Abstract
Prediction error plays an important role in modern associative learning theories. For example, the omission of an expected event (surprise) can enhance attention to cues that accompany those omissions, such that subsequent new learning about those cues is more rapid. Many studies from our laboratories demonstrated that circuitry that includes the amygdala central nucleus (CeA), the cholinergic neurons in the substantia innominata/nucleus basalis region (SI/nBM), and their innervation of the posterior parietal cortex, is critical for this surprise-induced enhancement of attention in learning. We recently showed that midbrain dopamine neurons, known to code prediction error, are also important for surprise-induced enhancement of learning through their interaction with CeA
The current study examined whether the communication between the substantia nigra pars compacta (SNc) and CeA is critical only at the time of surprise, for example to detect prediction error information, or is also needed to maintain and later express that information as enhanced learning. All animals received unilateral CeA lesions and unilateral cannula implants targeting the SNc located contralateral to the lesioned CeA. Since the SNc-CeA connections are mainly ipsilateral, inactivating SNc contralateral to the lesioned CeA provided transient blockage of SNc and CeA communication. The results show that SNc-CeA communication is critical for processing prediction error information at the time of surprise, but neither SNc nor SNc-CeA communication is necessary to express that information as enhanced learning later.
Keywords: Rat, prediction error, midbrain dopamine, associative learning
Consistent with many learning theories, considerable behavioral data show that learning is enhanced when outcome expectancies are violated. For example, unexpected rewards are often more effective than well-predicted rewards in establishing new learning (Rescorla and Wagner, 1972). Similarly, the surprising presentation or omission of a reward may also enhance attention to other events that accompany those surprising episodes (e.g., Pearce and Hall, 1980). Because the firing rates of midbrain dopamine (DA) neurons increase or decrease when unpredicted rewards are presented or predicted rewards are omitted, respectively (Schultz et al., 1997), activity of these neurons has been suggested as a neural basis for the role of prediction error in learning (Schultz and Dickinson, 2000). However, empirical data on the mechanisms by which prediction error coding by DA neurons influences learning are sparse.
Previous research from our laboratories identified components of circuitry critical for the enhancement of attention to stimuli that accompany the omission of expected events. In intact rats, after extended training with a light→tone sequence, omission of the tone increased attention to the light, as manifested in the rate of subsequent learning of light-food associations. However, such surprise-accelerated learning was not displayed by rats with lesions or pharmacological interventions that disrupted the function of, or communication between, the amygdala central nucleus (CeA), the cholinergic neurons in the substantia innominata/nucleus basalis region (SI/nBM), and their innervation of the posterior parietal cortex (Bucci et al., 1998; Chiba et al., 1995; Han et al., 1999; Holland & Gallagher, 1993; 1999).
Lee et al. (2006) showed that the midbrain substantia nigra pars compacta (SNc), through its connectivity with CeA, is also critical to this example of surprise-induced enhancement of learning. Rats with SNc lesions in one hemisphere combined with contralateral CeA lesions, which prevented communication between those 2 structures, showed no surprise-enhanced learning. Here we used lesion and transient inactivation procedures to examine the roles of SNc and its connectivity with CeA in the initial processing of surprise, and in the expression of the consequences of that surprise as enhanced learning later.
Holland and Gallagher (2006) found that in this training protocol, intact CeA function was required only at the time of surprise. Pharmacological inactivation of CeA function at the time of surprise prevented enhanced learning in test, but inactivation at test had no effect. In the present study, if SNc-CeA communication at the time of surprise alters processing of the light in SNc itself, which enables enhanced learning later, then disruption of SNc function at either the time of surprise or at test will produce impairments. By contrast, if communication between SNc and CeA serves only to process prediction error information, and information about the light that affects subsequent learning is not maintained in SNc, then disruption of SNc-CeA communication at the time of surprise will prevent enhanced learning in test, but alteration of SNc function at test will have little impact.
Materials and Methods
Subjects
Experimentally naive, male Long-Evans rats (Charles River Laboratories, Raleigh, NC), initially weighing 300-350 g, were individually housed in a climate-controlled vivarium on a 12:12-hr light/dark cycle (lights on at 07:00) with ad libitum access to water. They were fed ad libitum during acclimation and a 10-day postoperative recovery period. Starting 5 days prior to training until the completion of the study, they were placed on a restricted diet in order to maintain 85% of free-feeding body weight.
