Abstract
Background
There is growing evidence for the familiality of pediatric bipolar disorder (BPD) and its association with impairments on measures of processing speed, verbal learning and “executive” functions. The current study investigated whether these neurocognitive impairments index the familial risk underlying the diagnosis.
Methods
Subjects were 170 youth with BPD (mean age 12.3 yrs), their 118 non-mood disordered siblings and 79 non mood-disordered controls. Groups were compared on a battery of neuropsychological tests from the Wechsler Intelligence Scales, the Stroop, the Wisconsin Card Sorting Test, the Rey-Osterreith Complex Figure, an auditory working memory CPT and the California Verbal Learning Test (Child Edition). Measures were factor analyzed for data reduction purposes. All analyses controlled for age, sex and ADHD.
Results
Principal components analyses with a promax rotation yielded three factors reflecting: 1) processing speed/verbal learning; 2) working memory/interference control and 3) abstract problem solving. A CPT working memory measure with interference filtering demands (WM INT) was only administered to subjects 12 and older and thus analyzed separately. BPD youth showed impairments versus controls and unaffected relatives on all three factors and the WM INT. Unaffected relatives exhibited impairments versus controls on the abstract problem-solving factor and the WM INT. They also showed a statistical trend (p=.07) toward worse performance on the working/memory interference control factor.
Conclusions
Neurocognitive impairments in executive functions may reflect the familial neurobiological risk mechanisms underlying pediatric BPD and may have utility as endophenotypes in molecular genetic studies of the condition.
Keywords: pediatric bipolar disorder, executive functions, endophenotype
INTRODUCTION
Pediatric-onset bipolar disorder (BPD) is a disabling, under-studied condition characterized by extreme affective and behavioral dysregulation (Biederman 2003). Understanding its etiology is a public health priority given that it impacts up to 20% of child psychiatric referrals in some settings (Biederman and James 2004) and is associated with increased risk for suicide attempts (Goldstein et al. 2005) and hospitalization (Meyer et al. 2004).
Although neurobiological studies of this condition are limited, a growing literature has documented neurocognitive weaknesses in youth with BPD on measures that tap prefrontal-subcortical circuits. Consistent with adult BPD studies, impairments in affected youth have been observed on measures of attention, verbal and visuospatial memory, processing speed and “executive” functions (e.g. (Dickstein et al. 2004; Doyle, Wilens et al. 2005; Glahn et al. 2005; McClure et al. 2005; Pavuluri et al. 2006; Bearden et al. 2007). Recently, a meta-analysis (Torres et al. 2007) confirmed neurocognitive weaknesses in euthymic adult samples, suggesting that they reflect trait-like rather than state-related impairments. Although fewer studies have addressed this issue in young samples, Henin et al. (2007) have shown that impairments in unmedicated BPD youth were not generally associated with BPD symptomatology. Others have also that neurocognitive impairments in juvenile samples are independent of the severity of manic symptomatology (Glahn et al. 2005; McClure et al. 2005; Pavuluri et al. 2006; Bearden et al. 2007), comorbidity with other disorders (Glahn et al. 2005; Bearden et al. 2007) and medication status (Pavuluri et al. 2006).
Given that the neurocognitive functions found to be impaired in BPD youth are critical to self-regulation, such decrements may index the etiological risk mechanisms underlying the behavioral symptoms of the disorder. If so, since the condition itself has been shown to be familial (Faraone et al. 2003), neurocognitive impairments should be observed in unaffected relatives of affected individuals. In the handful of such studies of adults with BPD, unaffected relatives have shown compromised processing speed (Sobczak et al. 2002) executive functions (Zalla et al. 2004; Clark et al. 2005), verbal memory (Gourovitch et al. 1999; Keri et al. 2001) and divided attention (Sobczak et al. 2003), though inconsistencies exist as to impaired domains and measures across studies (Savitz et al. 2005). To date, no studies have examined neurocognition in unaffected relatives of youth with BPD. Confirmation of a familial link between pediatric BPD and neurocognitive impairments has theoretical and practical implications. For example, distinguishing the impairments that are related to familial risk from those that are sequelae of the disorder could shed light on the pathophysiology of the condition. Such data could also reveal intermediate “endophenotypes” to serve as targets in molecular genetic studies that, in turn, could reveal the origins of the condition and etiologic overlap with the adult-onset disorder.
The current study investigated these issues using youth ascertained through family studies of pediatric-onset BPD. We compared affected probands, their non-mood-disordered relatives and unrelated non-mood disordered controls on a large battery of measures that have been shown to be impaired in BPD in the literature. Because a substantial proportion of BPD youth meet criteria for comorbid attention-deficit/hyperactivity disorder (ADHD) (Borchardt and Bernstein 1995), which itself is associated with neurocognitive deficits (Pennington and Ozonoff 1996), and the familial transmission of BPD (Faraone et al. 1998; Geller et al. 2006) all analyses controlled for ADHD. Our primary hypothesis was that impairments in “executive” functioning and verbal learning index a latent trait (or traits) that shares familial etiology with the disorder. Thus, we predicted that unaffected siblings of youth with BPD would show significantly greater impairments than controls after controlling for ADHD.
Methods & Materials
Subjects came from two ongoing family studies of pediatric BPD conducted at the Massachusetts General Hospital (MGH) (Wilens et al. 2004; Wozniak et al. 2005). Cases were unrelated youth who met full DSM-IV criteria for BPD I or II at some point in their lifetime. In accordance with our case-control family study design, controls were unrelated youth who had no history of BPD, major depression or dysthymia. Cases were 4 to 18 years old, though only youth ≥ 7 were included in the current analyses, due to age constraints for completing the full neurocognitive battery. Cases were referred via local clinicians or self-referred through internal hospital postings and Internet/community advertisements. Control probands were drawn from comparable advertisements and hospital postings. Potential subjects were screened over the telephone. Probands were excluded if they had major sensorimotor handicaps, a suspected IQ < 70 or first-degree biological family relatives unavailable for study. Subjects passing these screens were invited to enroll in the project along with their first-degree biological relatives. Probands and relatives were told the aims, procedures, risks or discomforts, and benefits of the study. For children, information was conveyed using developmentally appropriate language. Written informed consent was obtained from individuals ≥18 years and from parents for minor children. Written assent was obtained from minors. Families whose proband diagnostic status was confirmed by interview and blind clinician review were maintained in the study. Procedures were approved by the MGH Human Research Committee.
