Abstract
Objective
Our aim was to elucidate mechanisms involved in the acquisition of lipid transport properties during enterocyte differentiation.
Methods and Results
We show that lipid mobilization via apolipoprotein B-lipoproteins is dependent on the expression of microsomal triglyceride transfer protein (MTP) during differentiation of Caco-2 cells into enterocyte-like cells. Mechanistic studies showed that binding of the nuclear receptor family 2 group F member 1 (NR2F1) to the DR1 element in the MTTP promoter suppresses MTTP expression in undifferentiated cells. During cellular differentiation, NR2F1 expression and its binding to MTTP promoter decline and MTP induction ensues. Moreover, undifferentiated cells express inositol-requiring enzyme 1β (IRE1β), a protein that post-transcriptionally degrades MTP mRNA, and its expression substantially decreases during differentiation contributing to MTP induction. Immunohistochemical studies revealed a significant negative relationship between the expressions of MTP and Nr2f1/Ire1β in undifferentiated and differentiated Caco-2 cells as well as in crypt-villus and jejunum-colon axes of mouse intestine.
Conclusions
We propose that transcriptional and post-transcriptional mechanisms involving NR2F1 and IRE1β ensure low MTP expression in undifferentiated intestinal cells and avoid apoB-lipoprotein biosynthesis.
Keywords: lipoprotein assembly, apolipoprotein B, microsomal triglyceride transfer protein, intestine, gene transcription, enterocytes, differentiation, Nr2F1, Ire1β
INTRODUCTION
Intestinal crypts harbor stem/progenitor cells that divide, migrate, and differentiate into enterocytes 1 and absorb dietary constituents such as carbohydrates and lipids. Expression of carbohydrate hydrolyzing enzymes during enterocyte differentiation is dependent on Cdx2 2. Lipid absorption depends on the biosynthesis of triglyceride-rich lipoproteins that requires apolipoprotein B (apoB), a structural protein, and microsomal triglyceride transfer protein (MTP), an endoplasmic reticulum resident chaperone 3, 4. Mechanisms promoting induction of apoB-lipoprotein biogenesis and lipid absorption during differentiation of enterocytes are unknown.
Human colon carcinoma Caco-2 cells are used extensively to study cellular differentiation 5–7. Undifferentiated Caco-2 cells do not synthesize or secrete apoB-lipoproteins. However, during culture these cells spontaneously differentiated into enterocyte-like cells and produce chylomicron-size apoB-containing lipoproteins when supplemented with oleic acid 6, 8–14. We report that two proteins, NR2F1 and IRE1β, prevent MTP expression at transcriptional and post-transcriptional levels in undifferentiated intestinal cells and restrict apoB-lipoprotein biosynthesis.
Materials and Methods
Chemicals and reagents, primary antibodies, and secondary antibodies were purchased from Sigma, Santa Cruz Biotechnology, and Invitrogen, respectively. Caco-2 cells were allowed to differentiate on Transwells and studied for the secretion of apoB-lipoproteins. These cells were also infected with adenoviruses expressing human MTP or transfected with various plasmids or siRNA. For various methods please see http://atvb.ahajournals.org. Experiments were performed in triplicate and repeated at least twice. Data are presented as mean ± SEM. Statistical significances (p < 0.05) were determined using the Student t test, or one-way analysis of variance (ANOVA) followed with the Bonferroni test (GraphPad Prism).
Results
ApoB-lipoproteins are secreted after MTP induction in differentiated Caco-2 cells
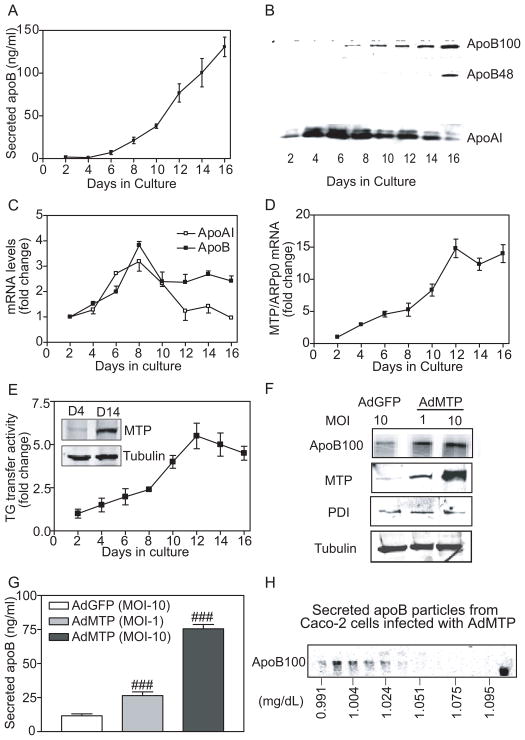
Caco-2 cells were plated at 30% to 40% confluence and growth media were collected on alternate days to measure apoB and apoAI secretions. These cells reached confluence around day 6 as observed under light microscope. ApoB was hardly detectable before day 6 (Figure 1A); its secretion increased 5-fold from day 8 to 16. ApoB100, a major form secreted by these cells 15, was detectable on day 8 and amounts increased thereafter (Figure 1B). ApoB48, another form of apoB arising from post-transcriptional editing of apoB mRNA, was evident on day 16. In contrast, apoAI was present throughout the culture period, with highest amounts seen on days 4 to 8 (Figure 1B) consistent with its mRNA profile (Figure 1C, apoAI) and other studies 16. These data are in agreement with previous observations that proliferative Caco-2 cells do not secrete apoB-lipoproteins; ability to synthesize lipoproteins is acquired during differentiation into enterocyte-like cells 8, 17.
