Abstract
The human pathogen Schistosoma mansoni exhibits a highly evolved and intricate relationship with its host, evading immune destruction while co-opting CD4+ T cell–driven mechanisms to facilitate parasite development and egg excretion. Because the common γ(γc) chain cytokine interleukin (IL)–7 is also implicated in modulating schistosome development, we investigated whether this effect is mediated indirectly through the essential role that IL-7 plays in CD4+ T cell growth and survival. We demonstrate that attenuated schistosome development in the absence of IL-7 results from dysregulated T cell homeostasis and not from disruption of direct interactions between schistosomes and IL-7. We also identify an indirect role that another γc chain cytokine plays in schistosome development, demonstrating that IL-2 expression by CD4+ T cells is essential for normal parasite development. Thus, cytokines critical for CD4+ T cell survival and function can mediate indirect but potent effects on developing schistosomes and underscore the importance of CD4+ T cells in facilitating schistosome development.
The blood fluke Schistosoma mansoni is a causative agent of schistosomiasis and a major global health problem. After penetrating the skin of the host, schistosomula migrate via the bloodstream to the portal circulation, arriving during the second week of infection [1]. On reaching the liver, migration ceases, and rapid growth and development ensues [2], culminating in the sexual maturation of the parasites, pairing, and the onset of oviposition at ~5 weeks after infection. Eggs are deposited in and transit across the wall of the intestine, emerging into the lumen and passing from the body in feces. Deposition of eggs in host tissues and the resulting inflammatory and immune responses are the primary cause of the pathological and clinical manifestations associated with schistosome infection.
Deposition of schistosome eggs in the bowel wall and other tissues induces formation of granulomas around the eggs, a process that is mediated by CD4+ Th2 responses to egg antigens [3]. Formation of granulomas around schistosome eggs appears to facilitate the transit of eggs across the intestinal wall and their egress from the body [4]. Interestingly, CD4+ T cells also facilitate S. mansoni development during the prepatent period, because parasite development is severely impaired in immunodeficient mice and normal development is restored by adoptive transfer of CD4+ T cells into immunodeficient mice [5]. Taken together, these findings may explain why patients with schistosomiasis who are coinfected with HIV excrete fewer eggs in their feces than patients who are HIV negative [6]. Observations from patients with schistosome-HIV coinfection highlight a general recurring theme in helminth-HIV coinfections—that, in contrast to bacteria, fungi, and protozoa, immunosuppression by HIV does not lead to fulminant helminth infections [7]. Rather, HIV coinfection either has little effect on helminth infections, as is the case with most intestinal helminths [7], or may create conditions that are unfavorable to helminth development in the host, as is the case for Strongyloides stercoralis infection [8]. The latter finding correlates well with the notable lack of association between HIV infection and disseminated strongyloidiasis [7].
Attenuated schistosome development was also reported in IL-7–deficient (IL-7−/−) mice [9], suggesting that this host factor may play a direct role in promoting parasite development. Because IL-7 is not expressed by T lymphocytes but is produced by endothelial and epithelial cells such as thymic stromal cells, it is possible that IL-7 represents a distinct mechanism by which host factors modulate schistosome development. However, IL-7 is critical for the thymic development of T lymphocytes and their subsequent survival in the periphery, as demonstrated by the severely lymphopenic phenotype of IL-7−/− [10] and IL-7 receptor (IL-7R)−/− [11] mice. We, therefore, hypothesized that the defective schistosome development reported in IL-7−/− mice was the indirect result of impaired T cell development and T lymphopenia in the absence of IL-7 signaling rather than the result of a direct effect that IL-7 has on schistosomes. To test this hypothesis, we examined schistosome infections in mice that lack components of the IL-7R complex (IL-7Rα and common γ [γc] chains) because IL-7 signaling is abrogated in these mice [11, 12] but IL-7 synthesis is intact, as demonstrated by their ability to sustain homeostatic proliferation of adoptively transferred IL-7R–expressing T cells [13]. Our data support the conclusion that the effects that IL-7 has on schistosome development are indeed mediated indirectly via the effects that IL-7 has on T cell homeostasis. Furthermore, our analyses identify an indirect role that another γc cytokine, IL-2, plays in schistosome development. Thus, although expression of Th1 or Th2 phenotypes and their associated effector cytokines by CD4+ T cells does not play a role in schistosome development [5, 14], autocrine production of the T cell growth factor IL-2 by CD4+ T cells is essential to the mechanism by which these cells modulate blood fluke development.
MATERIALS AND METHODS
Experimental mice
Wild-type, IL-7Rα−/− γc−/−, IL-2−/−, CD25/IL-2Rα−/−, and recombination activating gene (RAG)–1−/− mice were purchased from Jackson Laboratory. All mice possessed the C57BL/6 genetic background. Mice were age and sex matched in each experiment and were infected when ~8 weeks of age. All IL-2−/− [15] and IL-2Rα−/− [16] mice were infected at an early age, before the onset of autoimmune disease, and were assessed for gross and histologic evidence of autoimmune disease at necropsy. All mice were infected percutaneously via the tail skin with 150 S. mansoni cercariae (Puerto Rican strain) shed from infected Biomphalaria glabrata snails [17]. All experiments included 4–5 mice per group, were performed at least twice, and were in accordance with protocols approved by our institutional animal care and use committees.
