements and global integration of the constituent elements into a texture flow. Our demonstration of a differential response to transitions between two different global patterns of organization of line elements constitutes positive evidence for high-level pattern discrimination in infants. Nevertheless, infant sensitivity to global structure is quite fragile, as evidenced by their weak responses to Glass patterns. Slight reductions in the quality of the local orientation cues (e.g. removal of 2 central pixels, larger inter-dot separations (the present study) or the introduction of orientation noise (Pei et al., 2007) causes global integration to fail in infants. In the broader developmental context, Glass pattern sensitivity is adult-like only by 9 years of age (Lewis, Ellemberg, Maurer, Dirks, Wilkinson & Wilson, 2004) when studied behaviorally consistent with the notion that the processes underlying Glass pattern recognition have a protracted developmental sequence.
Limitations in the detection of local elements
The detection of global structure in Glass patterns involves integrative mechanisms at multiple spatial scales and developmental immaturities at any level or combination of levels could limit performance. At the lowest level, infants are known to have poor spatiotemporal contrast sensitivity (Banks & Bennett, 1988, Hartmann & Banks, 1992, Norcia et al., 1990). Infant contrast sensitivity for gratings of comparable spatial scale as that of the local elements in our Glass pattern (2 c/deg) is about three times worse than adult contrast sensitivity (Norcia et al., 1990) and immaturities of contrast sensitivity early in the visual system could thus preclude extraction of higher-level features.
Two of our results suggest that element visibility is not the critical limiting factor in Glass pattern processing in infants. First, the infants produced robust evoked responses at the element update rate (Fig. 4b, Fig. 5b and Fig. 6n, p) and the evoked responses for dots and lines with equivalent spatial spans were the same for all separations measured. Thus the local element responses for dots are not systematically weaker than those from line element displays. Second, Glass pattern detection does not depend strongly on contrast and inter alia visibility: evoked response amplitude and psychophysical thresholds for global structure in Glass patterns were each largely independent of stimulus contrast in the adults (Fig. 7–Fig. 8).
Limitations in extracting local parallel structure
Beyond simply detecting the presence of the dots in a Glass pattern, successful discrimination requires the extraction of an orientation signal from the dot pairs. This process will depend on the properties of receptive fields in early visual areas and any immaturities at this level will propagate forward to higher-level processes responsible for the discrimination of different patterns of global organization. We next consider whether the infants’ relative insensitivity to Glass patterns by may be due to immaturities in the properties of classical and non-classical receptive fields in early visual areas.
The classical receptive field is conventionally defined as that part of the visual field over which presentation of a small stimulus can elicit spiking (Barlow, Blakemore & Pettigrew, 1967). Orientation tuning within classical receptive fields arises independently of visual experience (Hubel & Wiesel, 1963, Wiesel & Hubel, 1974), and orientation-tuning bandwidth in macaque V1 is constant from 1 week of age to adulthood (Kiorpes & Movshon, 2004). Assuming the usual 4:1 conversion factor for macaque to human developmental age, classical receptive field orientation tuning is not likely to be a critical limiting factor at 4–6 months of age in humans.
The spatial extent of notional classical fields is shown as the small yellow circles surrounding some of the dot pairs in Fig. 1. Independent processing of orientation cues within such small integration areas would not elicit a differential population response in the VEP: each location in the visual field is populated with classical receptive fields of all orientations and thus equivalent responses would be generated by both random and organized patterns as exchanges of the patterns would activate equal numbers of cells.
More specialized receptive field structures could give rise to a differential response to organized versus random patterns that is detectable in by the VEP. As already noted, strictly local processing of individual orientation elements, even if they are lines, would not result in a differential population response. In adults, we suggest that non-classical receptive fields that preferentially integrate contrast along the orientation axis of explicit line stimuli, or along the implicit orientation defined by sparse dot pairs may contribute to the differential population response we record for organized versus random displays. A schematic depiction of these more extensive receptive fields is shown as yellow ellipses in Fig. 1. These more extensive receptive fields would be sensitive to the consistency of orientation information across multiple elements in the organized state of the pattern, but would be poorly driven by random stimuli.