Surgery
Rats were anesthetized with isoflurane gas (Abbott Laboratories, North Chicago, IL), placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) and surgery was conducted under aseptic conditions. All rats received unilateral lesions of CeA and cannula implants targeting SNc in the contralateral hemisphere. The hemisphere of lesion and cannula implant sites was counterbalanced. This combined lesion and cannula implant procedure allowed transient inactivation of SNc unilaterally, in the hemisphere opposite to the site of the lesioned CeA. Because the connections between SNc and CeA are predominantly ipsilateral, this procedure allowed for temporary disruption of communication between SNc and CeA when SNc is transiently inactivated pharmacologically. In the absence of such inactivation, normal SNc-CeA communication would occur in the hemisphere with intact CeA and cannulated SNc; elsewhere we have shown that rats with unilateral lesions of CeA show surprise-enhanced learning in this behavioral protocol (Holland & Gallagher, 2006; Lee et al., 2006). For unilateral CeA lesions, 0.4 μl of 10 μg/μl ibotenic acid (Biosearch Technologies, Novato, CA) in PBS solution (i.e. 0.1 M phosphate buffer with 0.9 % saline) was infused to two sites (0.2. μl over 2 min per site) using a Hamilton 2.0 μl syringe (25 gauge needle). For cannula implants, a 26 gauge guide cannula (Plastic One, Roanoke, VA) was chronically implanted 2.0 mm dorsal to the targeted SNc site, which allowed a 33 gauge injection needle (Plastic One) to be lowered 2.0 mm beyond the guide cannula to target SNc. The stereotaxic coordinates used for CeA were 2.2 mm and 2.6 mm posterior to bregma, 4.2 mm lateral from the midline, and 8.0 mm ventral from the skull surface, and for SNc were 5.3 mm posterior to bregma, 2.4 mm lateral from the midline, and 5.4 mm ventral from the skull surface. After surgery, all rats received a single 0.02 mg/kg subcutaneous injection of buprenorphine hydrochloride (Sigma) for amelioration of pain, and were allowed to recover from surgery for 15 days before behavioral testing.
Apparatus
The behavioral training apparatus consisted of four individual chambers (22.9 × 20.3 × 20.3 cm). Each chamber had aluminum front and back walls, clear acrylic sides and top, and a floor made of stainless steel rods (0.48 cm in diameter spaced 1.9 cm apart). A food cup, fitted with phototransistors for detecting head entries, was recessed in the center of the front wall 2 cm above the floor. A jeweled 6-w lamp, mounted on the front panel of the chamber, 15 cm above the food cup, served as the source of the light conditioned stimulus (CS). Each chamber was enclosed in a sound-attenuated box where ventilation fans provided masking noise (70 dB) and constant dim illumination was provided by a 6-w red light. A speaker mounted on the inside wall of the sound-attenuated box, behind and above the chamber, was used to present the auditory CS. A television camera was mounted within each box and images were recorded during behavioral training and testing.
Behavioral procedures
The behavioral task, first developed by Wilson et al. (1992) and used extensively in our laboratories (e.g., Baxter et al., 1997; Bucci et al. 1998; Chiba et al., 1995; Holland & Gallagher, 1993, 1999, 2006) is designed to assess enhanced attention and learning driven by prediction error, as postulated by the Pearce-Hall (1980) model. In the first phase (Table 1), rats are trained with a serial light →tone compound on a 50% food reinforcement schedule. Although the light →tone compound is followed by food on only half of the trials, the light is always followed by the tone. The tone is expected to acquire substantial conditioned responding (CR) because of its contiguity with food, and the light is expected to acquire minimal CR because of its relatively poor temporal and logical relationship with food, compared to the tone. Importantly, as the serial conditioning continues, the light comes to predict the tone accurately, which, within the Pearce-Hall (1980) model, results in the gradual decline of “controlled attention” to the light. In the second phase, rats in the “consistent” condition receive the same training as in the first phase, whereas for the rats in the “surprise” condition, the tone is omitted after the light presentations on nonreinforced trials. Thus, although the food reinforcement schedule remains the same, the light no longer accurately predicts the tone, and so, within the Pearce-Hall (1980) model, attention to the light is enhanced. This enhanced attention is manifested as more rapid learning about the light in a test phase, in which rats receive direct light → food pairings.
Table 1.
Behavioral and infusion procedures
| Behavioral Conditions | Infusion conditions | Phase I: L-T conditioning | Infusion prior to Phase II | Phase II: L-T prediction error | Infusion prior to test phase | Test phase: testing attention to L |
|---|---|---|---|---|---|---|
| Consistent | V-V | Vehicle | Vehicle | |||
| N-V | L-T-food; L-T | NBQX | L-T-food; L-T | Vehicle | L-food | |
| V-N | Vehicle | NBQX | ||||
| Surprise | V-V | Vehicle | Vehicle | |||
| N-V | L-T-food; L-T | NBQX | L-T-food; L | NBQX | L-food | |
| V-N | Vehicle | Vehicle | ||||
L, Light; T, Tone; The surprise omission of the tone in nonreinforced trials in Phase 2 in the surprise group (shown in bold) is intended to enhance attention to the light which is then expressed as enhanced conditioning to L in the test phase. V-V group received vehicle infusions during Phase 2 and the test phase. N-V group received NBQX during Phase 2 and vehicle during the test phase. V-N group received vehicle during phase 2 and NBQX during the test phase.