Assessment of parasite phenotype
Worms were perfused from the portal vasculature 42 days after infection and immediately fixed in 4% neutral buffered formaldehyde to prevent pairs from dissociating. Worms were counted and sexed, and the number of pairs was determined by microscopy. To determine parasite egg burden in the liver, livers were digested in 0.7% trypsin, and eggs were counted from aliquots of liver digest. For analysis of worm size, digital images were acquired using a Nikon Coolpix 4500 camera attached to a dissecting microscope, and worm length was measured using National Institutes of Health Image J software (available at: http://rsb.info.nih.gov/ij/). At least 15 randomly selected males were measured in each sample.
Cell isolation
Spleens and lymph nodes were removed aseptically and dispersed through 70-µm nylon filters to produce single cell suspensions. Splenocytes were treated with 0.16 mol/L ammonium chloride/0.17 mol/L Tris (pH 7.2) to lyse erythrocytes. Bulk CD4+ T cells were isolated using anti-CD4–labeled magnetic microbeads (Miltenyi Biotec). In experiments utilizing CD25+CD4+ and CD25−CD4+ T cells, these populations were separated by fluorescence-activated cell sorting on a MoFlo cytometer (Cytomation) after staining with fluorescein isothiocyanate–conjugated anti-CD4, phycoerythrin-conjugated anti-NK1.1, CyChrome-conjugated anti-TCRβ, and APC-Cy7–labeled anti-CD25 antibodies, in the presence of unlabeled antibodies to CD16 and CD32 (Fc Block; all from BD Biosciences).
Adoptive transfer experiments
Bulk CD4+ T cells and CD25+CD4+ or CD25−CD4+ cells were isolated, and 4 × 106 cells/mouse were transferred into RAG-1−/− mice by intravenous injection into a lateral tail vein 1 day before infection. Control mice received buffer alone. At 42 days after infection, parasite development was assessed, and splenic CD4+ T cell numbers were determined by flow cytometry.
Statistical analysis
Experimental groups in many experiments showed unequal variances based on the F test or analysis of variance. Hence, the Mann-Whitney U test and the Kruskal-Wallis test were used to determine the statistical significance of differences in median values from different experimental groups. In experiments involving 3 different experimental groups, Dunn’s multiple comparison tests were employed to evaluate differences between each pair of experimental groups. P < .05 was considered to be significant. Statistical analyses were performed using GraphPad Prism software (version 4; GraphPad Software).
RESULTS
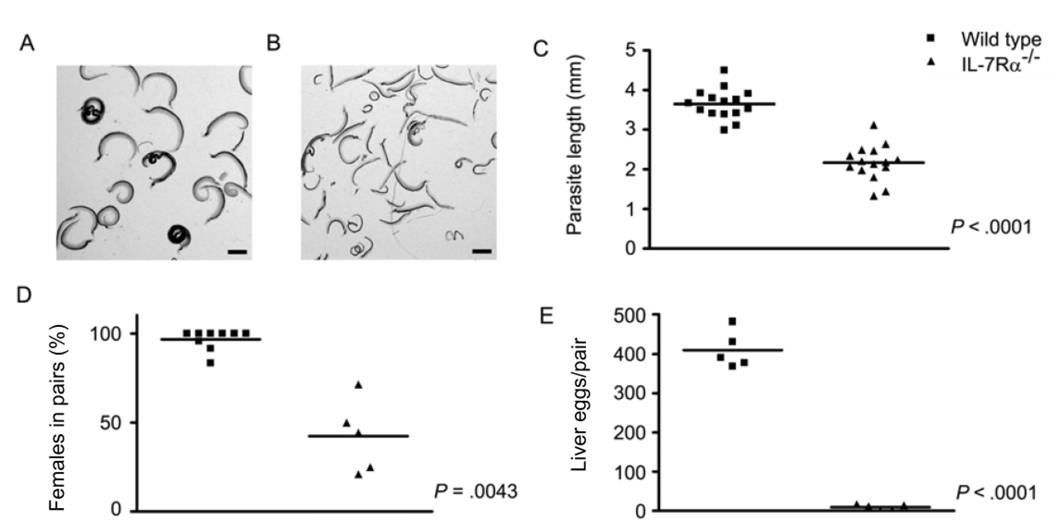
Infection of IL-7Rα−/− mice revealed that schistosome development is severely compromised in the absence of the IL-7Rα chain (figure 1). First, S. mansoni worms isolated from IL-7Rα−/− mice (figure 1B) were visibly smaller than those from wild-type controls (figure 1A) at the same time point after infection. Quantitation of parasite length from digital micrographs revealed that S. mansoni males attained only 60% of the length of males from wild-type mice by 6 weeks after infection (P < .0001) (figure 1C). The percentage of females participating in pairs (42%) in IL-7Rα−/− mice was also significantly less than in wild-type mice (97%) (P < .0001) (figure 1D). Finally, egg production by pairs that did form was drastically reduced in IL-7Rα−/− mice, compared with that in wild-type mice, with pairs from IL-7Rα−/− mice producing only 2% of the eggs that worm pairs in wild-type mice produced (P < .0001) (figure 1E). This degree of attenuation in parasite growth, development, and reproductive activity is similar to that seen in RAG-1−/− mice at 6 weeks after infection [5].