Non-classical receptive fields encompass a more extensive region of visual space around classical receptive fields. Stimuli presented in non-classical surrounds modify the response to stimuli presented within the classical receptive field, but do not evoke a spiking response when presented in isolation (Angelucci & Bressloff, 2006, Angelucci, Levitt & Lund, 2002). Stimulation of the non-classical surround sharpens orientation tuning (Chen, Dan & Li, 2005, Henry, Dreher & Bishop, 1974, Rose, 1977) which would be useful in extracting locally parallel structure (see Smith, et al, 2002 for a quantitative analysis of how effective receptive field size controls sensitivity to implicit orientation in Glass patterns).
Using small localized grating patches, we previously found that infants isotropically pool orientation information over extended regions of space (Hou, Pettet, Sampath, Candy & Norcia, 2003). Infants pooled two collinear patches equally well as non-collinear patches. Adults, in comparison, preferentially pooled contrast of collinear elements. This result suggests that infants have a less spatially specific extended summation areas than adults.
Early research on Glass patterns suggested that the probability of incorrect pairings of dots limited the extraction of local orientation signal, which consequently constrained Glass pattern perception (Stevens, 1978). As Stevens observed, this probability increases as the dot density in a Glass pattern increases. If we define a critical integration area following Stevens as the area whose radius is the dot pair separation, then on average, there would only be 1 dot pair in the critical integration area at our standard 49.6 min separation2. Spurious dots that could elicit false pairings are very unlikely to fall within an integration area of this size, which is similar in size to our classical receptive field. Line patterns make the pairing explicit and negate the need to interpolate the orientation defined by a given dot pair. If, however, the infant non-classical surround were isotropic rather than elongated, they would be more susceptible than adults to false matches in the dot stimuli (large yellow circles, Fig. 1). For a dot pair separation of 124 min, the maximum separation tested, there will be 6.29 dot pairs within the critical area of 13.42 deg2. This effect can be appreciated by comparing the large circular summation areas to the elliptical ones in Figure 1.
This mechanism may also explain why infants only show sensitivity to Glass patterns when the dots are very close together. As dot separation increases, the nearest neighbor dot within an extended isotropic pooling area becomes increasingly likely to signal a random orientation rather than a consistent one. In adults, this effect may be mitigated by preferential pooling of input that arise from appropriately aligned, albeit more distant, dots.
The structure and function of non-classical surround mechanisms is poorly understood in the adult, and is even more so during development. Surround mechanisms appear to be in place in infant macaque V1 by 2 weeks of age and in V2 by 4 weeks of age, but their modulatory effects on classical receptive fields become adult-like much later (Zhang, Zheng, Watanabe, Maruko, Bi, Smith & Chino, 2005). Anatomical tracer studies in human post-mortem samples have shown only weak evidence for horizontal connections at 4 months of age (Burkhalter, Bernardo & Charles, 1993). Similarly, feedback connections were also immature in humans at this age (Burkhalter, 1993). Thus it is likely that the anatomical substrate for non-classical receptive field refinement leading to enhanced orientation and spatial selectivity may be functionally immature in the infants we tested.
Global form processing limitations
Although the ability to globally integrate elements into a pattern in infants exists, the detailed characteristics of the infant evoked responses are qualitatively different from those of adults. Infants primarily have stronger odd harmonic responses to line patterns that transition from co-circular to horizontal structures (Fig. 4b), while adults show stronger even harmonic responses (Fig. 5b). Degrading local orientation signals by presenting dot pairs instead of lines (Expt. 1–2) presumably weakens the input to higher-level mechanisms that process the specific form of different global organizations. The fact that adult global responses survive this degradation, but that infant responses do not, suggests that the global integration stage in infant can only operate on strong inputs from earlier in the pathway. It is also possible that infants may require more time to extract an orientation signal from dot pairs than from lines, and a slower global update rate may be have been necessary. Notably, however, infants can successfully extract motion (Gilmore, Hou, Pettet & Norcia, 2007) and stereo (Birch & Petrig, 1996, Petrig, Julesz, Kropfl, Baumgartner & Anliker, 1981, Skarf, Eizenman, Katz, Bachynski & Klein, 1993) information from dot stimuli within the same time period of one second.