Figure 1. MTP expression and apoB-lipoprotein secretion are enhanced during differentiation.
(A–E) Conditioned media from Caco-2 cells cultured in Transwells were collected on alternate days for apoB measurements in triplicate (A) and Western blot detection of immunoprecipitated apoB and apoAI (B). RNA was isolated on indicated days and expression of apoB and apoAI (C) as well as MTP (D) was quantified with qRT-PCR using acidic ribosomal phosphoprotein 0 (ARPp0) as an internal control. Ratios of candidate mRNA and ARPp0 on day 2 were normalized to 1, and expression on other days is presented as fold changes. MTP activity was determined using a triglyceride transfer assay 31, 32 and by Western analysis (E and inset).
(F–H) Caco-2 cells seeded at 30% confluence in 6-well plates. The next morning cells were infected with AdGFP and AdMTP at indicated multiplicity of infection (MOI). Next day, growth media were replaced with DMEM containing 10% FBS and 100 μCi/mL 35S Met/Cys. After 16 h, apoB was immunoprecipitated with 10 μL of 1D1 antibody from 1 mL of media, separated on 5% polyacrylamide gels, and visualized using PhosphorImage (F, apoB100). Unlabeled cells were used to detect intracellular MTP, PDI, and tubulin by Western blotting (F). The conditioned media were used to quantify apoB with ELISA (G). About 4 mL of growth media from cells infected with AdMTP (MOI:10) and metabolically labeled was subject to density gradient ultracentrifugation to fractionate lipoproteins. ApoB was visualized after immunoprecipitation in each fraction (H). Mean ± SEM. ###, p < 0.001, one-way ANOVA.
We reasoned that induction of apoB-lipoprotein assembly might be linked to APOB gene transcription. However, apoB mRNA levels (Figure 1C, apoB) did not correlate with apoB secretion (Figure 1A) during culture. Hence, we hypothesized that APOB gene transcription might not be sufficient for its enhanced secretion after differentiation and other proteins are, perhaps, required. In addition to apoB, MTP is required for apoB-lipoprotein secretion. MTP mRNA, activity and protein increased slowly until day 8 and then rapidly until day 12; thereafter, triglyceride transfer activity either plateaus or falls modestly (Figure 1D, 1E). Therefore, MTP might be important for increases in apoB secretion during the early phase of cellular differentiation. Despite no further rise in MTP, apoB secretion continues to rise after day 12 indicating for another protein/factor/mechanism that augments apoB secretion and was not studied here.
To determine the need for MTP in apoB secretion, undifferentiated cells were infected with adenoviruses expressing human MTP (AdMTP) or green fluorescent protein (AdGFP). Cells infected with AdMTP secreted more apoB than did AdGFP-infected cells without altering expression of PDI or tubulin (Figure 1F). Cells infected with multiplicity of infection (MOI) of 1 and 10 AdMTP secreted 2- and 6-fold higher amounts of apoB, respectively, compared with AdGFP transduced cells (Figures 1G). Density gradient ultracentrifugation indicated that secreted particles had flotation density <1.024 g/ml (Figure 1H). Since MTP expression was very low in undifferentiated cells, no attempts were made to decrease MTP using siRNA. These data suggest that MTP transcription might be a limiting factor for apoB-lipoprotein secretion in undifferentiated Caco-2 cells.
HNF1, HNF4, and DR1 cis elements are critical for MTP induction during differentiation
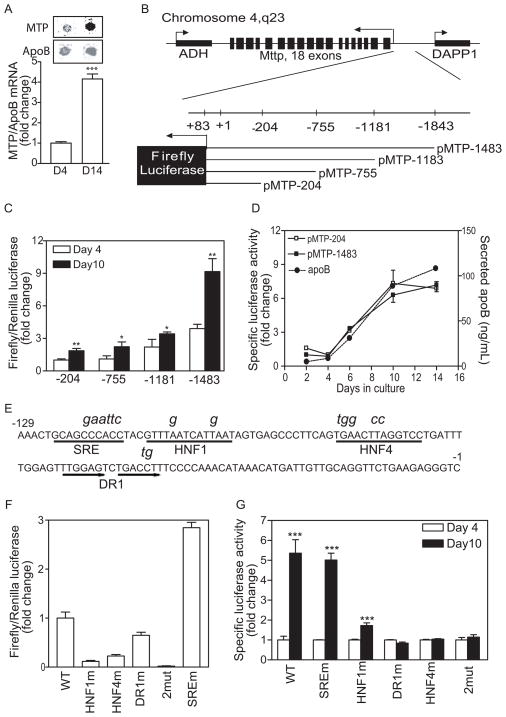
To investigate mechanisms of MTP induction, we carried out run-off assays using nuclei isolated from undifferentiated (day 4) and differentiated (day 14) Caco-2 cells. Amounts of apoB mRNA synthesized on day 14 were higher than those seen on day 4 (Figure 2A, apoB). However, amounts of newly transcribed MTP mRNA from day 14 nuclei were about 3 times higher after correction for increases in apoB mRNA than those from day 4 nuclei (Figure 2A) indicating that MTP gene transcription was induced significantly more than apoB gene transcription during differentiation.
Figure 2. HNF1, HNF4, and DR1 cis elements are required for MTP expression during differentiation.