Figure 1.
Schistosoma mansoni development in interleukin-7 receptor α–deficient (IL-7Rα−/−) mice. IL-7Rα−/− and wild-type mice were infected percutaneously with S. mansoni cercariae, and parasite development and egg production were assessed at 6 weeks after infection. A, S. mansoni worms isolated from wild-type mice. B, S. mansoni worms isolated from IL-7Rα−/− mice. C, Length of male S. mansoni worms isolated from wild-type and IL-7Rα−/− mice. D, Percentage of female worms participating in pairs in wild-type and IL-7Rα−/− mice. E, No. of eggs deposited per pair of worms in the livers of wild-type and IL-7Rα−/− mice. The scale bars in panels A and B represent a length of 1 mm. P values were calculated using the Mann-Whitney U test.
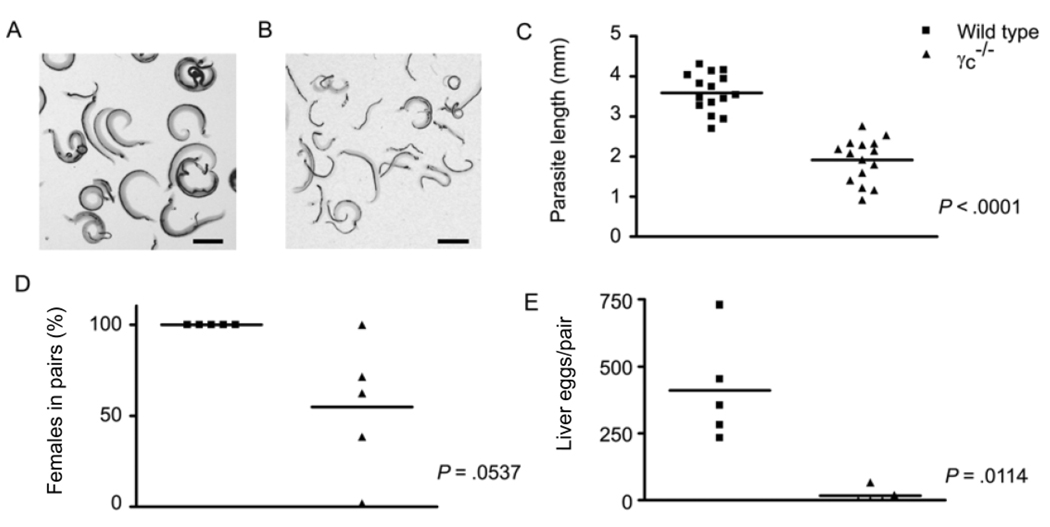
Deletion of the other component of the IL-7R complex, the γc chain, also severely impaired schistosome development (figure 2). First, worms recovered at 6 weeks after infection from γc−/− hosts (figure 2B) were visibly smaller than those from wild-type controls (figure 2A). Determination of parasite length showed that male schistosomes from γc−/− mice were only 53% of the size of those from wild-type controls (P < .0001) (figure 2C)—an even greater reduction than that observed in IL-7Rα−/− mice. The percentage of female worms in pairs was reduced in γc−/− mice, compared with that in wild-type controls (figure 2D), but this result narrowly avoided attaining statistical significance in the experiment shown (P = .0537) because of the considerable number of small paired worms that occurred in some γc−/− mice (a phenotype occasionally seen in some RAG−/− mice; see below). However, egg production by the pairs that did form in γc−/− mice was drastically reduced, compared with that in wild-type mice, with egg production reduced to only 5% of that seen in wild-type mice (P = .002) (figure 2E).
Figure 2.
Schistosoma mansoni development in common γ chain–deficient (γc−/−) mice. γc−/− and wild-type mice were infected percutaneously with S. mansoni cercariae, and parasite development and egg production were assessed at 6 weeks after infection. A, S. mansoni worms isolated from wild-type mice. B, S. mansoni worms isolated from γc−/− mice. C, Length of male S. mansoni worms isolated from wild-type and γc−/− mice. D, Percentage of female worms participating in pairs in wild-type and γc−/− mice. E, No. of eggs deposited per pair of worms in the livers of wild-type and γc−/− mice. The scale bars in panels A and B represent a length of 1 mm. P values were calculated using the Mann-Whitney U test.