Structured Diagnostic Interviews
Raters had undergraduate degrees in psychology or a related field and were blind to subjects’ ascertainment status. Interviews were conducted using the Schedule for Affective Disorders in Children – Epidemiologic Version (KSADS-E; Orvaschel 1994) for youth 17 and younger and the Structured Clinical Interview for DSM-IV (SCID; First et al. 1997) and KSADS-E childhood disorders for individuals ≥18. Information was obtained from independent interviews with the primary caregiver as well as youth ≥12. Diagnoses were scored positive if all DSM-IV criteria were met in either interview and were confirmed by board-certified child psychiatrists and psychologists who were unaware of the subject’s ascertainment group, neuropsychological data and family history. Interviewers/clinicians did not depart from or modify DSM-IV criteria when diagnosing a manic episode.
Interviews were audiotaped with permission. To assess reliability, we randomly selected 20 interviews to be re-rated by an expert in the field of pediatric and adult BPD (JW) who was blind to all subject information. Results showed high agreement between the interviewer and expert for ratings of mania (kappa=.95).
Neuropsychological Testing
We estimated Full Scale IQ (FSIQ; Sattler 1988) from the Vocabulary and Block Design subtests of the Wechsler Intelligence Scale for Children – Third Edition (WISC-III; Wechsler 1991) for individuals < 17 years and the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III; Wechsler 1997) for individuals ≥17. Remaining tests were selected to represent measures and constructs found to be impaired in adult and youth with BPD in the literature, many of which are thought to be indirect indices of frontal-system and pathways. These include components of executive functions (ie working memory, interference control, abstract problem solving/set shifting and planning/visuospatial organization) as well as attention, processing speed and verbal learning. Subtests from well-studied clinical instruments were employed including: the Arithmetic, Digit Span, Digit Symbol/Coding and Symbol Search subtests from the WISC-III/WAIS-III; the color naming and interference scores from the Stroop Color Word Test (Golden 1978); perseverative and non-perseverative errors on the computerized Wisconsin Card Sorting Test (WCST; Heaton et al. 1993); copy organization from Bernstein and Waber’s (Bernstein and Waber 1996) scoring of the Rey-Osterrieth Complex Figure (ROCF; Osterrieth 1944), the trials 1–5 score on the California Verbal Learning Test- Child Edition (Delis et al. 1994) and Second Edition (CVLT-II) for subjects 17 years or older (Delis et al. 2000); and the ‘vigilance’, ‘working memory’ and ‘working memory plus interference control’ measures from the Seidman auditory CPT (Seidman et al. 1998). All scores reflect age-based standard scores from published manuals, with the exception of the Seidman CPT and ROCF, which were translated into age-based T scores using data on 1,142 individuals from control families assessed through studies at MGH (normative data available from the first author).
Tests were administered and, except for the ROCF, scored by psychometricians trained and supervised by licensed neuropsychologists (AED, RF, LJS). The ROCF was scored by individuals with Master’s degrees in Psychology who were blind to subject characteristics.
Statistical Analysis
Three groups were created based on proband and relative diagnostic status for families and individuals whose data had been collected, cleaned and scored at the time of analysis-- specifically the unrelated affected probands, the biological siblings of the affected youth and the unrelated control probands. The first group included index cases with full DSM-IV BPD I or BPD II diagnoses. The second group included the “unaffected” full biological siblings of these individuals with no history of BPD I or II, major depressive disorder or dysthymia. The third group included the non-BPD, non-mood disordered control probands. This stratification resulted in 170 BPD probands, 118 unaffected relatives of BPD probands and 79 controls. Among the BPD families, sixty-eight had one unaffected sibling, and twenty-five included two unaffected siblings.
We conducted two sets of analyses. First, for data reduction purposes, we conducted a principle components factor analysis of neurocognitive measures. We used a promax rotation to allow for correlation among factors, given potentially overlapping neurocognitive domains. This strategy aimed to reduce Type I error and to capitalize on increased validity derived from the shared variance among measures. Support for combining measures comes from twin studies (e.g. Kuntsi et al. 2005) in which composite scores have been shown to yield greater familial aggregation with behavioral symptoms than scores on individual tests. The “Interference” score of the Seidman CPT (WM INT) was analyzed separately because it was administered to subjects ≥12 years (N = 189) and thus would have excluded younger subjects from the factor analysis.
Second, we used logistic regression to assess the association between group status and performance on the three factor scores and the Seidman WM INT. Specifically, we used generalized estimating equation models with the appropriate link and family specification. To account for correlation within families, we used robust estimates of variance so that p-values would not be underestimated (Zeger and Liang 1986). Models were fit with the statistical software package STATA. The statistical significance of each covariate was determined by Wald’s Chi-Square test. Alpha was set at 0.05; all tests were two-tailed.
RESULTS
Table 1 shows descriptive features of the sample. No significant group differences emerged on SES. Differences were found for age and sex, and thus, all GEE models included these variables as covariates. BPD I and II cases were analyzed together because only 11% of cases met criteria for BPD II. The mean age of onset of BPD was in early childhood. All cases onset at age 16 or earlier, with 94% of the BPD probands reporting an onset of 14 or earlier. Youth with BPD had significantly lower estimated FSIQ compared to unaffected relatives and controls, although all groups scored within the Average range. Because correcting for IQ may remove a portion of the variance directly attributable to our outcomes, primary analyses did not covary for IQ; however, we report results after correction for IQ for purposes of discussion. Rates of comorbid disorders differed across groups. Although we made an a priori decision to include ADHD as a covariate in GEE models in order to identify impairments specifically relevant to BPD, given the possibility of shared familial risk factors between ADHD and BPD, we also comment on how results differ when ADHD is not controlled.
Table 1.