(A) Nuclei (5 × 106) from day 4 and 14 cultures were labeled with digoxigenin-UTP at 37°C for 30 minutes. The nascent RNA was hybridized to MTP and apoB48 cDNA cross-linked to nylon membranes. The RNA-DNA complexes were visualized using monoclonal antibodies against digoxigenin (Roche) and anti-mouse Alexa Fluor 633 antibody (top panel). Data from 3 experiments are presented as fold change compared to day 4 (bottom panel).
(B) A scheme showing the MTTP locus and four constructs with different 5′ ends cloned in a pGL2-Basic background so that expression of firefly luciferase was now under the control of MTP promoter sequences (B). ADH, alcohol dehydrogenase; DAPP1, dual adaptor of phosphotyrosine and 3-phosphoinositides 1
(C) Each promoter construct was transiently transfected along with pCMV-RL that served as a transfection control. Cellular luciferase activities were measured after 4 or 14 days and ratios of firefly/Renilla luciferase were obtained. Units are presented as fold change of pMTP-204 ratio on day 4.
(D) Cells stably expressing pMTP-204 or -1483 were cultured for 14 days. The specific luciferase activities (light unit/mg/sec) were presented as fold change from day 4. In addition, apoB secretion was determined using ELISA.
(E–G) cis elements SRE, HNF1, HNF4, and DR1 in the 204-bp promoter were subjected to site-directed mutagenesis. Mutant sequences are shown above the original (E). Activities of wild type and mutant promoters were evaluated in Caco-2 cells by transient co-transfection with pCMV-RL, a transfection control (F). Caco-2 cells stably expressing WT pMTP-204 and different mutant sequences were cultured for 4 or 10 days (F). Specific luciferase activities (light unit/mg/sec) on day 4 were normalized to 1. Mean ± SEM; *, p < 0.05, **, p < 0.01, ***, p < 0.001, Student t test.
To identify promoter sequences necessary for MTP induction, sequences upstream of the transcription initiation site were cloned to generate pMTP-1483, pMTP-1181, pMTP-75, and pMTP-204 in which firefly luciferase was under the control of the MTTP promoter sequences (Figure 2B). First, we analyzed the response of these promoter constructs to differentiation after transient expression using Renilla luciferase as transfection control. The MTP promoter activity, as measured by increases in luciferase activity, was enhanced with increasing promoter length (Figure 2C, open bars). All constructs showed approximately 2-fold higher promoter activity on day 10 compared with day 4 (Figure 2C, solid bars), indicating that the 204 bp promoter was as efficient as the longest in inducing luciferase expression during differentiation. Second, pMTP-204 and pMTP-1483 were stably expressed in Caco-2 cells and luciferase activity and apoB secretion were monitored over time. ApoB secretion increased during culture (Figure 2D) as seen in non-transgenic cells (Figure 1A). Moreover, luciferase expression in both cell lines increased by more than 4-fold (Figure 2D). This increase was similar to that observed in the run-off assay (Figure 2A) suggesting that the 204-bp promoter might be sufficient for MTP expression during Caco-2 cell differentiation.
To identify cis elements within the 204-bp critical for MTP transcription during differentiation, we mutated different cis-elements as shown in italics above the original sequence (Figure 2E). Mutation-bearing and wild type promoter constructs were transiently expressed in undifferentiated cells, and luciferase activities were measured (Figure 2F). Promoter activity was reduced by 89%, 78%, and 35%, respectively, when HNF1, DR1, and HNF4 sites contained mutations (Figure 2F). Double mutations of DR1 and HNF4 decreased activity by 98% (Figure 2F, 2mut) indicating their importance in basal promoter activity. In contrast, the SRE mutation increased promoter activity by nearly 2-fold, suggesting that SRE is a negative regulator confirming an earlier study 18. To identify the elements accountable for MTP induction during differentiation, mutation-bearing constructs were expressed stably in Caco-2 cells, and luciferase activity was measured on days 4 and 10 (Figure 2G). Cells expressing either WT or SREm sequences showed about 5-fold increase in promoter activity (Figure 2G). Mutations in the HNF1 site reduced luciferase activity by 80%. Mutagenesis at DR1 and HNF4 sites individually or in combination (Figure 2G) completely abolished differentiation-induced activation. These data indicate that cis elements HNF1, HNF4, and DR1 in the minimal promoter are critical for differentiation-dependent MTP induction.