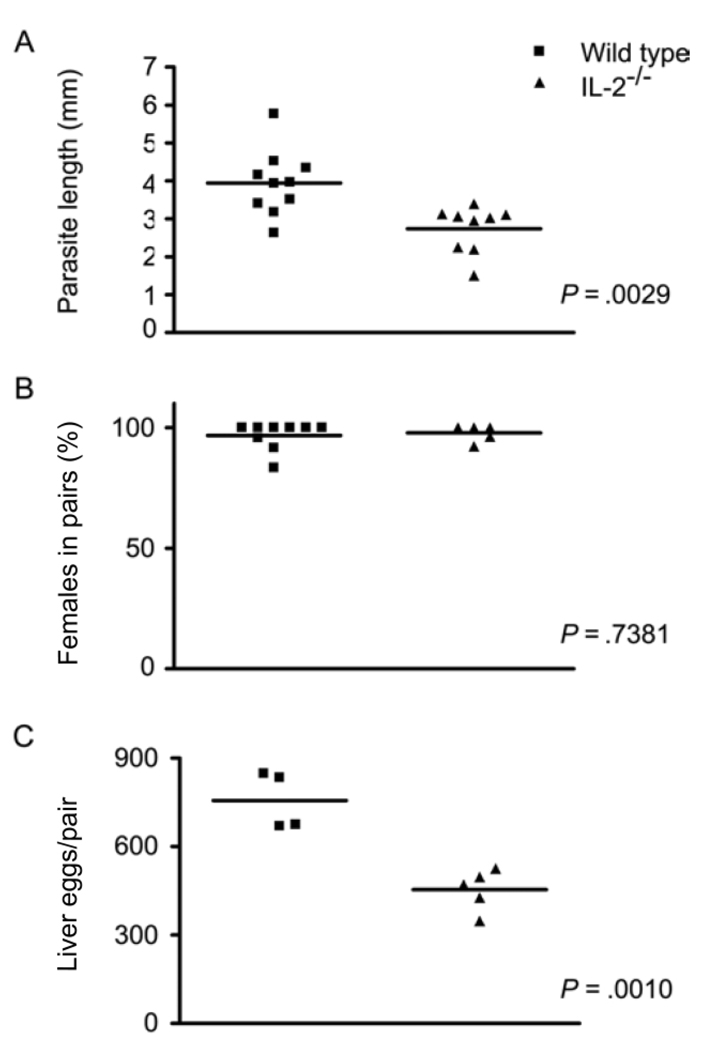
Because parasites isolated from γc−/− mice were even smaller than those from IL-7Rα−/− mice, compared with those from wild-type controls, we hypothesized that signaling by other γc chain cytokines might also influence schistosome development. To test this hypothesis, we examined schistosome development in mice in which IL-2 signaling is specifically disrupted. Although considerably less severe than those observed in IL-7Rα−/−, γc−/−, and RAG-1−/− mice, defects in the development of S. mansoni worms were detected in IL-2−/− mice (figure 3). Worms recovered from IL-2−/− mice were smaller than those from wild-type controls, with males being ~63% the size of males from wild-type mice (P = .0029) (figure 3A). More than 90% of all female worms recovered from IL-2−/− mice were paired (figure 3B), indicating that there is no detectable defect in worm pairing in IL-2−/− mice. However, egg production by worm pairs in IL-2−/− mice was significantly reduced to only 60% of the levels found in wild-type mice (P = .0010) (figure 3C).
Figure 3.
Schistosoma mansoni development in interleukin (IL)–2–deficient (IL-2−/−) mice. IL-2−/− and wild-type mice were infected percutaneously with S. mansoni cercariae, and parasite development and egg production were assessed at 6 weeks after infection. A, Length of male S. mansoni worms isolated from wild-type and IL-2−/− mice. B, Percentage of female worms participating in pairs in wild-type and IL-2−/− mice. C, No. of eggs deposited per pair of worms in the livers of wild-type and IL-2−/− mice. P values were calculated using the Mann-Whitney U test.
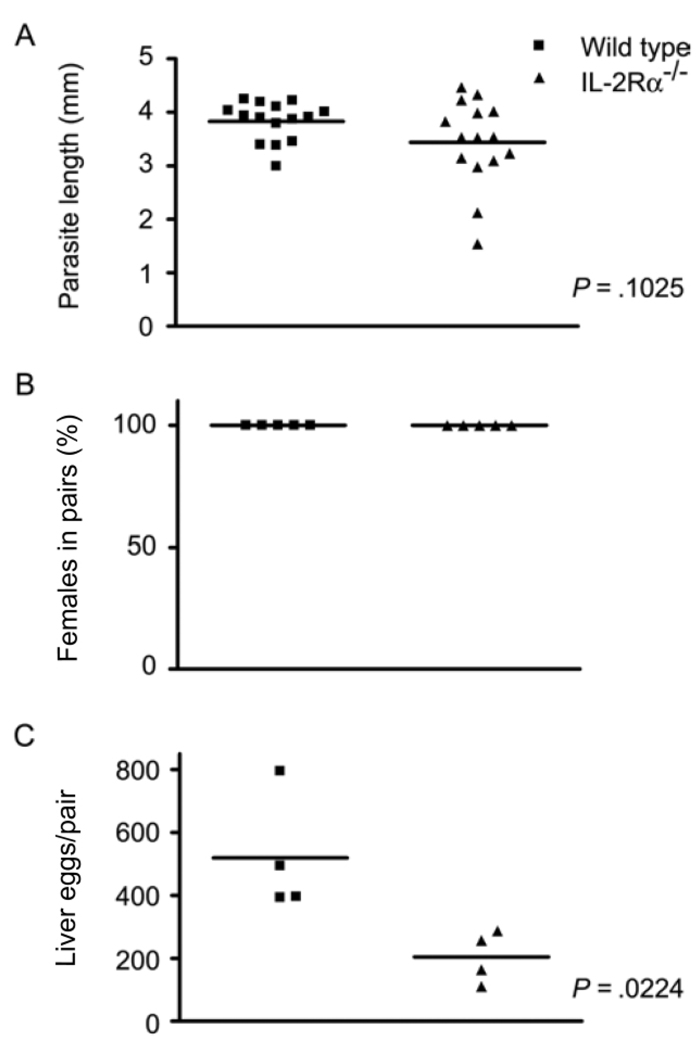
To determine whether IL-2 acted directly on the parasite or whether IL-2 signaling via its receptor was required for normal schistosome development, parasite development was examined in IL-2Rα−/− (CD25−/−) mice [16], in which IL-2 is produced but transduction of IL-2 signals is impaired. The phenotype of S. mansoni worms isolated from IL-2Rα−/− mice (figure 4) was similar to that of worms from IL-2−/− mice (figure 3), with evidence of developmental impairment that was less pronounced than that observed in IL-7Rα−/− (figure 1), γc−/− (figure 2), and RAG-1−/− mice [5]. Worms recovered from IL-2Rα−/− mice were smaller than worms from wild-type controls, (figure 4A), although this difference was not statistically significant (P = .1025) when the lengths of male parasites were directly compared. However, the variances of the 2 populations were significantly different (P = .0028, F test), suggesting that schistosome growth in IL-2Rα−/− mice is altered, compared with that of worms in wild-type mice. Similar to IL-2−/− mice, worm pairing was not decreased in IL-2Rα−/− mice (figure 4B), but egg production was reduced to 39% of that in wild-type controls in IL-2Rα−/− mice (P = .0224) (figure 4C).