Rats were first trained to eat the food that would be used as reinforcer in training by delivering two 45 mg pellets (Research Diets, New Brunswick, NJ) to the food cup with intertrial intervals (ITI) ranging from 2-6 min over one or two 64-min sessions. Then, all rats received 10 daily Phase 1 conditioning sessions. In each 64-min session, rats received eight reinforced and eight nonreinforced trials of a light → tone compound CS, randomly intermixed with variable ITI averaging 4 min. The compound CS comprised a 10-sec illumination of the panel light followed immediately by a 10-sec presentation of a 1,500-hz, 80 dB tone. Two 45 mg food pellets were delivered immediately after the tone presentation on reinforced trials. In Phase 2, rats in the Consistent condition continued to receive training sessions identical to those given in Phase 1 for an additional 2 sessions. In these sessions, the rats in the Surprise condition received eight reinforced trials of the light → tone compound CS, randomly intermixed with eight trials with the light alone. In Phase 3, all rats received five daily 64-min test sessions, in which 16 presentations of the 10-sec light were each followed by two food pellets.
The measure of learning was the percentage of time during each recording period that the food-cup photocells indicated head entry. We report this food-cup CR during the last 5-s periods of CSs because such CRs occur primarily during that time period (Holland 1977). To reduce the contribution of within-group variation in baseline responding, pre-CS responding (i.e. responding during the 5-s empty interval prior to the light CS) was subtracted from CS responding to form an elevation score.
Infusion procedures
In the current preparation, inactivating SNc unilaterally should result in the transient blockage of communication between SNc and CeA because CeA in the opposite hemisphere was permanently lesioned. Unilateral inactivation of SNc was produced by infusions of 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX), a competitive ionotropic glutamate receptor antagonist for alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate. AMPA receptors GluR1 and GluR2/3 are especially abundant on SNc DA neurons (Fallon and Loughlin, 1995; Yung, 1998). In addition to mediating excitatory glutamate transmission in SNc, AMPA and kainate receptors can also influence GABAergic inputs to SNc (Chen and Rice, 2002; Nakamura et al., 2002). Even though it is not known how the NBQX concentration used in the current study affects SNc physiology in vivo, NBQX at lower concentration (e.g., 1 mM) has been shown to effectively block glutamate effects on SNc in anesthetized animals (Christoffersen and Meltzer, 1995). Notably, El-Amamy and Holland (2006) used the same dosage of NBQX used here to inactivate SNc and reported similar behavioral deficits produced by lidocaine infusion to SNc.
All handling and infusion procedures were performed 20 min prior to behavioral training and testing sessions. Infusions (0.2 μl) were delivered over one minute via a 33-gauge injector cannula that was connected to a 10 μl Hamilton syringe placed in a multiple syringe pump (KD Scientific, Holliston, MA). Prior to sessions 7 and 8 in Phase 1, each rat's dummy cannula was removed and replaced with a clean one to acclimate the rats to manipulation of the cannula headset. Prior to sessions 9 and 10, each rat received an infusion of 0.1 M PBS vehicle solution to familiarize them with the infusion procedure. For Phase 2 and the test phase infusion, rats were assigned to three infusion conditions within each of the behavioral treatment (Consistent and Surprise) conditions, resulting in 6 groups (Table 1). Group V-V (vehicle – vehicle) served as infusion controls; they received PBS vehicle infusions during both Phase 2 and the test phase. Group N-V (NBQX – vehicle) received NBQX (20 mg/ml, Sigma) infusions during Phase 2 and PBS vehicle infusions during the test phase, which inactivated SNc (presumably blocking SNc-CeA communication) only at the time of surprise. Finally, Group V-N (vehicle – NBQX) received vehicle infusions during Phase 2 and NBQX during the test phase, which inactivated SNc and blocked SNc-CeA communication only at the time of testing. Thus, all animals received either PBS and/or NBQX infusions prior to each session during Phase 2 (two sessions) and the test phase (5 sessions).
Histology
After completion of behavioral testing, the rats were deeply anesthetized with pentobarbital (100 mg/kg) and perfused with 0.9 % saline followed by 4% formaldehyde in 0.1 M PB. Brains were removed, post-fixed and cryoprotected overnight in 4% formaldehyde in 0.1 M PB containing 12% sucrose, frozen with powdered dry ice, and stored at −80°C. Brains were sliced on a freezing microtome and 40-μm coronal sections through CeA and SNc were collected. Every third section was mounted on slides and stained with thionin in order to verify lesions made in CeA and cannula tracks targeting SNc.