Global integration in a static glass pattern task has been found to occur in a network of extra-striate visual areas including ventral stream areas V4, areas anterior to V4 and LOC (Ostwald et al., 2008). A similar network of areas is also activated in a contour-integration task that involves the linking of nearly collinear chains of Gabor patches that are embedded in a background of randomly oriented patterns (Altmann, Deubelius & Kourtzi, 2004). Like Glass patterns, Gabor-defined contours elicit only weak VEPs in infants (Norcia et al., 2005), suggesting that ventral stream areas are late developing. Dynamic Glass patterns are processed in a range of early visual areas, including V1-V4 as well as in the LOC. However, dynamic Glass patterns also engage V3A and hMT+ (Krekelberg, Vatakis & Kourtzi, 2005). It is currently not known whether the dynamic Glass pattern activity in dorsal areas is dependent on input from ventral stream areas, or if it arises independently. Development may not proceed uniformly over the extended network of areas are activated by dynamic Glass patterns. Future developmental comparisons of static and dynamic Glass pattern sensitivity may in help to resolve this question.
Relationship to other developmental studies of global integration
Our Glass and line pattern study resemble several other studies that involve hierarchical integration of a local cue into a global configuration, such as detection of orientation coherence from Gabor textures, texture segmentation of line-based elements, and detection of motion and stereo cues from random dot kinematograms.
We have also used Gabor-defined textures to test for global integration. We found robust differential VEP responses between coherent and random textures in infants who were unresponsive to Glass patterns (Supplementary data; see also (Norcia et al., 2005, Pei et al., 2007)). Since it has frequently been argued that interaction between widely separated Gabor elements depends on mechanisms that extend the size of the classically defined V1 receptive fields (Cavanaugh, Bair & Movshon, 2002, Kapadia, Westheimer & Gilbert, 1999, Polat, Mizobe, Pettet, Kasamatsu & Norcia, 1998, Polat & Norcia, 1998), activity in V1 non-classical surrounds and/or extrastriate areas (Altmann et al., 2003) must contribute to texture processing in infants. Feedback processes from higher areas acting on lower areas may help to select matches that lead to a more consistent global organization.
The ability to segment a patch of coherent texture from line elements has also been found to be present in infants over 3 months of age (Braddick & Atkinson, 2007) measured behaviorally and with VEPs. Our current study extends these findings in three ways. First, we show in Experiment 2 that infants are sensitive to the global organization of line textures even without local line crossings from random textures. In Braddick and Atkinson (2007), there were numerous line crossings in the random stimulus state that could be processed at a local level. Second, we demonstrate that infants are sensitive to global structure of texture in the absence of local texture segmentation cues, steep texture discontinuities between organized and random fields. Third, we show that infants can detect changes in global structure between two organized texture flows, not just detection of differences between organized and random textures. This would suggest that young infants truly integrate global structure across a large span of visual field, rather than simply detecting locally coherent patches of line elements.
More is known about the cortical networks involved in two other global integration tasks that resemble the dynamic Glass pattern task in many ways: coherent motion and random dot stereogram tasks. In the coherent motion task, a series of random-dot elements is systematically displaced according to a global constraint much like that used to generate Glass patterns. Here the pairing of dots occurs over time, rather that over space. Coherent motion BOLD activations predominate in hMT+ and V3A and are not prominent in ventral surface areas such as V4 (Koyama et al., 2005, Smith et al., 2006, Tootell et al., 1997). Infant VEPs are more robust to coherent motion displays at 4–6 months of age than are Glass pattern responses comprised of dots of similar size and update rates (Gilmore et al., 2007). Behavioral sensitivity for coherent motion displays also develops earlier for coherent motion than for Glass patterns in infant macaques (Kiorpes & Movshon, 2003), suggesting that development is more advanced for the complement of dorsal stream visual areas.