Figure 4.
Schistosoma mansoni development in interleukin-2 receptor α–deficient (IL-2Rα−/−) mice. IL-2Rα−/− and wild-type mice were infected percutaneously with S. mansoni cercariae, and parasite development and egg production were assessed at 6 weeks after infection. A, Length of male S. mansoni worms isolated from wild-type and IL-2Rα−/− mice. B, Percentage of female worms participating in pairs in wild-type and IL-2Rα−/− mice. C, No. of eggs deposited per pair of worms in the livers of wild-type and IL-2Rα−/− mice. P values were calculated using the Mann-Whitney U test.
Because IL-2−/− and IL-2Rα−/− mice are prone to develop autoimmune disease with age, young IL-2−/− mice were used for all experiments, and all were examined at necropsy for signs of immunopathological damage [15]. Experimental mice showed no overt autoimmune disease, and we, therefore, conclude that the altered parasite phenotype in these mice was not secondary to autoimmune disease.
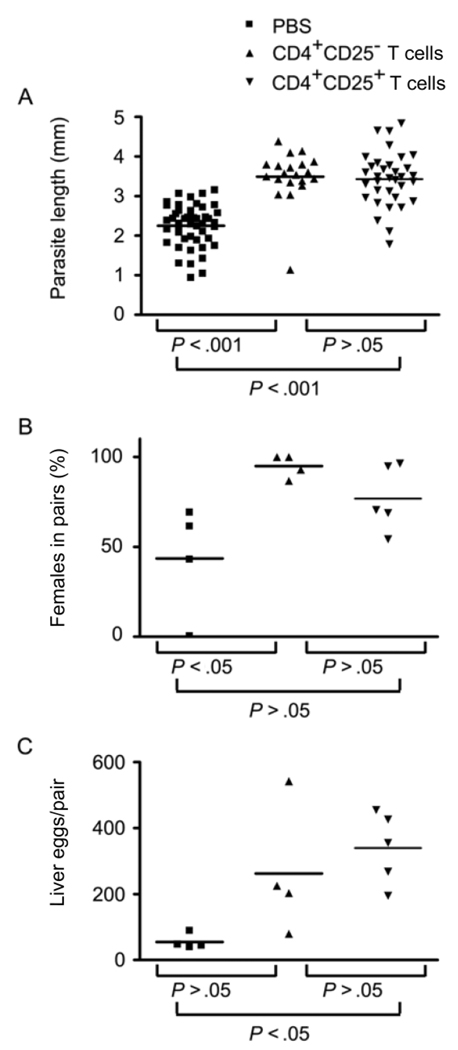
Because IL-2 has been shown to play an essential role in the survival and function of CD4+CD25+ regulatory T (Treg) cells in the periphery [18], we hypothesized that the impaired schistosome development observed in IL-2−/− and IL-2Rα−/− mice might be due to a deficit in peripheral Treg cell populations. To test this hypothesis, we assessed the ability of wild-type CD4+ CD25+ Treg cells and of CD4+ T cells depleted of CD4+CD25+ Treg cells to rescue S. mansoni development in RAG−/− mice (figure 5). CD4+CD25− T cells and CD4+CD25+ T cells repopulated RAG-1−/− recipients to similar levels (data not shown). Adoptive transfer of either CD4+CD25− T cells or CD4+CD25+ T cells into RAG-1−/− mice successfully restored parasite growth (figure 5A), with worms from both groups of recipients being similar in size and significantly bigger than worms from non-reconstituted RAG-1−/− mice. Both cell populations also increased levels of parasite pairing (figure 5B), with a greater proportion of females participating in pairs in both adoptively transferred groups. Although this result was significantly different from that in nonreconstituted RAG-1−/− mice only when CD4+CD25− T cells were transferred, transfer of CD4+CD25+ T cells produced an intermediate result that was not significantly different from that in either nonreconstituted mice or those receiving CD4+CD25− T cells. Both cell populations also enhanced schistosome egg production (figure 5C), although, in this instance, egg production was significantly higher only in mice receiving CD4+CD25+ T cells, with adoptive transfer of CD4+CD25− T cells producing an intermediate phenotype that was not significantly different from that in either nonreonstituted RAG-1−/− mice or those receiving CD4+CD25+ T cells. Thus, adoptive transfer of either CD4+CD25+ or CD4+CD25− T cells significantly enhanced parasite development as measured by 2 of the 3 parasitological parameters we analyzed and produced an intermediate phenotype in the third.