Increased MTTP transcription is due to reduced expression of NR2F1 during Caco-2 cell differentiation
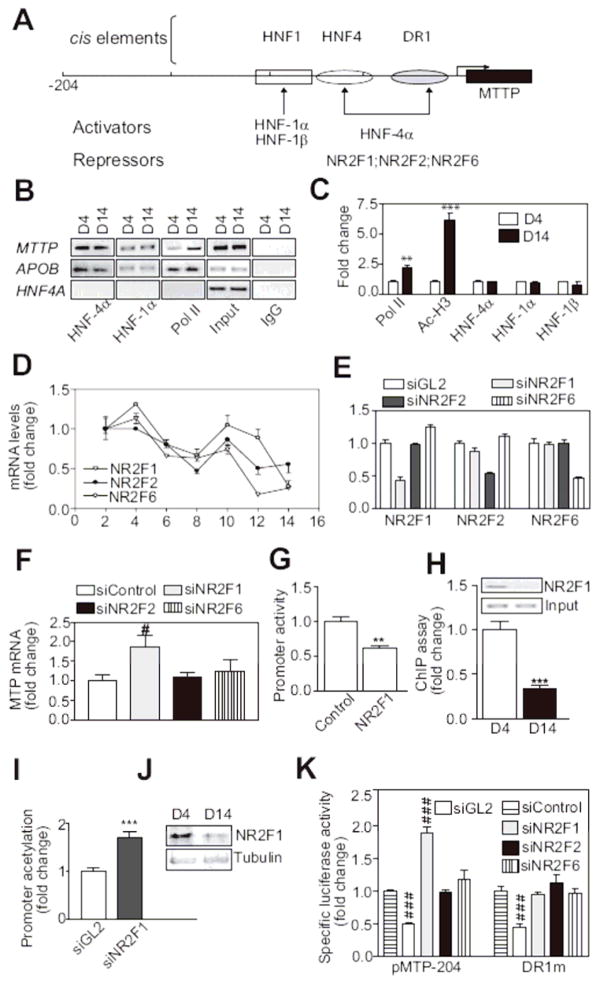
Attempts were then made to identify transcription factor(s) that bind to these cis elements and reduce MTP expression in undifferentiated Caco-2 cells. The enhanced binding of transcription factors HNF-4α, HNF-1α and HNF-1β (Figure 3A, activators) to the three identified cis elements could induce MTTP expression during differentiation. To determine whether these proteins exhibit differential binding to the MTTP promoter before and after differentiation, we used chromatin immunoprecipitation (ChIP) followed by standard polymerase chain reaction (PCR) (Figure 3B). Binding of HNF-4α and HNF-1α to the MTTP or APOB promoter did not change during differentiation. However, binding of RNA polymerase II (Pol II) to the MTTP promoter was enhanced after differentiation. Using ChIP and quantitative PCR (qPCR), we noted 2- and 5-fold elevations in the binding of Pol II and acetylated histone H3 to the MTTP promoter in day-14 compared with day-4 cultures (Figure 3C), respectively, indicating transcriptionally active MTTP promoter after differentiation. Again, the bindings of HNF-4α and HNF-1 proteins to the MTTP promoter were not significantly changed before and after differentiation (Figure 3C). These studies indicate that differential binding of HNF-4α and HNF-1α to the MTTP promoter does not determine differentiation-dependent induction of MTP.
Figure 3. NR2F1 supresses MTP expression in undifferentiated cells.
(A) Illustration of HNF1, HNF4, and DR1 elements in the 204-bp MTTP promoter. Activators and repressors that could bind to these elements were identified using MatInspector (Genomatix).
(B–C) Caco-2 cells were cross-linked (1% formaldehyde, 15 min, room temperature), sonicated, and incubated with specific antibodies against various DNA-binding proteins. The immunoprecipitated DNA fragments were amplified using primers targeted to MTTP, HNF4A, and APOB promoters and subjected to semiquantitative (B) or quantitative (C) PCR. Binding of different factors to MTTP promoter was normalized with their binding to apoB (C). Pol II, polymerase II; IgG, normal goat serum IgG; Input, 1% of total DNA
(D) Expression of repressors during differentiation was determined by measuring mRNAs using qRT-PCR.
(E, F) Undifferentiated Caco-2 cells were transfected with different siRNAs against NR2F1, NR2F2, and NR2F6. After 48 h, expressions of these repressors (E) and endogenous MTP (F) were determined.
(G) Caco-2 cells were co-transfected with pMTP-204 and pCMV-RL, a transfection control, along with pMT2-NR2F1 (plasmid expressing NR2F1) or pcDNA3.1 (control). Luciferase activities were measured 48 h later.
(H–I) ChIP followed by regular (inset) and qPCR determined NR2F1 binding to MTTP promoter in day 4 and 14 Caco-2 cells (H). Changes in acetylation of MTTP promoter were evaluated using Ac-H3 antibodies (sc-8655) (I).
(J) Immunoblotting evaluated nuclear NR2F1 protein in Caco-2 cell cultures before (D4) and after (D14) differentiation. Tubulin was a control.
(K) Undifferentiated Caco-2 cells stably transfected with pMTP-204 or the same plasmid carrying mutant DR1 element (DR1m) were transfected with different siRNAs.
Luciferase activities were measured using same amounts of protein. Data were normalized with siControl treated cells. Mean ± SEM; #, p < 0.05, ###, p < 0.001, One-way ANOVA; **, p < 0.01, ***, p < 0.001, Student t test.
We then hypothesized that the induction of MTTP transcription might be due to reduced bindings of putative repressors (Figure 3A) such as NR2F1 (COUP-TF1 or Ear3), NR2F2 (COUP-TFII or Arp-1) and NR2F6 (Ear2). To test this hypothesis, we first determined their mRNA levels during Caco-2 cell differentiation. Expression of these repressors was high in undifferentiated cells but low after differentiation (Figure 3D). To identify their role in MTP gene regulation, we reduced their expression using small interference RNAs (siRNA); siNR2F1, siNR2F2 and siNR2F6 specifically reduced expression of their respective target genes (Figure 3E). We then evaluated the effect of these siRNAs on MTP (Figure 3F). While siNR2F1 increased MTP mRNA, siNR2F2 and siNR2F6 had no effect suggesting that NR2F1 might act as a repressor. Consistent with this impression, NR2F1 overexpression in undifferentiated Caco-2 cells significantly inhibited MTTP promoter activity (Figure 3G). Moreover, ChIP showed more than 60% reduction in NR2F1 binding to the MTTP promoter after differentiation (Figure 3H) as well as increased acetylation of the MTTP promoter in siNR2F1-treated Caco-2 cells (Figure 3I). Furthermore, NR2F1 protein levels were diminished after differentiation (Figure 3J). These observations indicate that NR2F1 acts as a repressor in undifferentiated cells. Decreases in NR2F1 during differentiation might be linked to MTTP promoter activation.