Results
Histology
Fifty one rats were judged as having acceptable lesions of CeA and cannula placements over SNc (see Table 2 for distribution of 51 rats among the 6 groups) and 24 rats were eliminated from the study based on histological examination. Four sections containing CeA, each matching the levels from 25 to 27 according to Swanson's brain map (1992), were picked from each animal and the lesion area was outlined. Only the animals that had at least 30% damage to the medial CeA on each level were judged as having sufficient CeA lesions. A series of studies carried out by our laboratories examining the central amygdala functions in attention and learning consistently showed that 30% damage to the medial subdivision of central amygdala was sufficient to produce behavioral effects (e.g. Gallagher et al., 1990; Holland & Gallagher, 1993). The average of the medial CeA (as identified in levels 25-27 of Swanson, 1992) damage was 70 %, with no differences among the 6 groups (F 5, 45 = 0.82, p = 0.5). The majority of the CeA lesions were restricted to the medial portion of CeA, but for 13 rats, the damage extended to part of the lateral portion of CeA. In addition, in some rats there was also minor damage to the basolateral nucleus of the amygdala (n = 14) and/or medial amygdala (n = 8). SNc cannula placements were rejected if the injection cannula track was located more than 0.5 mm away from SNc or if substantial damage to SNc neurons was evident. All cannula tracks were located in the lateral part of SNc, as represented in Fig 1a. Figure 1 shows histology from a typical rat that received a cannula implant targeting the left SNc and a lesion of CeA in the right hemisphere.
Table 2.
Food-cup responding in Phases 1 and 2
| Phase 1 | Phase 2 | ||||
|---|---|---|---|---|---|
| Group | n | Light | Tone | Light | Tone |
| Consistent | |||||
| V-V | 7 | 8.3 ± 3.8 | 57.1 ± 8.7 | 9.2 ± 3.4 | 61.6 ± 10.7 |
| N-V | 9 | 3.9 ± 2.3 | 52.9 ± 7.5 | -0.4 ± 0.9 | 23.1 ± 3.0 |
| V-N | 10 | 4.0 ± 4.1 | 48.7 ± 7.3 | 7.7 ± 3.7 | 57.3 ± 7.7 |
| Surprise | |||||
| V-V | 9 | 8.0 ± 4.3 | 57.3 ± 6.8 | 6.2 ± 2.9 | 65.2 ± 6.3 |
| N-V | 7 | -0.1 ± 3.1 | 55.5 ± 4.3 | -1.2 ± 1.1 | 16.9 ± 7.3 |
| V-N | 9 | 6.6 ± 3.2 | 54.7 ± 6.6 | 4.1 ± 2.6 | 60.9 ± 6.3 |
Data are the mean (± SEM) percentage of time with head in the food cup during the last 5-sec of light or tone presentation minus the time spent in the food cup during the 5-sec empty interval prior to the light presentation. Phase 1 shows averaged responses on all trials in sessions 9-10 and Phase 2 shows averaged responses on light-tone-food trials. Animals in all groups received vehicle infusions during sessions 9-10 in Phase 1. Animals in V-V and V-N continued to receive vehicle infusions in Phase 2 while animals in N-V received NBQX infusions in Phase 2. n, Number of subjects; V-V, vehicle infusion in Phase 2 and the test phase; N-V, NBQX infusion in Phase 2 and vehicle infusion in the test phase; V-N, vehicle infusion in Phase 2 and NBQX infusion in the test phase.
Figure 1.
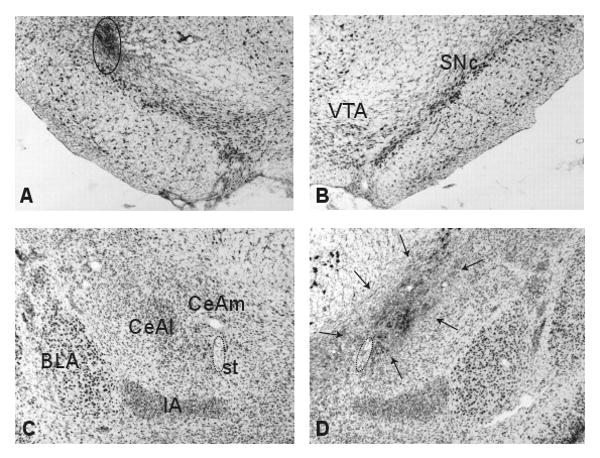
Photomicrographs showing representative brain sections of SNc and CeA of an animal that received a cannula implant targeting SNc in the left hemisphere and a CeA lesion in the right hemisphere. A, B Nissl-stained sections of the left and right SNc, respectively. Open circle shows mild gliosis caused by the tip of injection cannula. C, D, Nissl-stained sections of the intact and lesioned (arrows) CeA, respectively. BLA, basolateral amygdala; CeAl, lateral CeA; CeAm, medial CeA; IA, intercalated nucleus of the amygdala; st, stria terminalis (encircled by dotted line); VTA, ventral tegmental area.