Figure 5.
Schistosoma mansoni development in recombination activating gene (RAG)–1–deficient (RAG-1−/−) mice reconstituted with CD4+CD25− and CD4+CD25+ T cells from wild-type donors. One day before infection, RAG-1−/− recipients were reconstituted with 4 × 106 CD4+CD25− or CD4+CD25+ T cells from wild-type donors, and S. mansoni worm development and egg production were assessed at 6 weeks after infection. A, Length of male S. mansoni worms isolated from RAG-1−/− recipients reconstituted with PBS alone, CD4+CD25− T cells, or CD4+CD25+ T cells; overall P < .0001, Kruskal-Wallis test. B, Percentage of female worms participating in pairs in RAG-1−/− recipients reconstituted with PBS alone, CD4+CD25− T cells, or CD4+CD25+ T cells; overall P = .0306, Kruskal-Wallis test. C, No. of eggs deposited per pair of worms in the livers of RAG-1−/− recipients reconstituted with PBS alone, CD4+CD25− T cells, or CD4+CD25+ T cells; overall P = .0266, Kruskal-Wallis test. In panels A, B, and C, P values for each experimental group pair were calculated using Dunn’s multiple comparison test.
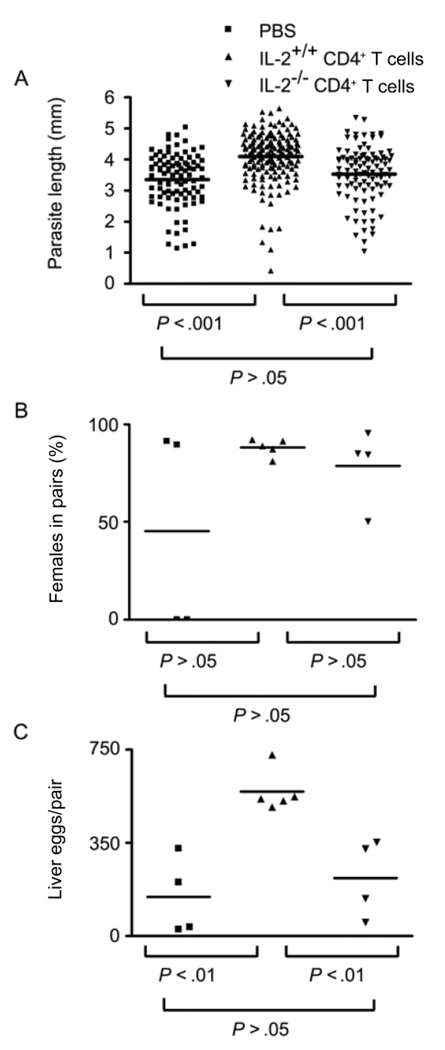
To test whether autocrine IL-2 production by CD4+ T cells is required to rescue schistosome development in RAG−/− mice, RAG-1−/− mice were reconstituted with CD4+ T cells isolated from either IL-2−/− or wild-type IL-2+/+ donors. Flow cytometric analysis of all recipients revealed that IL-2−/− CD4+ T cells repopulated RAG-1−/− recipients to the same levels as wild-type CD4+ T cells (data not shown). However, adoptive transfer of IL-2−/− T cells failed to rescue schistosome growth (figure 6A), with male parasites being identical in size to those from nonreconstituted RAG−/− mice and significantly smaller than those from RAG−/− mice reconstituted with wild-type CD4+ T cells (figure 6A). We could also find no evidence that adoptive transfer of IL-2−/− CD4+ T cells increased parasite pairing beyond those levels observed in nonreconstituted RAG-1−/− mice (figure 6B), although, in the experiment shown, neither non-reconstituted RAG-1−/− mice nor those receiving IL-2−/− CD4+ T cells showed significantly lower levels of pairing than those observed in IL-2+/+ T cell–reconstituted mice because of the unusually high proportion of small, paired female worms seen in some of the non-reconstituted and IL-2−/− T cell–reconstituted RAG-1−/− mice. However, egg production by worm pairs that did form in IL-2−/− CD4+ T cell–reconstituted RAG-1−/− recipients was very low, approximating that of pairs in non-reconstituted RAG-1−/− mice and significantly less than that of pairs from RAG-1−/− recipients of wild-type CD4+ T cells (figure 6C), indicating that IL-2−/− CD4+ T cells could not restore the fecundity of schistosome pairs in RAG-1−/− mice.
Figure 6.