To confirm whether the MTTP promoter is repressed by NR2F1 and to identify cis elements required for this repression, Caco-2 cells stably expressing pMTP-204- and DR1m-luciferase constructs were treated with various siRNAs. Compared with siControl, siGL2 reduced luciferase activity by about 50% indicating transfection efficiency. Knockdown of NR2F1 increased MTP promoter activity (Figure 3K, pMTP-204), while siNR2F2 and siNR2F6 had no effect. Caco-2 cells stably expressing a DR1m promoter construct did not respond to siNR2F1 (Figure 3K, DR1m). These observations indicate that the DR1 element is required for NR2F1 to repress MTTP transcription in undifferentiated Caco-2 cells.
Reduction in IRE1β expression contributes to increased MTP mRNA during differentiation
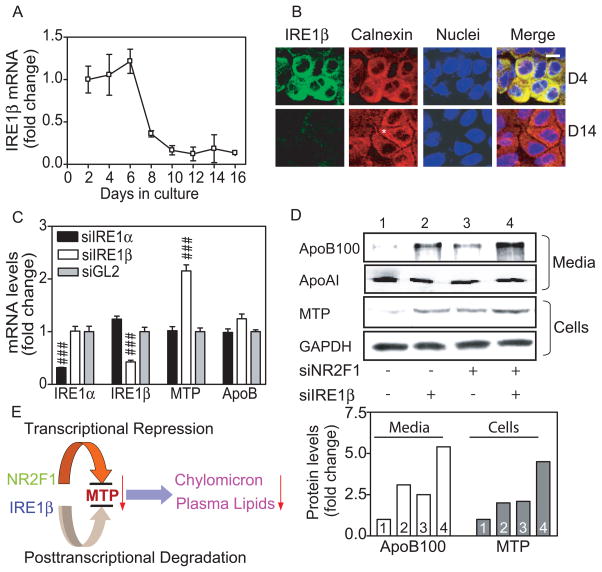
MTP mRNA levels were enhanced by 15-fold after differentiation (Figure 1D). This was higher than the increases seen in MTTP promoter activities (4–5 fold; Figures 2D; 2G) suggesting that mechanisms other than transcriptional induction are also in play. Therefore, we looked for post-transcriptional mechanisms. Recently, IRE1β has been shown to regulate MTP mRNA post-transcriptionally 19. We found that undifferentiated Caco-2 cells highly express IRE1β mRNA; its amounts fall significantly (~4-fold decline) after day 6 indicating differentiation-dependent reduction (Figure 4A). Next, we examined IRE1β protein by immunostaining (Figure 4B). Proliferating cultures at day 4 exhibited abundant IRE1β protein that co-localized with an ER marker, calnexin, as shown in yellow on the merge image. On day 14, Caco-2 cells showed a polarized morphology with the staining of calnexin suggestive of differentiated phenotype (Figure 4B, asterisk). IRE1β was significantly reduced in these differentiated cells. To understand the relationship between IRE1β reduction and MTP induction, we studied MTP expression in undifferentiated cells treated with siIRE1β. As a control, we used siRNA against its homologue, IRE1α (Figure 4C). IRE1β knockdown increased MTP mRNA without altering apoB mRNA when compared with siGL2, while siIRE1α had no effect indicating that suppression of IRE1β increases MTP mRNA.
Figure 4. IRE1β expression is reduced during differentiation.
(A) IRE1β and ARPp0 mRNA were quantified and ratios on day 2 were normalized to 1. Fold change in IRE1β mRNA during Caco-2 cell differentiation is shown.
(B) Caco-2 cells were fixed (1% formaldehyde, 30 min), treated with methanol (5 minutes), blocked with PBS containing 1% horse serum and 3% BSA (30 minutes), incubated (1 h) first with antibody against IRE1β (sc-20575) in blocking buffer and then with anti-calnexin (sc-11397). Two secondary antibodies, anti-rabbit Alexa Fluor 555 and anti-goat Alexa Fluor 488, were added and incubated (45 min). Nuclear DNA was stained with Topro3 blue (Invitrogen). Scale bar, 10 μm.
(C) Cells were treated with different siRNA and mRNA levels of IRE1α, IRE1β, MTP, and apoB were determined. Data were normalized to siGL2 and presented as means ± SEM.
(D) Caco-2 cells (100-mm dishes) were transfected with siGL2, siNR2F1, and siIRE1β, alone or in combination. Intracellular MTP and GAPDH were determined with Western blotting. Cells were labeled with 35S-methionine/cysteine and secreted apoB and apoAI were immunopreciptated with 1D1 or 4H1 antibodies 8, respectively. Bands were scanned and quantified using ImageQuant (lower panel). Representative bands from 2 separate experiments are shown in the upper panel.
(E) Hypothetical model of intestinal MTP regulation. NR2F1 and IRE1β suppress MTTP expression via transcriptional and post-transcriptional mechanisms. These two regulatory proteins might restrict MTP expression and chylomicron production in undifferentiated/dividing intestinal cells.