Demographic and descriptive characteristics of sample stratified by relative group and diagnoses
| BPD families |
Control families |
Omnibus Test |
||
|---|---|---|---|---|
| Affected Probands | Unaffected Relatives | Control Probands | ||
| (n=170) Mean(SD) |
(n=118) Mean(SD) |
(n=79) Mean(SD) |
Test Statistic (df), p-value | |
| Age | 12.2(3.1)a** range: 7–19 yrs |
12.8 (4.0) range: 7–26 yrs |
13.7 (2.1) range: 10–18 yrs |
χ2(2)=19.96,p<.001 |
| SES | 1.9 (0.9) | 2.1 (0.9) | 1.8 (0.9) | χ2(2)=3.73,p=.15 |
| Sex [N male(%)] | 128 (76.7) a**b** | 61 (53.4) | 46 (58.2) | χ2(2)=17.83,p<.001 |
| Estimated IQ | 102.0 (14.3) a**b* range: 71–136 |
106.1 (15.1) range: 71–140 |
108.7 (15.0) range: 74–143 |
χ2(2)=13.25,p=.001 |
| BPD Characteristics | ||||
| BPDI/BPDII | 89%/11% | N/A | N/A | ----- |
| BPD onset | 7.1 (3.9) | N/A | N/A | |
| # manic episodes [mean (SD)] | 27.6 (64.5) | N/A | N/A | ----- |
| # DSM-IV mania symptomsc [mean (SD)] | 5.8 (1.3) | N/A | N/A | ----- |
| age at onset | 7.1 (3.9) | N/A | N/A | ----- |
| ADHD [N(%)] | 128 (76.7) a**b** | 30 (25.6) | 14 (17.7) | χ2(2)=92.93,p<.001 |
| PSUD | ||||
| 38 (22.8) a**b** | 3 (3.4) | 4 (3.8) | χ2(2)=24.81,p<.001 | |
| ≥ 2 Anxiety | 06(63.5) a**b** | 18.6 | 14(17.7) | χ2(2)=73.78,p<.001 |
= vs, Controls;
= vs, unaffected relatives;
= symptoms refer to most severe manic episode by interviewee report;
= p≤.05
= p≤.01;
BPD = bipolar disorder; SES= socioeconomic status; ADHD= attention deficit-hyperactivity disorder; PSUD = psychoactive substance use disorders (alcohol/drug abuse/dependence);
Table 2 shows mean scores of the three groups on each neurocognitive measure, as well as primary loadings in the factor analysis. Three factors were retained (based on Eigenvalues >1.0 and the scree plot change in slope) which together accounted for 70% of the total variance. Measures of processing speed from the Wechsler and Stroop and the single measure of verbal learning loaded on Factor 1. Measures of working memory from the Wechsler tests and CPT loaded on Factor 2, along with Stroop Interference subtest and the Vigilance subtest. Measures of abstract problem solving/planning from the WCST and the ROCF loaded on Factor 3.
Table 2.
Neurocognitive measures – mean scores and factor loadings
| BPD Families |
Control Families |
Factor 1 | Factor 2 | Factor 3 | |||
|---|---|---|---|---|---|---|---|
| Affected Probands | Unaffected Relatives | Control Probands | Processing Speed/Verbal Learning | Working Memory/Interference Control | Abstract Problem Solving | ||
| Eigenvalue | 4.0 | 1.6 | 1.0 | ||||
| (n=170) Mean (SD) |
(n=118) Mean (SD) |
(n=79) Mean (SD) |
Unique Variance Explained | 26% | 24% | 20% | |
| Wechsler | Factor Loadings |
||||||
| Arithmetic | 9.4 (3.4) | 10.9 (3.3) | 11.7 (3.7) | .49 | |||
| Digit Span | 9.2 (3.0) | 10.3 (2.9) | 11.3 (2.9) | .49 | |||
| Digit Symbol/Coding | 6.8 (3.4) | 10.3 (3.2) | 9.6 (3.5) | .83 | |||
| Symbol Search | 8.7 (3.4) | 10.5 (3.3) | 11.2 (3.3) | .69 | |||
| Stroop | |||||||
| Color Naming | 36.9 (7.0) | 42.7 (6.9) | 41.0 (6.9) | .76 | |||
| Color Word Interference | 48.7 (6.1) | 49.7 (6.8) | 52.7 (7.8) | .75 | |||
| WCST | |||||||
| Perseverative Errors | 49.4 (11.0) | 53.2 (10.4) | 57.9 (9.5) | .91 | |||
| Non- perseverative Errors | 47.5 (11.0) | 51.4 (11.0) | 55.8 (7.6) | .91 | |||
| ROCF Copy Organization | 44.5 (9.6) | 47.7 (8.9) | 48.2 (10.4) | .35 | |||
| CVLT | 43.7 (14.8) | 51.8 (13.3) | 52.6 (11.4) | .60 | |||
| SEIDMAN CPT | |||||||
| Vigilance | 43.9 (14.4) | 51.0 (11.0) | 51.7 (8.2) | .54 | |||
| Memory | 43.0 (14.1) | 49.8 (12.5) | 51.9 (10.4) | .76 | |||
| Interference | 42.9 (12.8) | 47.7 (9.9) | 51.9 (10.1) | (.55)a | |||
Note: All data reflect T scores except for WISC-III/WAIS-III measures which are scale scores. WISC-III = Wechsler Intelligence Scale for Children – Third Edition; WAIS-III = Wechsler Adult Intelligence Scale – Third Edition; WCST = Wisconsin Card Sorting Test; ROCF = Rey-Osterreith Complex Figure; CVLT = California Verbal Learning Test; CPT = Continuous Performance Test.
= Seidman CPT Interference score not included in primary factor analysis because only administered to individuals 12 and over; secondary analysis using only youth 12 and over showed loading on factor 2.