In the dynamic random-dot stereogram task, successful performance requires extraction of dot displacements that occur across the two eyes (binocular disparity). Evoked responses to dynamic random dot stereograms have been be demonstrated by 3–5 months of age in infants (Birch & Petrig, 1996, Petrig et al., 1981, Skarf et al., 1993). This task activates many cortical areas, including V1, V2, V4 and MT and IT in macaque (Parker, 2007). In human, activation is particularly strong in V3A and caudal IPS (Backus, Fleet, Parker & Heeger, 2001, Tsao, Vanduffel, Sasaki, Fize, Knutsen, Mandeville, Wald, Dale, Rosen, Van Essen, Livingstone, Orban & Tootell, 2003, Wall & Smith, 2008). The fact that robust evoked responses can be recorded in infants for coherent motion or stereogram displays that are based on the same spatiotemporal local elements used to generate Glass patterns further indicates that performance on the Glass pattern task is not primarily limited by immature spatiotemporal contrast sensitivity. Rather, significant immaturities must exist in the anatomically and functionally specialized networks leading to and including ventral stream areas such as V4 and the LOC where fMRI studies indicate the presence of strong activation.
Given the complex, hierarchical nature of the Glass pattern task, it is perhaps not surprising that performance may be limited by multiple developmental immaturities operating at the level of local orientation generation and global integration of these orientation signals over large areas of the visual image. Our analysis suggests that single, critical limitations do not exist at any one of these stages, but rather, weaknesses at each of these levels may conspire to degrade infants’ sensitivity.
Acknowledgments
This research was funded by the National Institutes of Health (EY014536 and EY06579) and the Pacific Vision Foundation. We thank Margaret McGovern and Justin Ales for help in recruiting and running participants. Preliminary data from this manuscript were presented at the 2008 Vision Sciences Society Conference in Naples, FL.
Footnotes
In Braddick, et al (1986), the phase of a grating refreshed every 40 ms (25 Hz), while the orientation of the grating periodically changed every 120 ms (8.33 Hz). A full cycle of orientation changes thus occurred at 4.16 Hz). They recorded an orientation-change response at 8.33 Hz, which was their second harmonic. Because the population response to a change in orientation from left oblique to right oblique is the same, they did not observe odd harmonic responses. The fourth harmonic in their experiment (in principle) also contained an orientation-specific response In the present study, the frequency ratio between local presentation of the dots and change in global structure of our stimuli is 20:1. Thus, there are many more harmonics of the global update rate that could indicate a significant response to changes in the global pattern.
Critical area=πr2, where r is the dot pair separation. For the standard separation of 49.6 min=0.83 deg, the critical area is π(0.83 deg)2 or 2.14 deg2. For a density of 0.47 dot pairs/deg2, the number of dots in the critical area is 0.47 dot pairs x 2.2 deg or about 1 dot pair within the critical area.