The studies described above identified transcriptional and posttranscriptional mechanisms involving NR2F1 and IRE1β that control MTTP expression during Caco-2 cell differentiation. Next, we determined individual and combined effects of these mechanisms on MTP expression and apoB secretion (Figure 4D). Treatment of undifferentiated cells with siNR2F1 (lane 3) or siIRE1β (lane 2) increased MTP protein in cells and apoB100 in media compared with untreated cells (lane 1). Combined treatment of siNR2F1 and siIRE1β increased MTP protein and apoB100 secretion (lane 4) equal to the sum of individual treatments (lane 2 and 3). Similar experiments were not performed in differentiated cells because they express small amounts of these proteins and are difficult to transfect. These data suggest that both transcriptional and post-transcriptional mechanisms might additively suppress MTP and curb apoB lipoprotein production in undifferentiated cells (Figure 4E).
Expression of Nr2f1, Ire1β, and Mtp along the crypt-villus and jejunum-colon axes
To obtain in vivo corollary to the cell culture experiments, we studied Mtp, Nr2f1, and Ire1β expression in mouse intestine by immunohistochemistry (Figure 5A). Mtp was more abundant in jejunum than in ileum, and was low in colon. In the jejunum and ileum, it was present mainly in the villi consistent with previous findings 20–23. Ire1β protein (Figure 5B) expression followed the order of jejunum < ileum < colon. More Ire1β was present in crypts than in villi of the jejunum (Figure 5B). Ire1β expression gradient from jejunum to colon, and from villus to crypt in the jejunum was reciprocal to intestinal Mtp expression. Similarly, the expression patterns of Nr2f1 (Figure 5C) were also reciprocal to those of Mtp. Its expression was high in cells that express Ki67, a marker for dividing cells, and low in villi. These data indicate an inverse relationship in the expression of Mtp with Nr2f1 and Ire1β in the intestine.
Figure 5. Mtp, Ire1β and Nr2f1 expression in mouse intestine.
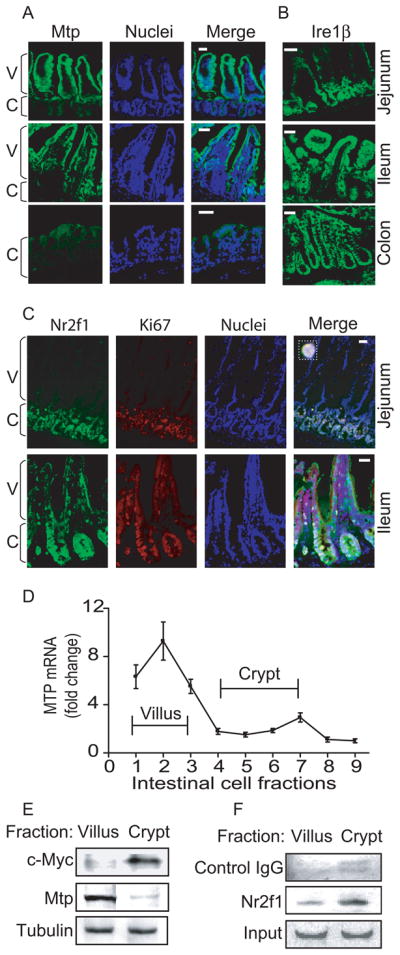
Frozen mouse proximal jejunum, distal ileum, and colon were sectioned (10 μm) and processed for staining as in Figure 4B.
(A–B) Goat anti-MTP (sc-331166) (A) and IRE1β (sc-10512) (B) were incubated (1h) with sections. Anti-goat Alexa Flour 488 antibody was added and nuclei were stained with Topro3 blue. Bar, 100 μm; C, crypt; V, villus
(C) Goat anti-NR2F1 and rabbit anti-Ki67 (Vector Labs, Burlingame, CA) antibodies were used for staining the intestinal sections.
(D–F) Mouse jejunum (n=3, 16 cm from duodenum) was incubated with 1% EDTA for various times to separate villus and crypt cells from the intestinal wall as described in Supplementary Materials and Methods. Mtp mRNA was determined with qRT-PCR (D). Cells representing villi (fractions 1–3) and crypts (fractions 4–7) were pooled and presence of c-Myc, Mtp, and tubulin was determined by western blotting (E). Binding of NR2F1 to the MTTP promoter in villus and crypt cells was analyzed by ChIP followed by regular PCR. Goat serum IgGs were used as a negative control (F).
To document further the reciprocal relationship between Mtp and Nr2f1 at functional level, we separated villus and crypt cells from mouse intestine. Mttp mRNA and c-Myc, a marker for diving cells, protein levels were high in villus and crypt cells, respectively, indicating separation of these cells (Figure 5D–E). To determine whether Nr2f2 interacts with the mttp promoter, we performed ChIP in these two fractions (Figure 5F). Binding of Nr2f1 to the mttp promoter was high in the crypt but low in the villi, similar to that observed in undifferentiated and differentiated Caco-2 cells, respectively. Therefore, we suggest that the binding of Nr2f1 to mttp promoter in crypt cells might suppress Mtp expression in mouse intestine.
Discussion
MTP expression is limiting for lipoprotein biosynthesis in undifferentiated intestinal cells
We show that undifferentiated Caco-2 cells neither express MTP nor synthesize lipoproteins. In contrast, both undifferentiated and differentiated cells contain apoB mRNA. Adenoviral mediated expression of human MTP induces apoB-lipoprotein secretion in undifferentiated cells. Therefore, we propose that MTP is the limiting factor for the biosynthesis of apoB-lipoproteins in undifferentiated cells. It is unclear why the regulation of MTP is related to lipid mobilization in enterocytes. Possibly, regulation of the synthesis of a shorter MTTP transcript than that of APOB might be more efficient.