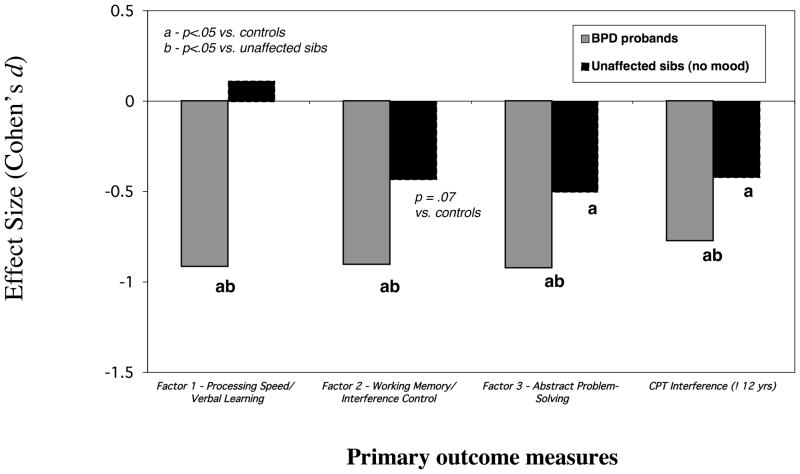
Figure 1 illustrates effect sizes (Cohen’s d) reflecting the performance of BPD probands and unaffected relatives versus controls on the four primary measures. The omnibus tests for the GEE models showed group differences on each (Factor 1- Processing Speed/Verbal Learning χ2(2)=56.40, p<.001; Factor 2- Working Memory/Interference Control χ2(2)=16.64, p<.001; Factor 3-Abstract Problem Solving χ2(2)=23.59, p<.001; Seidman WM INT measure χ2(2)=14.39,p<.001). Pairwise comparisons revealed that BPD probands performed more poorly than controls on all four (Factor 1 χ2(1)=30.69,p<.001; Factor 2 χ2(1)=15.92,p<.001; Factor 3 χ2(1)=22.75 ,p<.001; WM INT χ2(1)=13.99,p<.001). Unaffected relatives showed significantly worse performance versus controls on the Abstract Problem Solving factor (χ2(1)= 4.88, p =.03) and the Seidman WM INT measure (χ2(1)4.20 =,p =.04). Although the performance of unaffected relatives was also worse than that of controls on the Working Memory/Interference Control factor, this comparison fell short of statistical significance (χ2(1)=3.20,p =.07. Affected siblings also showed more impairments than unaffected siblings on each measure (Factor 1 χ2(1)=47.48, p<.001; Factor 2 χ2(1)=7.29, p<.01; Factor 3 χ2(1)=9.21, p=.002;WM INT χ2(1)=4.42, p=.04).
Figure 1.
Performance of BPD probands & unaffected siblings versus controls
It is noteworthy that the overall pattern of results would have been the same had ADHD not been included as a covariate, except that the performance of unaffected relatives on the Working Memory/Interference Control factor would have differed significantly from controls (χ2(1)4.12 =,p=.04) instead of emerging as a statistical trend. Had we controlled for IQ, the majority of significant findings would have remained, including the difference between unaffected relatives and controls on the Abstract Problem Solving/Organization factor (χ2(1)= 4.49,p =.03); however, that the difference between unaffected relatives and controls on the WM-INT would have fallen just short of statistical significance (χ2(1) 3.02 =,p =.08), possibly due to the smaller sample size of the group.
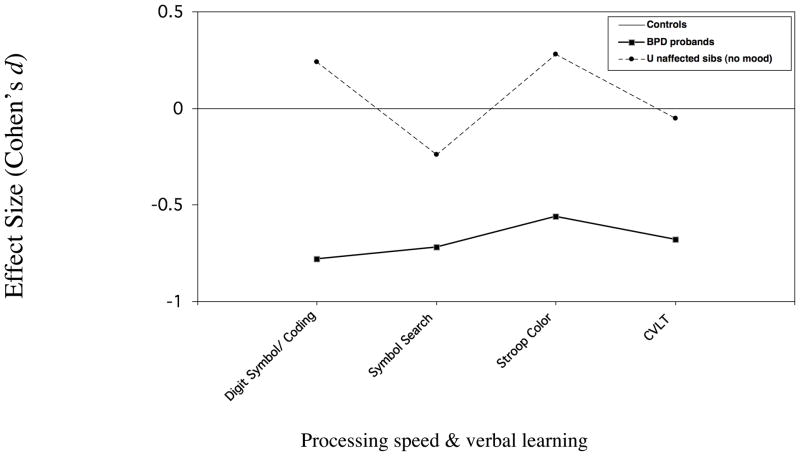
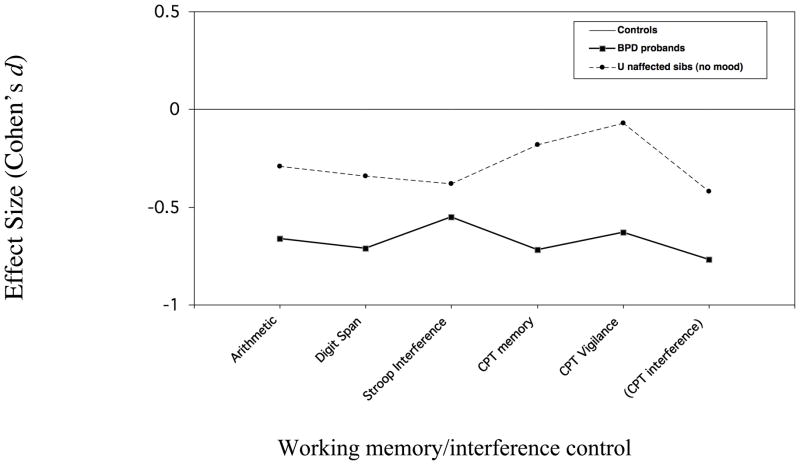
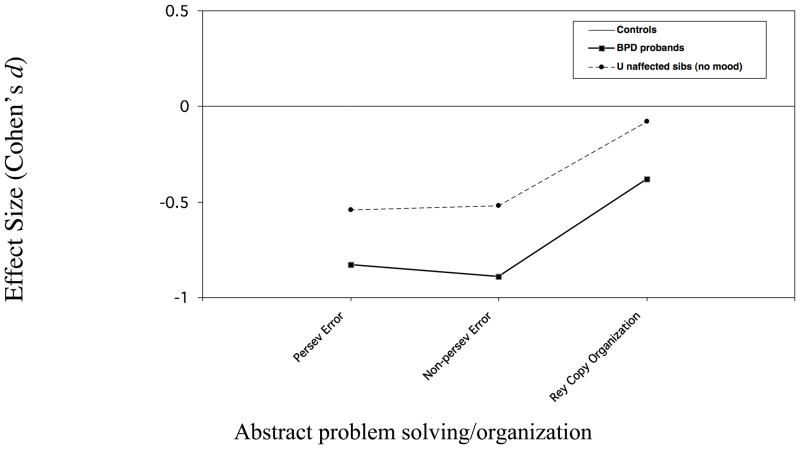
Although comparisons were not made on individual subtests, effect sizes for the subtests that loaded on each factor are shown in Figure 2 for discussion purposes.