References
- Altmann CF, Bulthoff HH, Kourtzi Z. Perceptual organization of local elements into global shapes in the human visual cortex. Curr Biol. 2003;13(4):342–349. doi: 10.1016/s0960-9822(03)00052-6. [DOI] [PubMed] [Google Scholar]
- Altmann CF, Deubelius A, Kourtzi Z. Shape saliency modulates contextual processing in the human lateral occipital complex. J Cogn Neurosci. 2004;16(5):794–804. doi: 10.1162/089892904970825. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Prog Brain Res. 2002;136:373–388. doi: 10.1016/s0079-6123(02)36031-x. [DOI] [PubMed] [Google Scholar]
- Backus BT, Fleet DJ, Parker AJ, Heeger DJ. Human cortical activity correlates with stereoscopic depth perception. J Neurophysiol. 2001;86(4):2054–2068. doi: 10.1152/jn.2001.86.4.2054. [DOI] [PubMed] [Google Scholar]
- Banks MS, Bennett PJ. Optical and photoreceptor immaturities limit the spatial and chromatic vision of human neonates. J Opt Soc Am A. 1988;5(12):2059–2079. doi: 10.1364/josaa.5.002059. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Blakemore C, Pettigrew JD. The neural mechanism of binocular depth discrimination. J Physiol. 1967;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch E, Petrig B. FPL and VEP measures of fusion, stereopsis and stereoacuity in normal infants. Vision Res. 1996;36(9):1321–1327. doi: 10.1016/0042-6989(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J. Development of brain mechanisms for visual global processing and object segmentation. Prog Brain Res. 2007;164:151–168. doi: 10.1016/S0079-6123(07)64008-4. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JM, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001;30(1):61–72. doi: 10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JM, Wattam-Bell J, Atkinson J, Turner R. Form and motion coherence activate independent, but not dorsal/ventral segregated, networks in the human brain. Curr Biol. 2000;10(12):731–734. doi: 10.1016/s0960-9822(00)00540-6. [DOI] [PubMed] [Google Scholar]
- Burkhalter A. Development of forward and feedback connections between areas V1 and V2 of human visual cortex. Cereb Cortex. 1993;3(5):476–487. doi: 10.1093/cercor/3.5.476. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Bernardo KL, Charles V. Development of local circuits in human visual cortex. J Neurosci. 1993;13(5):1916–1931. doi: 10.1523/JNEUROSCI.13-05-01916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Santoro L. Temporal integration of optic flow, measured by contrast and coherence thresholds. Vision Res. 2001;41(15):1891–1899. doi: 10.1016/s0042-6989(01)00072-4. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol. 2002;88(5):2530–2546. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- Chen G, Dan Y, Li CY. Stimulation of non-classical receptive field enhances orientation selectivity in the cat. J Physiol. 2005;564(Pt 1):233–243. doi: 10.1113/jphysiol.2004.080051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin SC. The detection of structure in glass patterns: psychophysics and computational models. Vision Res. 1997;37(16):2227–2246. doi: 10.1016/s0042-6989(97)00038-2. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Bex PJ. Local and global visual grouping: tuning for spatial frequency and contrast. J Vis. 2001;1(2):99–111. doi: 10.1167/1.2.4. [DOI] [PubMed] [Google Scholar]
- Eagle RA, Rogers BJ. Effects of dot density, patch size and contrast on the upper spatial limit for direction discrimination in random-dot kinematograms. Vision Res. 1997;37(15):2091–2102. doi: 10.1016/s0042-6989(96)00153-8. [DOI] [PubMed] [Google Scholar]
- Gerhardstein P, Kovacs I, Ditre J, Feher A. Detection of contour continuity and closure in three-month-olds. Vision Res. 2004;44(26):2981–2988. doi: 10.1016/j.visres.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Gilmore RO, Hou C, Pettet MW, Norcia AM. Development of cortical responses to optic flow. Vis Neurosci. 2007;24(6):845–856. doi: 10.1017/S0952523807070769. [DOI] [PubMed] [Google Scholar]
- Glass L. Moire effect from random dots. Nature. 1969;223(206):578–580. doi: 10.1038/223578a0. [DOI] [PubMed] [Google Scholar]