NR2F1 suppresses MTTP expression in undifferentiated cells
Our studies indicate that HNF-4α and HNF-1α required for the synergistic activation of MTP in the liver 24 bind to the MTTP promoter in both undifferentiated and differentiated Caco-2 cells. Despite their binding to the MTTP promoter, MTTP gene is not expressed in undifferentiated intestinal cells. Mechanistic studies revealed that NR2F1 suppresses MTP expression in undifferentiated cells by binding to the DR1 element in its promoter. During differentiation, NR2F1 expression is reduced and it’s binding to the MTTP promoter is decreased. Therefore, MTTP induction during differentiation depends on decreased binding of NR2F1 to its promoter.
Nr2f1 and Nr2f2 behave similarly during differentiation of neuronal cells into glial cells in mice 25. Consequently, we were surprised to observe that NR2F1, but not its homologues NR2F2 and NR2F6, modulates MTP expression during differentiation of Caco-2 cells. It has been shown that NR2F2 suppresses MTP expression in hepatoma cells 26 but very little is known about the role of NR2F1 in hepatic mttp gene regulation. Our preliminary studies (not shown) indicate that Nr2f1 is not expressed in hepatocytes. These observations indicate that Nr2f1 might play a specific role in the suppression of mttp gene in enterocytes.
Post-transcriptional mechanisms reduce MTTP mRNA levels
In addition to the transcriptional suppression by NR2F1, we found that post-transcriptional mechanisms involving IRE1β might be operative in undifferentiated intestinal cells. IRE1β has been shown to post-transcriptionally degrade MTP mRNA and play a role in suppressing high fat diet-induced MTP induction 19. This study shows that high expression of IRE1β in undifferentiated cells might ensure low MTP mRNA abundance. Knockdown of Ire1β alone or in combination with NR2F1 increases MTP expression and apoB secretion in undifferentiated cells. Therefore, reduced expression of proteins involved in MTTP gene repression and mRNA degradation may pave the way for MTP expression during differentiation.
MTTP induction in the intestine involves unique mechanism
Mechanisms elucidated in this study for the induction of MTP during differentiation are different than those reported for the induction of sucrase-isomaltase 2 and apoAI 16 in intestinal epithelial cells. Induction of sucrase-isomaltase is dependent on the expression of Cdx2 2. ApoAI induction is high on day 8 and is preceded by significant induction in HNF-4α expression 16. Barrero and Malik have shown that, in addition to HNF-4α, PRMT1 acts as a coactivator of HNF-4α 16 in the induction of apoAI during Caco-2 cell differentiation. Although MTP expression requires HNF-4α 27, we observed that MTP induction was not correlated with HNF-4α binding. Instead, MTP expression was dependent on the loss of NR2F1 and IRE1β expression during differentiation of intestinal cells. Thus, intestinal cells use different mechanisms to induce various genes during differentiation.
In addition to the intestine, MTP is also highly expressed in the liver. Very little is known about the induction of MTP during hepatic differentiation or regeneration. However, adenovirus-mediated expression of PGC1β, Foxa2, and FoxO1 in hepatocytes increases MTP expression 28, 29. On the other hand, SHP reduces its expression 18, 30. Surprisingly, NR2F2, which has been shown to repress Mttp transcription in rat liver-derived cell lines 26, does not play a role in MTTP induction in Caco-2 cells (Figure 2E–F). Since Nr2f1 and Ire1β are not expressed in hepatocytes, the mechanisms identified in this report might be specific to intestinal MTP expression.
Supplementary Material
Acknowledgments
a) Sources of funding: National Institutes of Health (DK-46900) and the American Heart Association.
b) Acknowledgment: We thank Dr. Titelman’s laboratory for help in confocal microscopy.
Abbreviations used
- apoAI
apolipoprotein AI
- apoB
apolipoprotein B
- ChIP
chromatin immunoprecipitation
- GFP
green fluorescent protein
- IRE
inositol-requiring enzyme
- MOI
multiplicity of infection
- MTP
microsomal triglyceride transfer protein
- MTTP
MTP gene
- NR2F
nuclear receptor family 2 group F
- PCR
polymerase chain reaction
Reference List
- 1.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008 March;134:849–64. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 2.Traber PG, Silberg DG. Intestine-specific gene transcription. Annu Rev Physiol. 1996;58:275–97. doi: 10.1146/annurev.ph.58.030196.001423. [DOI] [PubMed] [Google Scholar]
- 3.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apolipoprotein B-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 4.Hussain MM, Iqbal J, Anwar K, Rava P, Dai K. Microsomal triglyceride transfer protein: a multifunctional protein. Front Biosci. 2003;8:S500–S506. doi: 10.2741/1071. [DOI] [PubMed] [Google Scholar]
- 5.Hussain MM, Glick JM, Rothblat GH. In vitro model systems: Cell cultures used in lipid and lipoprotein research. Curr Opin Lipidol. 1992;3:173–8. [Google Scholar]
- 6.Hussain MM, Kancha RK, Zhou Z, Luchoomun J, Zu H, Bakillah A. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim Biophys Acta. 1996;1300:151–70. doi: 10.1016/0005-2760(96)00041-0. [DOI] [PubMed] [Google Scholar]