Figure 2.
Figure 2a. Neuropsychological performance of BPD probands & unaffected siblings Measures loading on factor 1
Figure 2b. Neuropsychological performance of BPD probands & unaffected siblings Measures loading on factor 2
Figure 2c. Neuropsychological performance of BPD probands & unaffected siblings Measures loading on factor 3
Because source studies were naturalistic, we could not assess the impact of medication on test performance.. Among BPD youth, 79% reported medication use at the time of testing. Of these, 19% were taking more than one medication. Medications taken by this group were: 8% lithium, 31% anti-epileptics, 17% atypical neuroleptics, 13.5 % SSRIs,1% antipsychotics, 19% psychostimulants, 5% buproprion, 4% alpha-adrenoreceptor agonists and 5.5% other. Six percent of unaffected relatives and 8% of controls were taking at least one psychotropic medication; exclusion of these individuals did not change results.
DISCUSSION
The current study examined whether neurocognitive performance in youth with BPD is linked to the familial risk for the disorder. Consistent with the growing literature on pediatric BPD samples, the diagnosis itself was associated with relative weaknesses in processing speed/verbal learning, working memory/interference control and abstract problem-solving. Unaffected siblings of youth with BPD showed impairments on measures of abstract problem-solving, and working memory/interference control. These findings suggest that executive dysfunction may index the familially-transmitted pathophysiology underlying the condition. To our knowledge, this is the first study to evaluate and identify candidate endophenotypes in youth with BPD.
The neurocognitive functions we examined, including abstract problem-solving/set shifting, working memory, attention, verbal learning and processing speed, have previously been shown to be impaired in pediatric (Bearden et al. 2007; Dickstein et al. 2007; Pavuluri et al. 2006; Rucklidge 2006), and adult (Quraishi and Frangou 2002) patients with BPD. The fact that these functions are critical to cognitive and behavioral self-control compels the question of whether impaired performance reflects the underlying risk for the disorder. Yet, in affected youth, compromised performance could represent sequelae of symptoms, a degenerative process related to the illness or even a consequence of treatment. Although neurocognitive weaknesses in euthymic samples (McClure et al. 2005; Pavuluri et al. 2006; Torres et al. 2007) suggest that compromised functions are not purely due to acute disorganization, impairments in unaffected relatives provide more direct support for the link between test performance and familial etiological mechanisms.
In the current study, only some of the measures that were impaired in affected youth were impaired in unaffected relatives. Measures of abstract problem solving showed the most definitive familial link to the disorder, with unaffected relatives showing impairment on the factor reflecting this construct, and the two subscales of the WCST that loaded on this factor showing the largest decrements of the battery. For measures reflecting working memory and interference control, the pattern of findings was more complex but indicated a familial link between these domains and the disorder as well. Specifically, the working memory CPT with interference (WM INT) was impaired in individuals ≥12 years who were old enough to be expected to accomplish the task. Additionally, while impairments on the working memory/interference factor itself fell short of statistical significance (p =.07), the majority of working memory and interference control measures (ie Digit Span, Arithmetic, Stroop Interference) that loaded on it showed decrements of similar magnitude to the WM INT (Cohen’s d: .3 – .4; Figure 2a). The exception was the working memory CPT subtest with no interference (Cohens d = .2), possibly due to ceiling effects. In contrast, the unaffected relatives’ performance on the vigilance subtest, which also loaded on Factor 2, was similar to that of controls, as was their performance on measures of processing speed and verbal learning that loaded on Factor 1.
The current data are consistent with several studies of unaffected relatives of BPD adults that show impairments in interference control (Zalla et al. 2004) and abstract problem-solving (Meyer et al. 2004; Clark et al. 2005). Yet, other studies of BPD adults have failed to find abstract problem solving (Keri et al. 2001; Zalla et al. 2004; Frangou et al. 2005) and working memory (Keri et al. 2001) impairments in unaffected relatives. It is not clear whether such discrepancies are due to small sample sizes or true neurocognitive heterogeneity in adult samples. Yet data suggest there is a range of neurocognitive impairments in adult samples, with the presence of more severe deficits associated with worse functional outcome (Barnett et al. 2006; Jaeger et al. 2006). Our findings raise the possibility that executive dysfunction may be etiologically relevant to the behavioral symptoms of BPD that onsets in youth, whereas weaknesses in vigilance, processing speed and verbal learning may be sequelae of the condition.
Because pediatric onset BPD is familially linked to ADHD (Faraone et al 1998; Gellar et al 1996), it is likely that the disorders share at least some risk factors. Measures of working memory/interference control have been found to be impaired in relatives of ADHD youth (Doyle, Biederman et al 2005), and measures of these constructs in our sample were significantly impaired in unaffected relatives prior to as well as after controlling for ADHD. It is notable that a familial link between abstract problem-solving and pediatric onset BPD emerged even after controlling for ADHD and that impairments in this domain have not previously been found in unaffected relatives of ADHD probands (Doyle, Faraone et al. 2005). Moreover, Meyer et al (2004) found that impairments on the WCST in adolescents who eventually developed BPD were not associated with early attentional problems. These data suggest the possibility that abstract problem solving taps risk factors for pediatric BPD that do not overlap with ADHD, whereas working memory interference control measures tap familial risk that may be shared between ADHD and pediatric BPD. More work is needed to test these hypotheses in other samples.
Conceptually, the possibility that the executive system is linked to the extreme behavioral dyscontrol of BPD youth is compelling. Deficits in abstract problem-solving, working memory and interference control may interfere with the ability to process information from the environment in conjunction with known rules and expectations, identify pro-social options for behavior and disregard provocative or distracting stimuli. Support for this latter issue is found in the neuroimaging literature—e.g with manic patients showing excessive ventral and medial prefrontal response to distractors with emotional valence (Elliott et al. 2004).