Behavior
In Phase 1, all rats acquired substantial food-cup CRs to the tone, which was immediately followed by food delivery, but responded little to the light, which was temporally remote from the food delivery. Table 2 shows food-cup CR elevation scores for the light and tone during the last two sessions of Phase 1. Although all rats were treated identically in Phase 1, for purposes of analysis they were grouped according to the behavioral and infusion treatments they would receive in Phase 2 and the test phase, to demonstrate that all groups began Phase 2 with identical performance. As expected, there were no between-group differences in baseline (pre-CS) responses, minimal food-cup CRs to the light, and acquisition of food-cup CRs to the tone during phase 1. Two (Behavioral training conditions: Consistent or Surprise) by three (infusion conditions:V-V, N-V, or V-N) factorial repeated measures (training sessions 1-10) ANOVAs confirmed these observations. For each stimulus period (pre-CS, light, and tone) there were no significant main effects of behavioral training condition (F 1, 45 = 1.97, p = 0.17 for pre-CS; F 1, 45 = 1-3, p = 0.97 for light; F 1, 45 = 7-5, p = 1.0 for tone) or infusion condition (F 2, 45 = 2.21, p = 0.12 for pre-CS; F 2, 45 = 1.2, p = 0.31 for light; F 2, 45 = 0.6, p = 0.5 for tone), and no training by infusion condition interactions (F 2, 45 = 1.10, p = 0.34 for pre-CS; F 2, 45 = 0.09, p = 0.92 for light; F 2, 45 = 0.14, p = 0.87 for tone).
In Phase 2, the rats continued to show substantial responding to the tone and minimal responding to the light (Table 2). Two (surprise conditions) × 3 (infusion conditions) factorial repeated measures (2 phase 2 sessions) ANOVAs showed that the surprise condition had no effect on this pattern for light (F 1, 45 = 0.72, p = 0.4) or tone (F 1, 45 = 3-3, p = 0.95) responding, but there was a main effect of infusion condition on responding to both light (F 2, 45 = 5.46, p < 0.01) and tone (F 2, 45 = 22.2, p < 0.001). Planned contrasts between groups with NBQX infusions (N-V) and vehicle infusion (V-V and V-N) confirmed the observation (see Table 2) that NBQX depressed the overall CR to both the light (F 1, 45 = 10.9, p < 0.01) and the tone (F 1, 45 = 44.3, p < 0.1-6). This NBQX-depressed responding was also observed in pre-CS responding: rats infused with NBQX showed lower baseline responding (5.6 %) than rats infused with vehicle (10.9%; F 1, 45 = 5.0, p < 0.05).
Video recordings made during behavioral training revealed motor effects of unilateral NBQX infusion to SNc, which probably interfered with the display of food cup entry. All rats tilted their heads to the side contralateral to the NBQX infusion site. In addition, intermittent contralateral turning behavior and disruption of exploratory/grooming behavior due to unbalanced posture were observed. However, it appeared that animals were still able to process the sensory information because they showed behavioral reactions comparable in form (startle and orienting) to those exhibited by intact animals at the onset of the tone and the light, respectively.
Figure 2 shows the primary data of this study, the acquisition of food-cup CRs to the light during the test phase. Fig 2a shows that differential effects of surprise and infusion conditions began to emerge by the second day of testing. A surprise condition (2) by infusion condition (3) by test sessions (5) ANOVA confirmed this observation. There was a significant interaction of infusion condition and test session (F 8, 180 = 4.97, p < 0.001) as well as a marginal interaction of surprise condition and test session (F 4, 180 = 2.20, p =0.071). Fig 2b shows the average food-cup CR of the last 4 test days for clearer representation of group differences. A surprise condition by infusion condition by test session (4) ANOVA showed a main effect of surprise condition (F 1, 45 = 6.24, p = 0.016), a main effect of infusion condition (F 2, 45 = 19.86, p < 0.001), and an interaction of these two conditions (F 2, 45 = 3.70, p = 0.032). Within each of the 3 infusion conditions, we conducted planned contrast of responding of surprise and consistent condition rats. As predicted, among the rats that received vehicle infusions only (Group V-V), those in the surprise condition showed enhanced learning of the light-food association compared to the rats in the consistent condition (F(1, 45) = 8.63, p =0.005). However, rats that received NBQX infusions during Phase 2 (Group N-V) exhibited similar food-cup CR to the light in test regardless of Phase 2 training procedure (F(1, 45) = 0.55, p = 0.461). In addition, rats in the surprise condition in Group N-V showed less learning in test than rats in the surprise condition in Group V-V (F(1, 45) = 5.58, p = 0.023). Thus, disruption of SNc-CeA communication at the time of surprise (Phase 2) prevented subsequent enhancement of learning, when that communication was no longer blocked.