- Glass L, Switkes E. Pattern recognition in humans: correlations which cannot be perceived. Perception. 1976;5(1):67–72. doi: 10.1068/p050067. [DOI] [PubMed] [Google Scholar]
- Hartmann EE, Banks MS. Temporal contrast sensitivity in human infants. Vision Res. 1992;32(6):1163–1168. doi: 10.1016/0042-6989(92)90018-e. [DOI] [PubMed] [Google Scholar]
- Henry GH, Dreher B, Bishop PO. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Hess RF, Dakin SC, Field DJ. The role of "contrast enhancement" in the detection and appearance of visual contours. Vision Res. 1998;38(6):783–787. doi: 10.1016/s0042-6989(97)00333-7. [DOI] [PubMed] [Google Scholar]
- Hou C, Pettet MW, Norcia AM. Abnormalities of coherent motion processing in strabismic amblyopia: Visual-evoked potential measurements. J Vis. 2008;8(4):1–12. doi: 10.1167/8.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Pettet MW, Sampath V, Candy TR, Norcia AM. Development of the spatial organization and dynamics of lateral interactions in the human visual system. J Neurosci. 2003;23(25):8630–8640. doi: 10.1523/JNEUROSCI.23-25-08630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive Fields of Cells in Striate Cortex of Very Young, Visually Inexperienced Kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Jenkins B. Spatial limits to the detection of transpositional symmetry in dynamic dot textures. J Exp Psychol Hum Percept Perform. 1983;9(2):258–269. doi: 10.1037//0096-1523.9.2.258. [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Westheimer G, Gilbert CD. Dynamics of spatial summation in primary visual cortex of alert monkeys. Proc Natl Acad Sci U S A. 1999;96(21):12073–12078. doi: 10.1073/pnas.96.21.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Extended developmental time course for global visual functions in primates [Abstract] Journal of vision. 2003;2(10):47a. [Google Scholar]
- Kiorpes L, Movshon JA. Neural Limitations on Visual Development in Primates. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2004. pp. 159–173. [Google Scholar]
- Koyama S, Sasaki Y, Andersen GJ, Tootell RB, Matsuura M, Watanabe T. Separate processing of different global-motion structures in visual cortex is revealed by FMRI. Curr Biol. 2005;15(22):2027–2032. doi: 10.1016/j.cub.2005.10.069. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Vatakis A, Kourtzi Z. Implied motion from form in the human visual cortex. J Neurophysiol. 2005;94(6):4373–4386. doi: 10.1152/jn.00690.2005. [DOI] [PubMed] [Google Scholar]
- Kurki I, Laurinen P, Peromaa T, Saarinen J. Spatial integration in Glass patterns. Perception. 2003;32(10):1211–1220. doi: 10.1068/p5102. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Ellemberg D, Maurer D, Dirks M, Wilkinson F, Wilson HR. A window on the normal development of sensitivity to global form in Glass patterns. Perception. 2004;33(4):409–418. doi: 10.1068/p5189. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci. 2000;3(12):1322–1328. doi: 10.1038/81860. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Pei F. Development of vision and visual attention. In: Cioni GM, Mercuri E, editors. Clinics in Developmental Medicine No. 176: Neurological assessment in the first two years of life: instruments for the follow-up of high-risk newborns. London: MacKeith Press; 2007. pp. 198–213. [Google Scholar]
- Norcia AM, Pei F, Bonneh Y, Hou C, Sampath V, Pettet MW. Development of sensitivity to texture and contour information in the human infant. J Cogn Neurosci. 2005;17(4):569–579. doi: 10.1162/0898929053467596. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Tyler CW, Hamer RD. Development of contrast sensitivity in the human infant. Vision Res. 1990;30(10):1475–1486. doi: 10.1016/0042-6989(90)90028-j. [DOI] [PubMed] [Google Scholar]
- Ostwald D, Lam JM, Li S, Kourtzi Z. Neural coding of global form in the human visual cortex. J Neurophysiol. 2008;99(5):2456–2469. doi: 10.1152/jn.01307.2007. [DOI] [PubMed] [Google Scholar]