It is well documented that prefrontal-subcortical networks govern performance on abstract problem solving and working memory/interference control tasks. For example, the processing of negative feedback that leads to set shifting on the WCST has been associated with a loop involving the mid ventrolateral prefrontal cortex (PFC), the basal ganglia and dorsal thalamus, as well as with the anterior cingulate cortex (Monchi et al. 2001). In this study, the processing of task feedback in general, which is necessary for rule acquisition and successful problem solving, activated regions (i.e. the mid dorsolateral PFC and the posterior parietal cortex) implicated in working memory tasks, including the WM-INT (Seidman et al. 1998). Together, these findings implicate dysfunctional prefrontal-subcortical circuits in the putative familial risk mechanism that mediates the expression of BPD. Such data are consistent with a functional neuroimaging study of pediatric BPD (Leibenluft et al. 2007) illustrating anomalies in prefrontal-striatal activation during an executive task. Neuroimaging studies with unaffected relatives are needed to pinpoint which aspects of these pathways show a familial relationship to the disorder.
Presuming our findings are confirmed in other samples, measures such as the WCST and WM INT represent potential targets for molecular genetic studies of pediatric BPD and more precisely reveal its pathophysiology. Although twin studies of pediatric BPD are lacking, high rates of BPD in relatives of affected youth (Strober et al. 1988; Neuman et al. 1997) and the heritability of BPD in adults suggest that the etiology of pediatric BPD is complex but includes genetic origins (Faraone et al. 2003). Endophenotypes, such as familially-linked neurocognitive measures, theoretically may increase statistical power to detect the effects of individual susceptibility genes in such conditions due to their reduced complexity compared to the disorder as a whole given their 1) greater proximity to gene products and 2) potential to parse heterogeneous processes (Gottesman and Gould 2003). Although it is likely that neurocognitive measures such as the WCST are themselves complex conditions, associations between the WCST and the gene for catechol-o-methyltransferase (Flint and Munafo 2007), plus data from our recent study showing suggestive linkage of a WCST subscale to a region on chromosome 22q11 (Doyle et al in press), indicate that it is reasonable to expect to be able to identify genes that influence this phenotype. Indeed, even if neurocognitive phenotypes are not drastically less complex than pediatric BPD itself, their familial (and potentially heritable) covariance with the disorder underscores their potential for increasing power via inclusion in multivariate genetic analyses.
A few issues are noteworthy when considering our results. Although we discuss our findings based on constructs our tests purport to measure, future studies are needed to determine whether other measures of working memory/interference control (e.g. N-back tests) and abstract problem-solving/set shifting (e.g.Tower of London/Hanoi tests) will show impairments in unaffected relatives and/or whether other constructs (e.g executive attention; Sheese et al 2008) better reflect the underlying impairments that our measures are tapping in relatives. Additionally, some researchers have argued that IQ should be controlled in neuropsychological studies to insure that deficits are not attributable to low ability (Lahey et al 1998; Werry et al 1987); however, it is conceivable that neuropsychological deficits contribute to poor performance on measures of IQ (Barkley 1997b). Moreover, data from twin (Polderman et al 2006) and molecular genetic studies (Doyle et al in press) suggest a partially shared genetic etiology between measures of IQ and measures of executive functions. Thus, we did not control for IQ so as not to remove potentially relevant genetic variance from our outcomes. Nonetheless, including IQ as a covariate would not have reduced the statistical significance of the differential performance of unaffected relatives and controls on the Abstract Problem Solving/Organization factor. Regarding diagnostic issues, although our current diagnostic approach did not depart from or modify DSM-IV criteria for mania, both broad and narrow definitions of pediatric BPD have been proposed. Understanding the cognitive correlates of varied diagnostic criteria would be of interest to the field.
Finally, our data should be considered in light of its limitations. First, because the majority of affected youth in our sample were medicated, we could not determine neurocognitive impairments in BPD youth that were present in the absence of medication. Similarly, approximately half of our BPD probands met criteria for mania at the time of assessment. Thus, it is possible that current mood state influenced impairments in this group. Yet, because medication status and current rates of mania did not differ between unaffected relatives and controls, these variables do not impact our main focus on impairments unaffected relatives compared to controls. Second, given the worse performance of the BPD youth and their siblings, it is logical to attribute the familiality of neurocognitive impairments to BPD. Yet the inclusion of at risk youth unrelated to the BPD probands or of relatives of the control probands would have provided an even stronger test of this hypothesis and helped to rule out the possibility that a factor other than BPD was accounting for the familiality. Third, as part of the case control design of the source studies, controls were selected based on the absence of mood disorders. Yet because they had not yet passed through the age of risk, it is possible that a small number of controls may go on to develop a mood disorder. Because there is no doubt that the familial loading for BPD risk factors is greater in the BPD youth than in controls, this issue does not undermine our main findings of greater impairments in unaffected relatives versus controls, and, if anything, would have served to reduce the differences between these groups. Fourth, although our analyses included a wide variety of neurocognitive measures that are relevant to pediatric BPD, several interesting candidate endophenotypes were not assessed, including biased processing of emotionally-charged stimuli (Murphy and Sahakian 2001), social cognition/face processing (McClure et al. 2005; Pavuluri et al. 2006) and visual-spatial memory (Dickstein et al. 2004). Thus, the candidate endophenotypes that we have identified are not exhaustive and likely reflect only a piece of the etiological puzzle that underlies the extreme emotional dysregulation of youth with BPD. Fifth, it is important to note that youth under age 12 were not interviewed directly. Nonetheless, our recent investigation in the current sample (Biederman et al in press) suggests that diagnoses based only on parental reports represent a highly morbid condition with comparable clinical correlates to diagnoses resulting from concordant reports. Even so, direct data from younger children would have been preferable.
Despite these considerations, we found clear neurocognitive deficits in the unaffected siblings of youth with BPD. These data suggest that measures of abstract problem solving, working memory and interference control may be linked to the familial risk for the disorder and thus useful targets in molecular genetic studies of the condition. These findings add to a growing body of work documenting the validity and neurobiological underpinnings of pediatric BPD.