Schistosoma mansoni development in recombination activating gene (RAG)–1–deficient (RAG-1−/−) mice reconstituted with interleukin (IL)–2−/− or wild-type CD4+ T cells. One day before infection, RAG-1−/− recipients were reconstituted with 4 × 106 CD4+ T cells from wild-type (IL-2+/+) or IL-2−/− donors, and S. mansoni worm development and egg production were assessed at 6 weeks after infection. A, Length of male S. mansoni worms isolated from RAG-1−/− recipients reconstituted with PBS alone, IL-2+/+ CD4+ T cells, or IL-2−/− CD4+ T cells); overall P <.0001, Kruskal-Wallis test. B, Percentage of female worms participating in pairs in RAG-1−/− recipients reconstituted with PBS alone, IL-2+/+ CD4+ T cells, or IL-2−/− CD4+ T cells; overall P = .5833, Kruskal-Wallis test. C, No. of eggs deposited per pair of worms in the livers of RAG-1−/− recipients reconstituted with PBS alone, IL-2+/+ CD4+ T cells, or IL-2−/− CD4+ T cells; overall P = .0474, Kruskal-Wallis test. In panels A, B, and C, P values for each experimental group pair were calculated using Dunn’s multiple comparison test.
DISCUSSION
We have previously shown that signals from the host’s adaptive immune system are essential for normal schistosome development and that CD4+ αβ T cells play a central role in supplying the host signals required by the parasite [5]. Elegant studies by Wolowczuk et al. [9, 19] have added considerably to our understanding of the requirements for schistosome development in the vertebrate host and have implicated several other host factors in modulating schistosome development, including the cytokine IL-7 [9]. Because IL-7 is a critical survival factor for T cells and plays a central role in T cell homeostasis [10, 11, 20], we hypothesized that the effects that IL-7 has on schistosome development are mediated indirectly via its effect on peripheral CD4+ T cells. To test this hypothesis, we analyzed worm development in mice that lacked the IL-7Rα and γc chains, both of which are required for IL-7 signaling. The developmental phenotype of schistosomes in IL-7Rα−/− and γc−/− mice (figure 1 and figure 2) was similar to that observed in IL-7−/− mice [9], indicating that signaling via the IL-7 receptor is required to promote worm development and that IL-7 itself does not act directly on the parasite. This conclusion is supported by our finding that schistosome development is severely attenuated in RAG-1−/− mice, which lack all B and T cells but express high levels of IL-7 that support massive homeostatic proliferation of donor T cells on adoptive transfer [13]. Thus, we conclude that the attenuated schistosome development observed in IL-7−/−, IL-7Rα−/−, and γc−/− mice is explained by the severely lymphopenic state of these mice that results from the loss of IL-7 signaling. The results from γc−/− mice also indicate that the abnormal schistosome development observed in RAG−/− mice is not due to overrepresentation of NK cells in these mice, because γc−/− mice display a similar parasite phenotype to RAG−/− mice yet lack all NK cells through loss of IL-15 signaling [12].
Because schistosome growth was more severely attenuated in γc−/− mice than in IL-7Rα−/− mice, we hypothesized that the other γc chain–dependent cytokines—IL-2, IL-4, IL-9, IL-15, and IL-21—might influence schistosome development. Because there is currently no evidence that parasite development is altered in IL-4−/− [21, 22], IL-9−/− [23], IL-15−/− (J.H.M. and S.J.D., unpublished data), or IL-21 signaling–deficient mice [24], we focused our attention on IL-2, which, in addition to its long-recognized autocrine role in the proliferation and function of Th1 cells [25, 26], is now known to be critical to the survival and functionality of CD4+ CD25+ Treg cells [18] and to the early differentiation of Th2 cells [27]. Significant alterations in worm development were observed on infection of IL-2−/− mice (figure 3), consistent with the central role that this cytokine plays in CD4+ T cell function. That similar modulation of parasite development is observed in IL-2Rα−/− mice (figure 4) supports the conclusion that, like IL-7, IL-2 mediates its effects on schistosome development indirectly via CD4+ T cells, because IL-2Rα−/− mice can express IL-2 but cannot respond to it. An indirect role for IL-2 in schistosome development is further supported by the fact that administration of large doses of IL-2 to infected RAG-1−/− mice has no effect on worm development (data not shown).
The diversity of roles played by IL-2 in CD4+CD25+ Treg cell function [20] and CD4+ T helper responses [25–27] suggests that the indirect effect that IL-2 has on schistosome development may be mediated by a number of different mechanisms. To test whether the defective parasite development observed in IL-2−/− and IL-2Rα−/− mice could be specifically attributed to a lack of CD4+CD25+ Treg cells, we assessed the ability of these cells to rescue schistosome development in RAG-1−/− mice. Interestingly, transfer of purified CD4+CD25+ Treg cells or CD4+CD25− T cells both rescued schistosome development in RAG-1−/− mice (figure 5), indicating that, although CD4+CD25+ Treg cells have the ability to restore parasite development, these cells are not specifically required and other CD4+ T cells can mediate the same effects. Because CD4+CD25+ Treg cells exhibit different cytokine production profiles and specificities from other CD4+ T cells [28], these results suggest that T cell specificity and cytokine expression patterns may not be important for schistosome development and that other, more fundamental properties of CD4+ T cells that are common to both regulatory and nonregulatory cells are the critical determinants for parasite development. These possibilities are currently under investigation.