Figure 2.
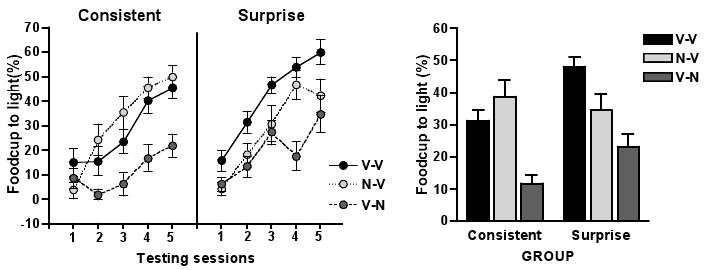
Mean (± SEM) time spent in the food cup during the light presentation in the test phase. The responding levels represent food-cup conditioned responses after subtracting pre-CS baseline responding (see methods for detailed description). A, The acquisition of food-cup conditioned response of animals in the consistent (left panel) and surprise (right panel) groups over the 5 sessions of the test phase. B, The mean food-cup conditioned response levels during the test phase (session 2-5) are plotted together for easier graphical comparison.
As we observed in Phase 2, NBQX infusion in the test phase depressed overall food-cup CRs to the light (V-N vs. V-V and N-V Planned contrast, F 1, 45 = 39.18, p < 0.001) as well as pre-CS food-cup behavior (V-N vs. V-V and N-V Planned contrast, F 1, 45 = 3.86, p = 0.055). However, it is notable that among the rats that received NBQX in the test phase (Group V-N), those that received Surprise treatment in Phase 2 (when vehicle infusions were given) still showed significantly more food-cup responding to the light than those that received Consistent treatment in Phase 2 (F(1, 45) = 4.86, p = 0.033). Thus, rats in Group V-N showed surprise-enhanced learning in test, even when responding in that test was depressed by NBQX infusion.
Discussion
Rats in which SNc-CeA communication was disrupted at the time of surprise, but intact at the time of testing (Group N-V), showed no evidence of surprise-enhanced learning, whereas that effect was observed in both control rats (Group V-V) and in rats in which SNc function was intact at the time of surprise but impaired at the time of testing (Group V-N). These results demonstrate that SNc-CeA connections are critical to processing surprise/prediction error information at the time of surprise but not to expressing that information as enhanced learning later. Furthermore, the performance of rats in Group V-N also suggests that once prediction error information has been processed within SNc-CeA circuitry, SNc itself (even apart from its connections with CeA) is no longer a critical site for maintenance or expression of information that produces accelerated learning. In Group V-N, surprise-enhanced learning was observed despite inactivation of the “trained” SNc (that is, SNc in the hemisphere with intact CeA) at the time of test. Although at test those rats had intact function of SNc in the hemisphere contralateral to NBQX infusion, that intact SNc was unlikely to have mediated enhanced learning because it was “untrained”. That is, the SNc that was intact in test had no opportunity to interact with CeA at the time of surprise, because CeA was lesioned in that hemisphere. Thus, any alterations of SNc function that may occur consequent to surprise were probably not critical to subsequent expression of accelerated learning. Taken together with Holland and Gallagher's (2006) observations, our results show that function of both SNc and CeA, and communication between those regions, are needed only for the processing of prediction error information and not for maintenance or expression of that information later.
Independent of surprise effects, unilateral infusion of NBQX into SNc caused motor disturbances that persisted for at least the duration of our 60-min sessions, presumably continuing until SNc regained function after dissipation of the NBQX effects. Rats tilted their heads contralateral to the side of the infusion, exhibited intermittent contralateral turning behavior, and showed disruption of exploratory and grooming behavior. These behaviors probably interfered with food-receptacle entry and hence depressed food-cup CRs. Transient contralateral rotation in rats has been observed previously following unilateral SNc lesions (Mintz et al., 1986). However, this unprovoked rotation behavior dissipated over a few days and animals recovered and exhibited normal motor behavior. In our previous study (Lee et al., 2006), permanent unilateral SNc lesions were made and the rats had 10 days to recover prior to the beginning of behavioral training, so it is not surprising that we observed no abnormal motor behavior in behavioral testing of those rats. In the current experiment, training sessions began 20 minutes after NBQX infusions into SNc and lasted an hour, which was apparently insufficient time for SNc function to recover. In addition, rats did not show adaptation to these transient motor effects over several days of NBQX infusions (e.g., in test in Group V-N). Behavioral recovery after unilateral SNc lesions is thought to arise from compensatory changes that occur in the remaining DA neurons (Emmi et al., 1997; Robinson et al., 1994). Thus, behavioral recovery (that is, display of food cup behavior) would not be expected in the current study, in which SNc neurons were only temporarily inactivated but not permanently destroyed. This lack of behavioral recovery over multiple infusions is consistent with the histological evidence that NBQX infusion did not cause permanent damage to SNc neurons. Most important, in the face of these substantial behavioral disruptions produced by NBQX infusion, it is striking that among the rats with NBQX infusion during the test phase only (Group V-N), rats in the surprise condition still showed higher levels of food-cup responding compared to rats in the consistent condition. This finding indicates that NBQX-induced depression of responding only reflected impairment at the motor output level, but not the expression of previously-processed information.