- Parker AJ. Binocular depth perception and the cerebral cortex. Nat Rev Neurosci. 2007;8(5):379–391. doi: 10.1038/nrn2131. [DOI] [PubMed] [Google Scholar]
- Pei F, Pettet MW, Norcia AM. Sensitivity and configuration-specificity of orientation-defined texture processing in infants and adults. Vision Res. 2007;47(3):338–348. doi: 10.1016/j.visres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei F, Pettet MW, Vildavski VY, Norcia AM. Event-related potentials show configural specificity of global form processing. Neuroreport. 2005;16(13):1427–1430. doi: 10.1097/01.wnr.0000177003.12322.9b. [DOI] [PubMed] [Google Scholar]
- Petrig B, Julesz B, Kropfl W, Baumgartner G, Anliker M. Development of stereopsis and cortical binocularity in human infants: electrophysiological evidence. Science. 1981;213(4514):1402–1405. doi: 10.1126/science.7268443. [DOI] [PubMed] [Google Scholar]
- Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual responses depending on cell's contrast threshold. Nature. 1998;391(6667):580–584. doi: 10.1038/35372. [DOI] [PubMed] [Google Scholar]
- Polat U, Norcia AM. Elongated physiological summation pools in the human visual cortex. Vision Res. 1998;38(23):3735–3741. doi: 10.1016/s0042-6989(97)00426-4. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Bhatt RS, Brush D, Grimes A, Sharpnack H. Development of form similarity as a Gestalt grouping principle in infancy. Psychol Sci. 2002;13(4):320–328. doi: 10.1111/1467-9280.00459. [DOI] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3(7):716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Rose D. Responses of single units in cat visual cortex to moving bars of light as a function of bar length. J Physiol. 1977;271(1):1–23. doi: 10.1113/jphysiol.1977.sp011987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y. Processing local signals into global patterns. Curr Opin Neurobiol. 2007;17(2):132–139. doi: 10.1016/j.conb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Skarf B, Eizenman M, Katz LM, Bachynski B, Klein R. A new VEP system for studying binocular single vision in human infants. J Pediatr Ophthalmol Strabismus. 1993;30(4):237–242. doi: 10.3928/0191-3913-19930701-05. [DOI] [PubMed] [Google Scholar]
- Smith AT, Wall MB, Williams AL, Singh KD. Sensitivity to optic flow in human cortical areas MT and MST. Eur J Neurosci. 2006;23(2):561–569. doi: 10.1111/j.1460-9568.2005.04526.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Bair W, Movshon JA. Signals in macaque striate cortical neurons that support the perception of glass patterns. J Neurosci. 2002;22(18):8334–8345. doi: 10.1523/JNEUROSCI.22-18-08334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KA. Computation of locally parallel structure. Biological Cybernetics. 1978;29(1):19–28. [Google Scholar]
- Tang Y, Norcia AM. An adaptive filter for steady-state evoked responses. Electroencephalogr Clin Neurophysiol. 1995;96(3):268–277. doi: 10.1016/0168-5597(94)00309-3. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17(18):7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15(4):3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Vanduffel W, Sasaki Y, Fize D, Knutsen TA, Mandeville JB, Wald LL, Dale AM, Rosen BR, Van Essen DC, Livingstone MS, Orban GA, Tootell RB. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron. 2003;39(3):555–568. doi: 10.1016/s0896-6273(03)00459-8. [DOI] [PubMed] [Google Scholar]
- Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78(5):378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- Wall MB, Smith AT. Sensitivithy of human visual cortical areas to the stereoscopic depth of a moving stimulus. Journal of Vision. 2008;8(10):1–12. doi: 10.1167/8.10.1. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neuol. 1974;158(3):307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zheng J, Watanabe I, Maruko I, Bi H, Smith EL, 3rd, Chino Y. Delayed maturation of receptive field center/surround mechanisms in V2. Proc Natl Acad Sci U S A. 2005;102(16):5862–5867. doi: 10.1073/pnas.0501815102. [DOI] [PMC free article] [PubMed] [Google Scholar]