Because we could not identify a specific role for CD4+CD25+ Treg cells in schistosome development, we tested whether expression of IL-2 by CD4+ T cells is required to rescue schistosome development by adoptively transferring IL-2−/− CD4+ T cells into RAG-1−/− mice. IL-2−/− CD4+ T cells failed to restore parasite growth or egg production in RAG-1−/− mice, with worms from these mice exhibiting a phenotype that was indistinguishable from that of worms obtained from nonrecontituted mice (figure 6). Thus, IL-2 expression by CD4+ T cells is crucial for their ability to facilitate schistosome development. This deficit is not related to defects in Th function, because schistosome development proceeds normally in mice in which the Th1 and Th2 arms of the CD4+ T cell response are disrupted [5, 14]. Rather, the function of IL-2 in schistosome development might be related to more fundamental roles that IL-2 play in CD4+ T cell survival, such as homeostatic maintenance mechanisms. Although IL-2 is required for the homeostasis of CD4+CD25+ Treg cells [18], a similar role has not been identified for nonregulatory populations, and this possibility is under investigation. Alternatively, transcription of the IL-2 gene is an early and vital step in the response of T cells to antigen [29], and thus, although we cannot identify a specific role for Th1 or Th2 responses in schistosome development, there may yet be a role for fundamental responses of CD4+ T cells to antigen in the process by which these cells facilitate parasite development. The role that CD4+ T cell activation by antigen plays in schistosome development is currently being investigated.
In summary, our data show that the cytokines IL-7 and IL-2 modulate schistosome development indirectly through their effects on CD4+ T cells. Because IL-7 is critical to T cell homeostasis and loss of IL-7 signaling results in severe T lymhopenia, defective schistosome development in this context (e.g., IL-7−/−, IL-7Rα−/−, and γc−/− mice) is predictable. However, that IL-2 is required for normal parasite development is intriguing, providing insights into the mechanisms by which CD4+ T cells facilitate schistosome development. A detailed understanding of these mechanisms may identify rational approaches to interrupting schistosome development for therapeutic and prophylactic purposes.
Acknowledgments
We thank Cliff McArthur and Karen Wolcott for assistance with flow cytometric cell sorting.
Financial support: National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants K22 AI053054 and R01 AI066227 to S.J.D.); Sandler Family Foundation (to J.H.M.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Georgi JR, Wade SE, Dean DA. Attrition and temporal distribution of Schistosoma mansoni and S. haematobium schistosomula in laboratory mice. Parasitology. 1986;93:55–70. doi: 10.1017/s0031182000049829. [DOI] [PubMed] [Google Scholar]
- 2.Georgi JR, Wade SE, Dean DA. Schistosoma mansoni: mechanism of attrition and routes of migration from lungs to hepatic portal system in the laboratory mouse. J Parasitol. 1987;73:706–711. [PubMed] [Google Scholar]
- 3.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 4.Doenhoff M, Musallam R, Bain J, McGregor A. Studies on the host-parasite relationship in Schistosoma mansoni-infected mice: the immunological dependence of parasite egg excretion. Immunology. 1978;35:771–778. [PMC free article] [PubMed] [Google Scholar]
- 5.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 6.Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya. I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56:515–521. doi: 10.4269/ajtmh.1997.56.515. [DOI] [PubMed] [Google Scholar]
- 7.Karp CL, Neva FA. Tropical infectious diseases in human immunodeficiency virus–infected patients. Clin Infect Dis. 1999;28:947–963. doi: 10.1086/514745. quiz 964�5. [DOI] [PubMed] [Google Scholar]
- 8.Viney ME, Brown M, Omoding NE, et al. Why does HIV infection not lead to disseminated strongyloidiasis? J Infect Dis. 2004;190:2175–2180. doi: 10.1086/425935. [DOI] [PubMed] [Google Scholar]
- 9.Wolowczuk I, Nutten S, Roye O, et al. Infection of mice lacking interleukin-7 (IL-7) reveals an unexpected role for IL-7 in the development of the parasite Schistosoma mansoni. Infect Immun. 1999;67:4183–4190. doi: 10.1128/iai.67.8.4183-4190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 13.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 14.Davies SJ, Lim KC, Blank RB, et al. Involvement of TNF in limiting liver pathology and promoting parasite survival during schistosome infection. Int J Parasitol. 2004;34:27–36. doi: 10.1016/j.ijpara.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 16.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 17.Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 18.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 19.Saule P, Adriaenssens E, Delacre M, et al. Early variations of host thyroxine and interleukin-7 favor Schistosoma mansoni development. J Parasitol. 2002;88:849–855. doi: 10.1645/0022-3395(2002)088[0849:EVOHTA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Yu Q, Erman B, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Pearce EJ, Cheever A, Leonard S, et al. Schistosoma mansoni in IL-4-deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 22.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 23.Fallon PG, Jolin HE, Smith P, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 24.Pesce J, Kaviratne M, Ramalingam TR, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellery JM, Nicholls PJ. Possible mechanism for the alpha subunit of the interleukin-2 receptor (CD25) to influence interleukin-2 receptor signal transduction. Immunol Cell Biol. 2002;80:351–357. doi: 10.1046/j.1440-1711.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 27.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–6327. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 29.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]