Acknowledgments
This work was supported in part by grants NIMH K08-MH66072 to Dr. Doyle, K08-MH01503 to Dr Wozniak, and NIH RO1 DA12945 and K24 DA016264 to Dr. Wilens. Support also came in part from the Neal/Kimmerly Fund for the Study of Cognition in Psychiatric Illness at the Massachusetts General Hospital. No authors have affiliations or financial arrangements with companies who may financially benefit from or potentially bias the manuscript’s content. In the interest of full financial disclosure, Dr. Doyle has received research support from NIH (NIMH) and consultation fees from Pfizer Pharmaceuticals. Dr. Wilens receives grant support, speaker or consultant fees from: Abbott, McNeil, Lilly, NIH (NIDA), Merck, Novartis and Shire. Dr. Wozniak receives income from her book Is Your Child Bipolar?”, published May 2008, Bantam Books. Her spouse, John Winkelman MD PhD receives research support, consultation fees, or speaker’s fees from Axon Labs Boehringer-Ingelheim, GlaxoSmithKline, Jazz Pharmaceuticals, Novartis, Neurogen, Pfizer, Sanofi-Aventis, Sepracor, Takeda, UCB (Schwarz) Pharma. Dr. Faraone reports having received lecture fees and research funding from Pfizer, and consulting and research funding from Shire. Dr. Biederman receives research support, consultation fees, or speaker’s fees from: Alza, AstraZeneca, Bristol Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals Inc., McNeil, Merck, Novartis, Organon, Otsuka, Shire, NIMH, NICHD and UCB Pharma, Inc. Aspects of this work were presented as a poster in the 2005 NIMH Pediatric Bipolar Conference in Chicago IL.
References
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997b;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychol Med. 2006;36(8):1053–64. doi: 10.1017/S0033291706007501. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Caetano S, Olvera RL, Fonseca M, Najt P, Hunter K, Pliszka SR, Soares JC. Evidence for disruption in prefrontal cortical functions in juvenile bipolar disorder. Bipolar Disord. 2007;9(s1):145–159. doi: 10.1111/j.1399-5618.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Bernstein JH, Waber DP. Developmental scoring system for the Rey-Osterreith Complex Figure [manual] Odessa, Florida: Psychological Assessment Resources, Inc; 1996. [Google Scholar]
- Biederman J. Pediatric bipolar disorder coming of age. Biological Psychiatry. 2003;53(11):931–934. doi: 10.1016/s0006-3223(03)00297-x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S, Wozniak J, Mick E, Kwon A, Aleardi M. Further evidence of unique developmental phenotypic correlates of pediatric bipolar disorder: Findings from a large sample of clinically referred preadolescent children assessed over the last 7 years. Journal of Affective Disorders. 2004;82(Suppl 1):S45–S58. doi: 10.1016/j.jad.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Biederman J, James RS. Furthering the scientific foundation of pediatric bipolar disorder. J Affect Disord. 2004;82(Suppl 1):S1–3. doi: 10.1016/j.jad.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Wilens TE, Spencer T, Henin A, Faraone SV, Mick E, Monuteaux M, Kenealey D, Mirto T, Wozniak J. Examination of concordance between maternal and youth reports in the diagnosis of pediatric bipolar disorder. Bipolar Disorders. doi: 10.1111/j.1399-5618.2009.00671.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt CM, Bernstein GA. Comorbid disorders in hospitalized bipolar adolescents compared with unipolar depressed adolescents. Child Psychiatry and Human Development. 1995;26(1):11–18. doi: 10.1007/BF02353226. [DOI] [PubMed] [Google Scholar]
- Clark L, Sarna A, Goodwin GM. Impairment of executive function but not memory in first-degree relatives of patients with bipolar I disorder and in euthymic patients with unipolar depression. Am J Psychiatry. 2005;162(10):1980–2. doi: 10.1176/appi.ajp.162.10.1980. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. The california verbal learning test -children’s version. San Antonio: Psychological Corporation; 1994. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corp; 2000. [Google Scholar]
- Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, Rich BA, Pine DS, Leibenluft E. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(3):341–55. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biological Psychiatry. 2004;55(1):32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Biederman J, Seidman LJ, Reske-Nielsen J, Faraone SV. Neuropsychological functioning in relatives of girls with and without ADHD. Psychological Medicine. 2005;35(8):1121–1132. doi: 10.1017/s0033291705004496. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, Pennington BF, Peart J, Biederman J. Are endophenotypes based on measures of executive functions useful for molecular genetic studes of ADHD? Journal of Child Psychology and Psychiatry. 2005;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Ferreira MAR, Sklar PB, Lasky-Su J, Petty C, Fusillo SJ, Seidman LJ, Willcutt E, Smoller JW, Purcell S, Biederman J, Faraone SV. Multivariate genomewide linkage scan of neurocognitive traits and ADHD symptoms: Suggestive linkage to 3q13. Am J Med Genet B Neuropsychiatr Genet. doi: 10.1002/ajmg.b.30868. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE, Wilens T, Kwon A, Seidman LJ, Faraone SV, Fried R, Swezey A, Snyder L, Biederman J. Neuropsychological functioning in youth with bipolar disorder. Biological Psychiatry. 2005;58(7):540–548. doi: 10.1016/j.biopsych.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55(12):1163–70. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Faraone S, Glatt S, Tsuang M. The genetics of pediatric onset bipolar disorder. Biological Psychiatry. 2003;53(11):970–977. doi: 10.1016/s0006-3223(02)01893-0. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mennin D, Russell RL. Bipolar and antisocial disorders among relatives of ADHD children: Parsing familial subtypes of illness. Am J Med Genet. 1998;81(1):108–116. [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37(2):163–80. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol Psychiatry. 2005;58(10):838–9. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Geller B, Tillman R, Bolhofner K, Zimerman B, Strauss NA, Kaufmann P. Controlled, blindly rated, direct-interview family study of a prepubertal and early-adolescent bipolar I disorder phenotype: morbid risk, age at onset, and comorbidity. Arch Gen Psychiatry. 2006;63(10):1130–8. doi: 10.1001/archpsyc.63.10.1130. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Caetano S, Fonseca M, Najt P, Hunter K, Pliszka SR, Olvera RL, Soares JC. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disord. 2005;7(6):546–54. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Use. Chicago: Stoelting, Co; 1978. [Google Scholar]