- 7.Sun H, Chow EC, Liu S, Du Y, Pang KS. The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol. 2008 April;4:395–411. doi: 10.1517/17425255.4.4.395. [DOI] [PubMed] [Google Scholar]
- 8.Luchoomun J, Hussain MM. Assembly and secretion of chylomicrons by differentiated Caco-2 cells: Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J Biol Chem. 1999;274:19565–72. doi: 10.1074/jbc.274.28.19565. [DOI] [PubMed] [Google Scholar]
- 9.Hussain MM. A proposed model for the assembly of chylomicrons. Atherosclerosis. 2000;148:1–15. doi: 10.1016/s0021-9150(99)00397-4. [DOI] [PubMed] [Google Scholar]
- 10.Hussain MM, Kedees MH, Singh K, Athar H, Jamali NZ. Signposts in the assembly of chylomicrons. Front Biosci. 2001;6:D320–D331. doi: 10.2741/hussain. [DOI] [PubMed] [Google Scholar]
- 11.Moberly JB, Cole TG, Schonfeld G. Oleic acid stimulation of apolipoprotein B secretion from HepG2 and Caco-2 cells occurs post-transcriptionally. Biochim Biophys Acta. 1990;1042:70–80. doi: 10.1016/0005-2760(90)90058-6. [DOI] [PubMed] [Google Scholar]
- 12.Liao W, Chan L, Apolipoprotein B. a Paradigm for Proteins Regulated by Intracellular Degradation, Does Not Undergo Intracellular Degradation in CaCo2 Cells. J Biol Chem. 2000 February 11;275:3950–6. doi: 10.1074/jbc.275.6.3950. [DOI] [PubMed] [Google Scholar]
- 13.Hughes TE, Sasak WV, Ordovas JM, Forte TM, Lamon-Fava S, Schaefer EJ. A novel cell line (Caco-2) for the study of intestinal lipoprotein synthesis. J Biol Chem. 1987;262:3762–7. [PubMed] [Google Scholar]
- 14.Dashti N, Smith EA, Alaupovic P. Increased production of apolipoprotein B and its lipoproteins by oleic acid in Caco-2 cells. J Lipid Res. 1990;31:113–23. [PubMed] [Google Scholar]
- 15.Lee DM, Dashti N, Mok T. Apolipoprotein B-100 is the major form of this apolipoprotein secreted by human intestinal Caco-2 cells. Biochem Biophys Res Commun. 1988;156:581–7. doi: 10.1016/s0006-291x(88)80882-9. [DOI] [PubMed] [Google Scholar]
- 16.Barrero MJ, Malik S. Two functional modes of a nuclear receptor-recruited arginine methyltransferase in transcriptional activation. Mol Cell. 2006 October 20;24:233–43. doi: 10.1016/j.molcel.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner RD, Krul ES, Moberly JB, Alpers DH, Schonfeld G. Apolipoprotein expression and cellular differentiation in Caco-2 intestinal cells. Am J Physiol. 1992;263:E374–E382. doi: 10.1152/ajpendo.1992.263.2.E374. [DOI] [PubMed] [Google Scholar]
- 18.Hirokane H, Nakahara M, Tachibana S, Shimizu M, Sato R. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J Biol Chem. 2004 October 29;279:45685–92. doi: 10.1074/jbc.M404255200. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1β inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7:445–55. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swift LL, Jovanovska A, Kakkad B, Ong DE. Microsomal triglyceride transfer protein expression in mouse intestine. Histochem Cell Biol. 2005 June;123:475–82. doi: 10.1007/s00418-005-0772-7. [DOI] [PubMed] [Google Scholar]
- 21.Shelton JM, Lee MH, Richardson JA, Patel SB. Microsomal triglyceride transfer protein expression during mouse development. J Lipid Res. 2000 April;41:532–7. [PubMed] [Google Scholar]
- 22.Swift LL, Kakkad B, Boone C, Jovanovska A, Jerome WG, Mohler PJ, Ong DE. Microsomal triglyceride transfer protein expression in adipocytes: a new component in fat metabolism. FEBS Lett. 2005 June 6;579:3183–9. doi: 10.1016/j.febslet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Levy E, Stan S, Delvin E, Menard D, Shoulders C, Garofalo C, Slight I, Seidman E, Mayer G, Bendayan M. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. J Biol Chem. 2002 May 10;277:16470–7. doi: 10.1074/jbc.M102385200. [DOI] [PubMed] [Google Scholar]
- 24.Sheena V, Hertz R, Nousbeck J, Berman I, Magenheim J, Bar-Tana J. Transcriptional regulation of human microsomal triglyceride transfer protein by hepatocyte nuclear factor-4alpha. J Lipid Res. 2005 February;46:328–41. doi: 10.1194/jlr.M400371-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008 August 24;11:1014–23. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- 26.Kang S, Spann NJ, Hui TY, Davis RA. ARP-1/COUP-TF II determines hepatoma phenotype by acting as both a transcriptional repressor of microsomal triglyceride transfer protein and an inducer of CYP7A1. J Biol Chem. 2003 August 15;278:30478–86. doi: 10.1074/jbc.M304201200. [DOI] [PubMed] [Google Scholar]
- 27.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001 February;21:1393–403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006 February;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008 June 2;118:2347–64. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, Moore DD, Wang L. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147–57. doi: 10.1002/hep.21632. [DOI] [PubMed] [Google Scholar]
- 31.Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res. 2004;45:764–72. doi: 10.1194/jlr.D300026-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Rava P, Athar H, Johnson C, Hussain MM. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res. 2005;46:1779–85. doi: 10.1194/jlr.D400043-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.