This study highlights the importance of SNc-CeA interaction in processing prediction error information. However, the nature of this interaction remains in question. For example, prediction error information from SNc DA neurons might alter information processing in CeA. Alternately, CeA might provide SNc with prediction information needed for its computation of prediction error (Holland & Gallagher, 2006). Heavy projections have been identified in both directions between SNc and CeA (Fallon and Moore, 1978; Fudge and Haber, 2000; Gonzales and Chesselet, 1990; Hasue and Shammah-Lagnado, 2002; Lee et al., 2005; Swanson, 1982), so information transmission in either or both directions may be important.
In other experimental paradigms, the surprising omission of an expected food reinforcer is accompanied by reduction in firing rates of DA neurons (Schultz et al., 1997). Thus, the surprising omission of the tone might similarly decrease SNc DA neuron activation, leading to a transient reduction in DA release in their CeA terminal fields. Because there is a heavy presence of GABAergic cells in CeA (Sun and Cassell, 1993; Swanson and Petrovich, 1998), decreased DA release in CeA might disinhibit CeA. The resultant increase in CeA activity might enhance attention to the light CS via CeA's projections to other brain areas, for example, to the magnocellular cholinergic neurons in SI/nBM (Jolkkonen et al., 2002; Groves, 1988), which modulate a range of cortical regions known to be involved in attention (Briand et al., 2007; Holland & Gallagher, 1999; Sarter and Parikh, 2005). As noted earlier, previous studies from our laboratories using the present training protocol have demonstrated that disconnection of CeA from SI/nBM (Han et al., 1999), and destruction of SI/nBM cholinergic neurons that project to PPC (Bucci et al., 1998) also eliminate surprise-enhanced learning. Recently, Bucci and MacLeod (2007) examined Fos expression at the time of surprise in the task used here. After extended Phase 1 consistent light→tone training, rats received a single surprise session (or an additional consistent session) and their brains were prepared for analysis of Fos protein. Bucci and MacLeod (2007) found significantly more neurons that expressed Fos in Surprise than in Consistent rats in CeA, SI/nBM and PPC, but not in visual cortex or SNc. While a decrease in Fos expression in SNc might have been predicted, those results are otherwise consistent with the model just described.
It is also possible that CeA provides SNc with prediction information needed for its computation of prediction error (Holland & Gallagher, 2006). Using a combination of retrograde tracing and immediate early gene activation techniques, Lee et al. (2006) found that a well-trained visual cue paired with food provoked a greater Fos response in CeA neurons that projected to SNc than a briefly-trained cue or a cue that had been explicitly unpaired with food. Thus, in the present experiment, when the light is presented, CeA might similarly provide SNc with information about the upcoming tone presentation. Notably, SNc also projects directly to cholinergic neurons in SI/nBM (Gaykema and Zaborszky, 1996) and hence could convey prediction error information, derived from CeA-SNc interaction, directly to the basal forebrain cholinergic system. It is also notable that other neural circuitry may contribute to SNc regulation. For example, lateral habenula neurons show increases in firing rate in response to reward omission and are known to exert inhibition of SNc DA neurons (Matsumoto and Hikosaka, 2007).
The present data add to our understanding of how interaction between SNc and CeA can influence attention and learning. Here, we found that enhanced attention to a light, produced by the surprising omission of an expected tone, required intact function of SNc and CeA at the time of surprise. However, intact function of SNc and CeA was not necessary to express enhanced attention to the light as faster learning later. By contrast, another attentional function (enhanced orienting responses to cues paired with food) demands a more persistent role of SNc. As with surprise-induced enhancement of learning, projections from CeA to SNc play an important role in mediating this aspect of attention (Lee et al., 2005), and CeA function alone is critical only at the time of learning and not at the time of expressing that learning (McDannald et al., 2004). However, El-Amamy and Holland (2006) found that SNc function is required for both the acquisition and expression of conditioned orienting responses. Interestingly, the display of conditioned orienting is more correlated with reinforcement prediction than prediction error. Even though we have no evidence that SNc DA neurons show activation changes in response to conditioned orienting responses or to the surprising omission of the tone in this learning paradigm, it is plausible that SNc DA cells may show differential activation to these two different conditions, as they do to reward delivery and reward omission (Shultz et al., 1997). This may lead to different interactions of SNc with various brain regions, including the CeA, in mediating these two attentional processes. Notably, in addition to SNc-CeA connections, conditioned orienting responses also rely on the nigrostriatal pathway (Han et al., 1997), whereas surprise-induced enhancement of learning requires cholinergic inputs to posterior parietal cortex (Bucci et al., 1998). Together, this suggests that SNc and CeA interact in multiple ways to influence information processing critical to different aspects of attention in associative learning.