- Goldstein TR, Birmaher B, Axelson D, Ryan ND, Strober MA, Gill MK, Valeri S, Chiappetta L, Leonard H, Hunt J, Bridge JA, Brent DA, Keller M. History of suicide attempts in pediatric bipolar disorder: factors associated with increased risk. Bipolar Disord. 2005;7(6):525–35. doi: 10.1111/j.1399-5618.2005.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry Apr. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry. 1999;45(5):639–46. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sort Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources, Inc; 1993. [Google Scholar]
- Henin A, Mick E, Biederman J, Fried R, Wozniak J, Faraone SV, Harrington K, Davis S, Doyle AE. Can Bipolar Disorder-Specific Neuropsychological Impairments in Children be Identified? Journal of Consulting and Clinical Psychology. 2007;75(2):210–20. doi: 10.1037/0022-006X.75.2.210. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Tatsuoka C, Berns S, Varadi F, Czobor P, Uzelac S. Associating functional recovery with neurocognitive profiles identified using partially ordered classification models. Schizophr Res. 2006;85(1–3):40–8. doi: 10.1016/j.schres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med. 2001;31(5):915–22. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biol Psychiatry. 2005;57(6):647–54. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Lahey B, Pelham W, Stein M, Loney J, Trapini C, Nugent K, et al. Validity of DSM-IV attention-deficit/hyperactivity disorder for younger children. Journal of the American Academy of Child and Adolescnet Psychiatry. 1998;37:695–702. doi: 10.1097/00004583-199807000-00008. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Memory and learning in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(5):461–9. doi: 10.1097/01.chi.0000156660.30953.91. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162(9):1644–51. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Carlson GA, Wiggs EA, Martinez PE, Ronsaville DS, Klimes-Dougan B, Gold PW, Radke-Yarrow M. A prospective study of the association among impaired executive functioning, childhood attentional problems, and the development of bipolar disorder. Dev Psychopathol. 2004;16(2):461–76. doi: 10.1017/s095457940404461x. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21(19):7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ. Neuropsychology of bipolar disorder. Br J Psychiatry. 2001;178(Suppl 41):S120–7. [PubMed] [Google Scholar]
- Neuman R, Geller B, Rice J, Todd R. Increased prevalence and earlier onset of mood disoders among relatives of prepubertal versus adult probands. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):466–473. doi: 10.1097/00004583-199704000-00008. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for Affective Disorder and Schizophrenia for School-Age Children Epidemiologic Version. Ft. Lauderdale: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe. Archives de Psychologie. 1944;30:206–256. [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163(2):286–93. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Gosso MF, Posthuma D, Van Beijsterveldt TC, Heutink P, Verhulst FC, Boomsma DI. A longitudinal twin study on IQ, executive functioning, and attention problems during childhood and early adolescence. Acta Neurol Belg Dec. 2006;106(4):191–207. [PubMed] [Google Scholar]
- Quraishi S, Frangou S. Neuropsychology of bipolar disorder: A review. J Affect Disord. 2002;72(3):209–26. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Impact of ADHD on the neurocognitive functioning of adolescents with bipolar disorder. Biol Psychiatry. 2006;60(9):921–8. doi: 10.1016/j.biopsych.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of Children. San Diego, CA: Jerome M. Sattler; 1988. [Google Scholar]
- Savitz JB, Solms M, Ramesar RS. Neurocognitive function as an endophenotype for genetic studies of bipolar affective disorder. Neuromolecular Med. 2005;7(4):275–86. doi: 10.1385/NMM:7:4:275. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Breiter HC, Goodman JM, Goldstein JM, Woodruff PW, O’Craven K, Savoy R, Tsuang MT, Rosen BR. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12(4):505–18. doi: 10.1037//0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Rothbart MK, Posner MI, White LK, Fraundorf SH. Executive attention and self-regulation in infancy. Infant Behav Dev Sep. 2008;31(3):501–10. doi: 10.1016/j.infbeh.2008.02.001. Epub Apr 11. [DOI] [PubMed] [Google Scholar]
- Sobczak S, Honig A, Schmitt JA, Riedel WJ. Pronounced cognitive deficits following an intravenous L-tryptophan challenge in first-degree relatives of bipolar patients compared to healthy controls. Neuropsychopharmacology. 2003;28(4):711–9. doi: 10.1038/sj.npp.1300055. [DOI] [PubMed] [Google Scholar]
- Sobczak S, Riedel WJ, Booij I, Aan Het Rot M, Deutz NE, Honig A. Cognition following acute tryptophan depletion: difference between first-degree relatives of bipolar disorder patients and matched healthy control volunteers. Psychol Med. 2002;32(3):503–15. doi: 10.1017/s0033291702005342. [DOI] [PubMed] [Google Scholar]
- Strober M, Morrell W, Burroughs J, Lampert C, Danforth H, Freeman R. A family study of bipolar I disorder in adolescence: Early onset of symptoms linked to increased familial loading and lithium resistance. Journal of Affective Disorders. 1988;15:255–268. doi: 10.1016/0165-0327(88)90023-7. [DOI] [PubMed] [Google Scholar]
- Torres IJ, V, Boudreau G, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand. 2007;(Suppl434):17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3. San Antonio: The Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale III [manual] San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Werry JS, Reeves JC, Elkind GS. Attention deficit, conduct, oppositional, and anxiety disorders in children: I. A review of research on differentiating characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:133–143. doi: 10.1097/00004583-198703000-00003. [DOI] [PubMed] [Google Scholar]
- Wilens T, Biederman J, Kwon A, Ditterline J, Forkner P, Chase R, Moore H, Swezey A, Snyder L, Morris M, Henin A, Wozniak J, Faraone SV. Risk for substance use disorders in adolescents with bipolar disorder. Journal of American Academy Child and Adolescent Psychiatry. 2004;43(11):1380–1386. doi: 10.1097/01.chi.0000140454.89323.99. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Biederman J, Kwon A, Mick E, Faraone SV, Orlovsky K, Schnare L, Cargol C, Van Grondelle A. How cardinal are cardinal symptoms in pediatric bipolar disorder?: An examination of clinical correlates. Biological Psychiatry. 2005;58(7):583–588. doi: 10.1016/j.biopsych.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zalla T, Joyce C, Szoke A, Schurhoff F, Pillon B, Komano O, Perez-Diaz F, Bellivier F, Alter C, Dubois B, Rouillon F, Houde O, Leboyer M. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res. 2004;121(3):207–17. doi: 10.1016/s0165-1781(03)00252-x. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]