Acknowledgments
This work was supported by the National Institutes of Health Grant MH53667.
Abbreviations
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- CeA
the central nucleus of the amygdala
- CR
conditioned response
- CS
conditioned stimulus
- DA
dopamine
- GABA
gamma-aminobutyric acid
- ITI
intertrial interval
- NBQX
1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide
- PBS
0.1 M phosphate buffer with 0.9 % saline
- SI/nBM
substantia innominata/nucleus basalis region
- SNc
substantia nigra pars compacta
References
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Macleod JE. Changes in neural activity associated with a surprising change in the predictive validity of a conditioned stimulus. Eur J Neurosci. 2007;26:2669–2676. doi: 10.1111/j.1460-9568.2007.05902.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Synaptic regulation of somatodendritic dopamine release by glutamate and GABA differs between substantia nigra and ventral tagmental area. J Neurochem. 2002;81:158–169. doi: 10.1046/j.1471-4159.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen CL, Meltzer LT. Evidence for N-methyl-D-aspartate and AMPA subtypes of the glutamate receptor on substantia nigra dopamine neurons: possible preferential role for N-methyl-D-aspartate receptors. Neuroscience. 1995;67:373–381. doi: 10.1016/0306-4522(95)00047-m. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Substantia nigra pars compacta is critical to both the acquisition and expression of learned orienting of rats. Eur J Neurosci. 2006;24:270–276. doi: 10.1111/j.1460-9568.2006.04896.x. [DOI] [PubMed] [Google Scholar]
- Emmi A, Rajabi H, Stewart J. Behavioral and neurochemical recovery from partial 6-hydroxydopamine lesions of the substantia nigra is blocked by daily treatment with D1/D5, but not D2, dopamine receptor antagonists. J Neurosci. 1997;17:3840–3846. doi: 10.1523/JNEUROSCI.17-10-03840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE. Substantia nigra. In: Paxinos G, editor. The rat nervous system. Academic Press; San Diego: 1995. pp. 215–237. [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Zaborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain. II. Substantia nigra-ventral tegmental area projections to cholinergic neurons. J Comp Neurol. 1996;374:555–577. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Chesselet MF. Amygdalonigral pathway: an anterograde study in the rat with Phaseolus vulgaris leucoagglutinin (PHA-L) J Comp Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Grove EA. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988;277:315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Han JS, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behav Neurosci. 1999;113:143–151. doi: 10.1037//0735-7044.113.1.143. [DOI] [PubMed] [Google Scholar]
- Han JS, McMahan RW, Holland PC, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Process. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav Neurosci. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Miettinen R, Pikkarainen M, Pitkanen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience. 2002;111:133–149. doi: 10.1016/s0306-4522(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. J Neurosci. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- McDannald M, Kerfoot E, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting. Eur J Neurosci. 2004;20:240–248. doi: 10.1111/j.0953-816X.2004.03458.x. [DOI] [PubMed] [Google Scholar]
- Mintz M, Douglas RJ, Tomer R, de Villiers AS, Kellaway L. Transient contralateral rotation following unilateral substantia nigra lesion reflects susceptibility of the nigrostriatal system to exhaustion by amphetamine. Life Sci. 1986;39:69–76. doi: 10.1016/0024-3205(86)90439-x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Jang IS, Ishibashi H, Watanabe S, Akaike N. Possible roles of kainate receptors on GABAergic nerve terminals projecting to rat substantia nigra dopaminergic neurons. J Neurophysiol. 2003;90:1662–1670. doi: 10.1152/jn.01165.2002. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variation in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. J Neurosci. 1994;14:2687–2696. doi: 10.1523/JNEUROSCI.14-05-02687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Wilson PN, Boumphrey P, Pearce JM. Restoration of the orienting response to a light by a change in its predictive accuracy. Q J Exp Psychol. 1992;44B:17–36. [Google Scholar]
- Yung KK. Localization of ionotropic and metabotropic glutamate receptors in distinct neuronal elements of the rat substantia nigra. Neurochem Int. 1998;33:313–326. doi: 10.1016/s0197-0186(98)00034-5. [DOI] [PubMed] [Google Scholar]


